Isolation, Identification and Antibacterial Characteristics of Lacticaseibacillus rhamnosus YT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Measurement of Antibacterial Activity
2.3. Screening of LAB with Antibacterial Activity
2.4. Molecular Identification
2.5. Growth and Antibacterial Curve of the Supernatant
2.6. Viable Count Method
2.7. Co-Incubation with Indicator Bacteria Assay
2.8. Sensitivity of Antimicrobial Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Screening and Identification of LAB Strain with Antibacterial Ability
3.2. Antibacterial Characteristics of the CFS from L. rhamnosus YT (CFS-YT)
3.2.1. Growth Kinetics and Antimicrobial Activity of the CFS-YT
3.2.2. The Characteristics of the Antimicrobial Activity of CFS-YT
3.3. Antibacterial Characteristics of the L. rhamnosus YT Cells (Cs-YT)
3.3.1. Antibacterial Activity of Cs-YT
3.3.2. Mode of Action
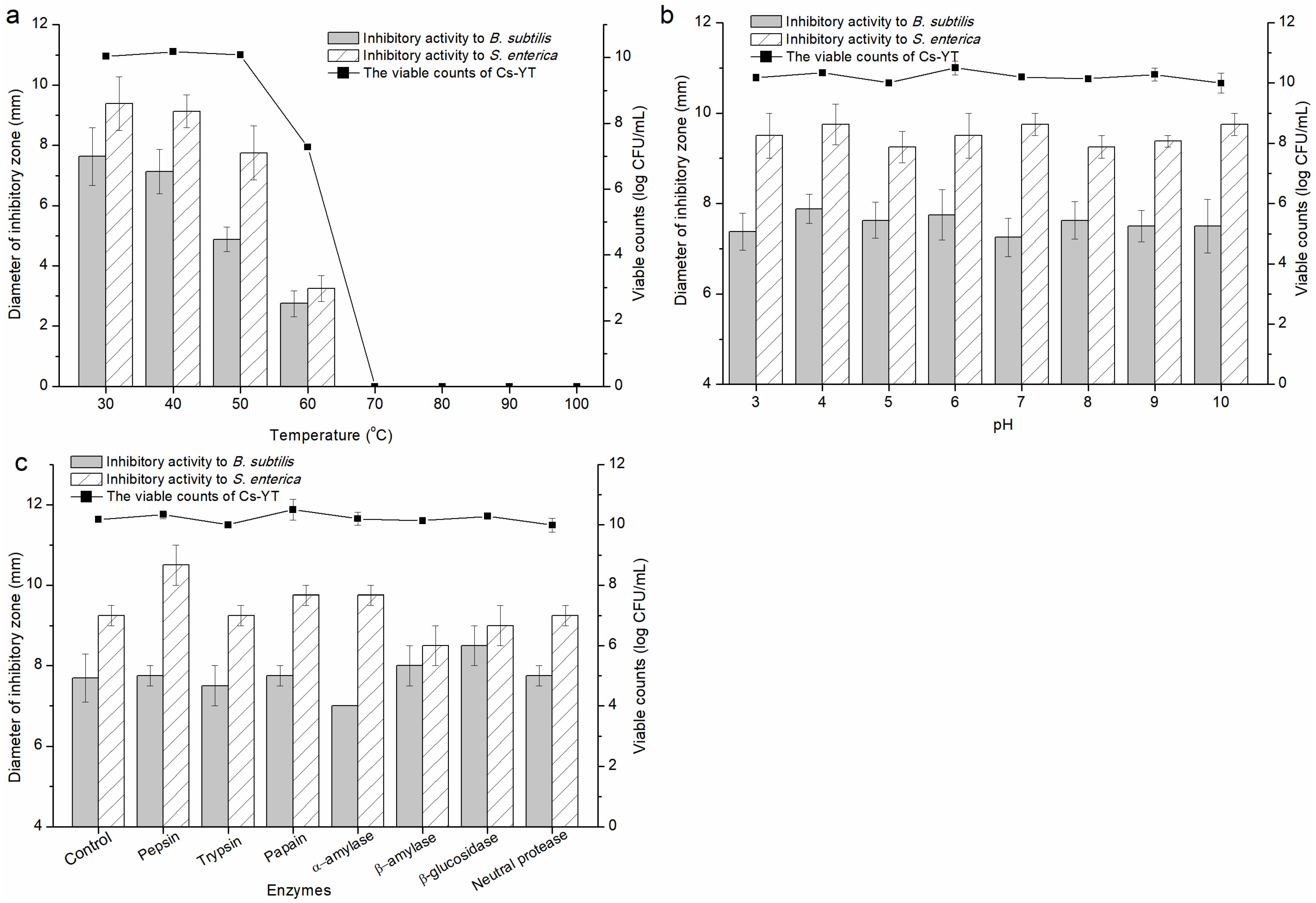
3.3.3. Factors Affecting the Activity of Cs-YT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Li, M.; Yue, X. Current research on probiotics and fermented products. Foods 2024, 13, 1406. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Skowron, K.; Budzyńska, A.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Andrzejewska, M.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Two faces of fermented foods-the benefits and threats of its consumption. Front. Microbiol. 2022, 13, 845166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.H.; Wu, L.H. Effects of governmental intervention on foodborne disease events: Evidence from China. Int. J. Environ. Res. Public Health 2021, 18, 13311. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Mardani, K.; Tajik, H. Characterization and application of postbiotics of Lactobacillus spp. on Listeria monocytogenes in vitro and in food models. LWT-Food Sci. Technol. 2019, 111, 457–464. [Google Scholar] [CrossRef]
- Zhao, S.; Han, J.; Bie, X.; Lu, Z.; Zhang, C.; Lv, F. Purification and characterization of plantaricin JLA-9: A novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a traditional Chinese fermented cabbage. J. Agric. Food Chem. 2016, 64, 2754–2764. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dicks, L.M.T. Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria. Enzyme Microb. Tech. 2005, 36, 318–326. [Google Scholar] [CrossRef]
- von Mollendorff, J.W.; Todorov, S.D.; Dicks, L.M.T. Comparison of bacteriocins produced by lactic-acid bacteria isolated from boza, a cereal-based fermented beverage from the Balkan Peninsula. Curr. Microbiol. 2006, 53, 209–216. [Google Scholar] [CrossRef]
- Ahmad, V.; Ahmad, K.; Baig, M.H.; AL-Shwaiman, H.A.; Al Khulaifi, M.M.; Elgorban, A.M.; Khan, M.S. Efficacy of a novel bacteriocin isolated from Lysinibacillus sp. against Bacillus pumilus. LWT-Food Sci. Technol. 2019, 102, 260–267. [Google Scholar] [CrossRef]
- Meng, J.; Gao, S.M.; Zhang, Q.X.; Lu, R.R. Murein hydrolase activity of surface layer proteins from Lactobacillus acidophilus against Escherichia coli. Int. J. Biol. Macromol. 2015, 79, 527–532. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Yang, K.M.; Kim, J.S.; Kim, H.S.; Kim, Y.Y.; Oh, J.K.; Jung, H.W.; Park, D.S.; Bae, K.H. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci. Rep. 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Chen, X.; Zhao, R.; Yuan, Y.; Huang, X.; Su, J.; Ding, X.L.; Chen, X.; Huang, Y.J.; Gu, R.X. A weak post-acidification Lactobacillus helveticus UV mutant with improved textural properties. Food Sci. Nutr. 2021, 9, 469–479. [Google Scholar] [CrossRef]
- Zhu, W.M.; Liu, W.; Wu, D.Q. Isolation and characterization of a new bacteriocin from Lactobacillus gasseri KT7. J. Appl. Microbiol. 2000, 88, 877–886. [Google Scholar] [CrossRef]
- Milioni, C.; Martinez, B.; Degl’Innocenti, S.; Turchi, B.; Fratini, F.; Cerri, D.; Fichetti, R. A novel bacteriocin produced by Lactobacillus plantarum LpU4 as a valuable candidate for biopreservation in artisanal raw milk cheese. Dairy Sci. Technol. 2015, 95, 479–494. [Google Scholar] [CrossRef]
- Cabo, M.L.; Braber, A.F.; Koenraad, P.M.F.J. Apparent antifungal activity of several lactic acid bacteria against penicillium discolor is due to acetic acid in the medium. J. Food Prot. 2002, 65, 1309–1316. [Google Scholar] [CrossRef]
- Hussain, N.; Tariq, M.; Saris, P.E.J.; Zaidi, A. Evaluation of the probiotic and postbiotic potential of lactic acid bacteria from artisanal dairy products against pathogens. J. Infect. Dev. Ctries. 2021, 15, 102–112. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y.; Sun, Y.; Gu, Q. Purification and characterisation of plantaricin ZJ008, a novel bacteriocin against Staphylococcus spp. from Lactobacillus plantarum ZJ008. Food Chem. 2014, 165, 216–223. [Google Scholar] [CrossRef]
- Lv, X.R.; Miao, L.H.; Ma, H.H.; Bai, F.L.; Lin, Y.; Sun, M.T.; Li, J.R. Purification, characterization and action mechanism of plantaricin JY22, a novel bacteriocin against Bacillus cereus produced by Lactobacillus plantarum JY22 from golden carp intestine. Food Sci. Biotechnol. 2018, 27, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Makras, L.; Triantafyllou, V.; Fayol-Messaoudi, D.; Adriany, T.; Zoumpopoulou, G.; Tsakalidou, E.; Servin, A.; Vuyst, L.D. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar typhimurium reveals a role for lactic acid and other inhibitory compounds. Res. Microbiol. 2006, 157, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Pan, T.M. Characterization of an antimicrobial substance produced by Lactobacillus plantarum NTU 102. J. Microbiol. Immunol. Infect. 2019, 52, 409–417. [Google Scholar] [CrossRef]
- Todorov, S.D.; Cavicchioli, V.Q.; Ananieva, M.; Bivolarski, V.P.; Vasileva, T.A.; Hinkov, A.V.; Todorov, D.G.; Shishkov, S.; Haertlé, T.; Iliev, I.N.; et al. Expression of coagulin A with low cytotoxic activity by Pediococcus pentosaceus ST65ACC isolated from raw milk cheese. J. Appl. Microbiol. 2020, 128, 458–472. [Google Scholar] [CrossRef]
- Ge, J.; Sun, Y.; Xin, X.; Wang, Y.; Ping, W. Purification and partial characterization of a novel bacteriocin synthesized by Lactobacillus paracasei HD1-7 isolated from Chinese sauerkraut juice. Sci. Rep. 2016, 6, 19366. [Google Scholar] [CrossRef]
- Kaya, H.I.; Simsek, O. Characterization of Pediococcus acidilactici PFC69 and Lactococcus lactis PFC77 bacteriocins and their antimicrobial activities in tarhana fermentation. Microorganisms 2020, 8, 1083. [Google Scholar] [CrossRef]
- Galvez, A.; Maqueda, M.; Valdivia, E.; Quesada, A.; Montoya, E. Characterization and partial purification of a broad spectrum antibiotic AS-48 produced by Streptococcus faecalis. Can. J. Microbiol. 1986, 32, 765–771. [Google Scholar] [CrossRef]
- Hassan, M.U.; Nayab, H.; Rehman, T.U.; Williamson, M.P.; Haq, K.U.; Shafi, N.; Shafique, F. Characterisation of bacteriocins produced by Lactobacillus spp. isolated from the traditional Pakistani yoghurt and their antimicrobial activity against common foodborne pathogens. Biomed. Res. Int. 2020, 8281623. [Google Scholar] [CrossRef]
- Danilova, T.A.; Adzhieva, A.A.; Danilina, G.A.; Polyakov, N.B.; Soloviev, A.I.; Zhukhovitsky, V.G. Antimicrobial activity of supernatant of Lactobacillus plantarum against pathogenic microorganisms. Bull. Exp. Biol. Med. 2019, 167, 751–754. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, C.; Tian, R.; Wang, W.; Ma, J.; Gu, L.; Liu, F.; Jiang, Z.; Hou, J. Screening beneficial bacteriostatic lactic acid bacteria in the intestine and studies of bacteriostatic substances. J. Zhejiang Univ. Sci. B 2021, 22, 533–547. [Google Scholar] [CrossRef]
- Leslie, V.A.; Mohammed Alarjani, K.; Malaisamy, A.; Balasubramanian, B. Bacteriocin producing microbes with bactericidal activity against multidrug resistant pathogens. J. Infect. Public Health 2021, 14, 1802–1809. [Google Scholar] [CrossRef]
- Abdul-Rahim, O.; Wu, Q.H.; Price, T.K.; Pistone, G.; Diebel, K.; Bugni, T.S.; Wolfe, A. Phenyl-lactic acid is an active Ingredient in bactericidal supernatants of Lactobacillus crispatus. J. Bacteriol. 2021, 203, 10–1128. [Google Scholar] [CrossRef]
- Jung, S.; Park, O.J.; Kim, A.R.; Ahn, K.B.; Lee, D.; Kum, K.Y.; Yun, C.H.; Han, S.H. Lipoteichoic acids of lactobacilli inhibit Enterococcus faecalis biofilm formation and disrupt the preformed biofilm. J. Microbiol. 2019, 57, 310–315. [Google Scholar] [CrossRef]
- Mouafo, T.H.; Mbawala, A.; Ndjouenkeu, R. Effect of different carbon sources on biosurfactants’ production by three strains of Lactobacillus spp. Biomed. Res. Int. 2018, 5034783. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Niu, M.M.; Yao, D.; Zhao, J.; Wu, Y.; Lu, B.X.; Zheng, X.Q. Physicochemical characteristics and in vitro and in vivo antioxidant activity of a cell-bound exopolysaccharide produced by Lactobacillus fermentum S1. Int. J. Biol. Macromol. 2019, 139, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Kang, M.; Yoo, Y.J.; Yun, C.H.; Perinpanayagam, H.; Kum, K.Y.; Han, S.H. Lactobacillus plantarum lipoteichoic acid disrupts mature Enterococcus faecalis biofilm. J. Microbiol. 2020, 58, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.P.; Palomino, M.M.; Allievi, M.C.; Rivas, C.S.; Ruzal, S.M. Murein hydrolase activity in the surface layer of Lactobacillus acidophilus ATCC 4356. Appl. Environ. Microbiol. 2008, 74, 7824–7827. [Google Scholar] [CrossRef]
- Paraszkiewicz, K.; Moryl, M.; Plaza, G.; Bhagat, D.; Satpute, S.K.; Bernat, P. Surfactants of microbial origin as antibiofilm agents. Int. J. Environ. Health Res. 2021, 31, 401–420. [Google Scholar] [CrossRef]
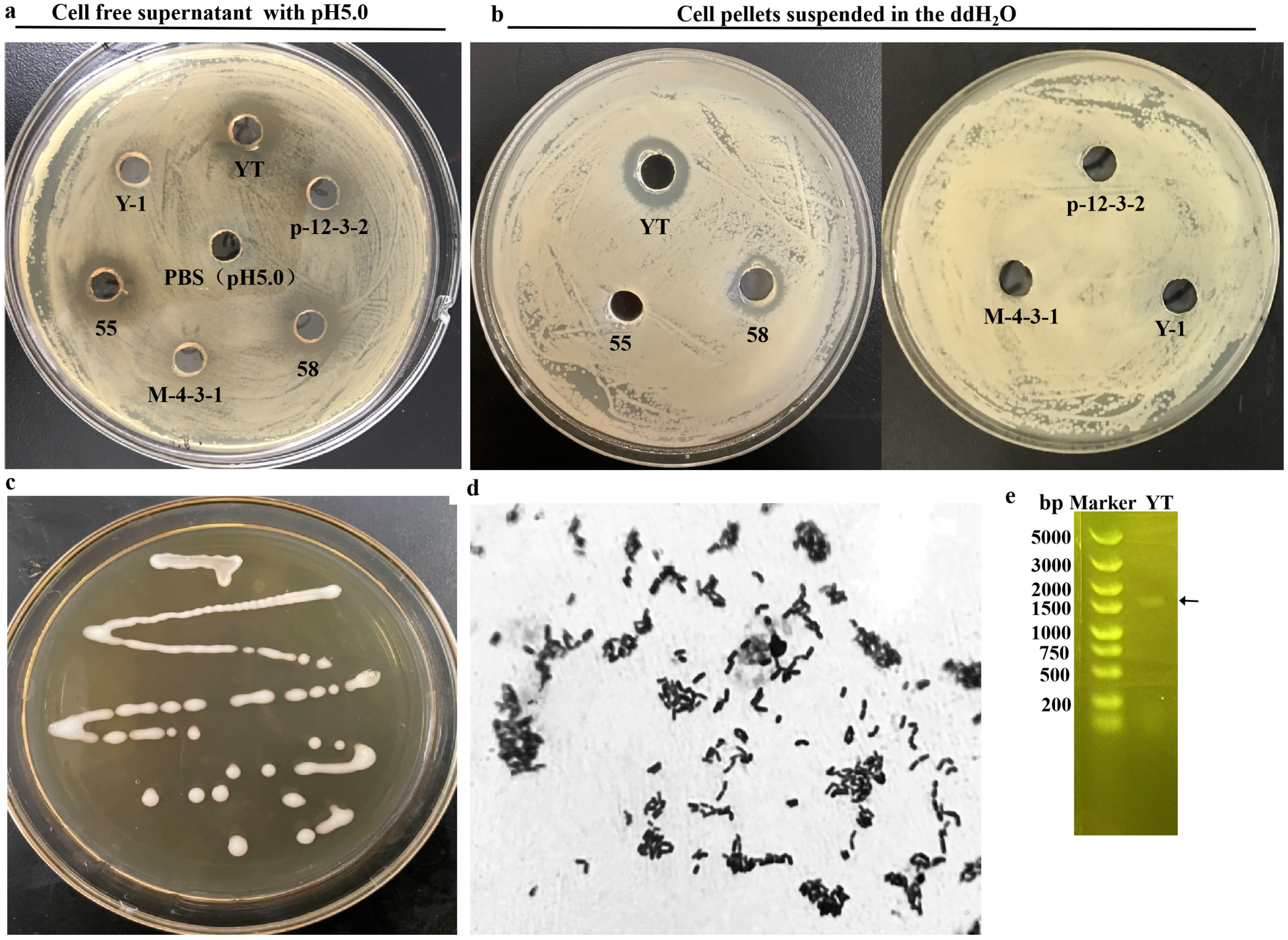
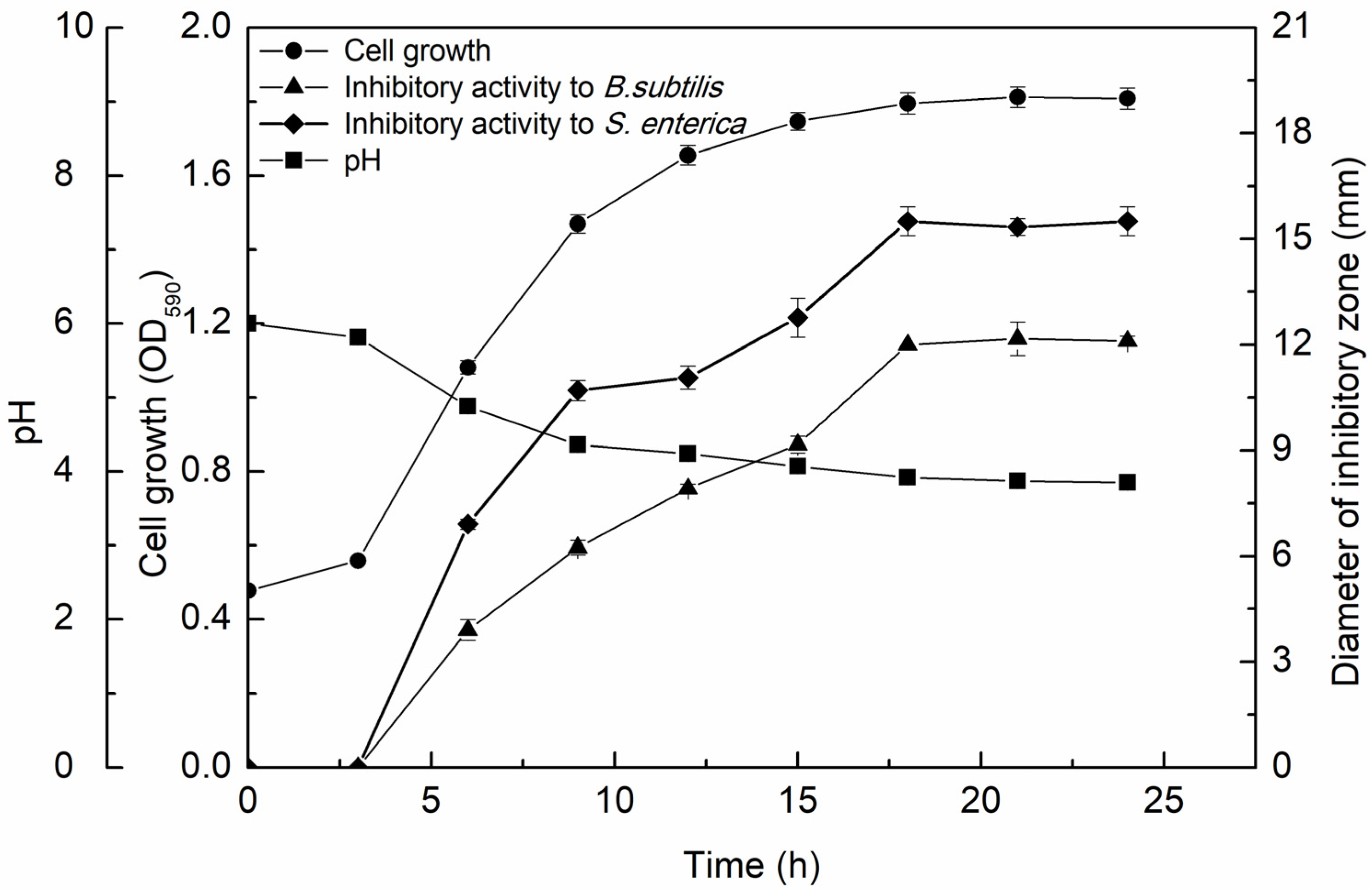
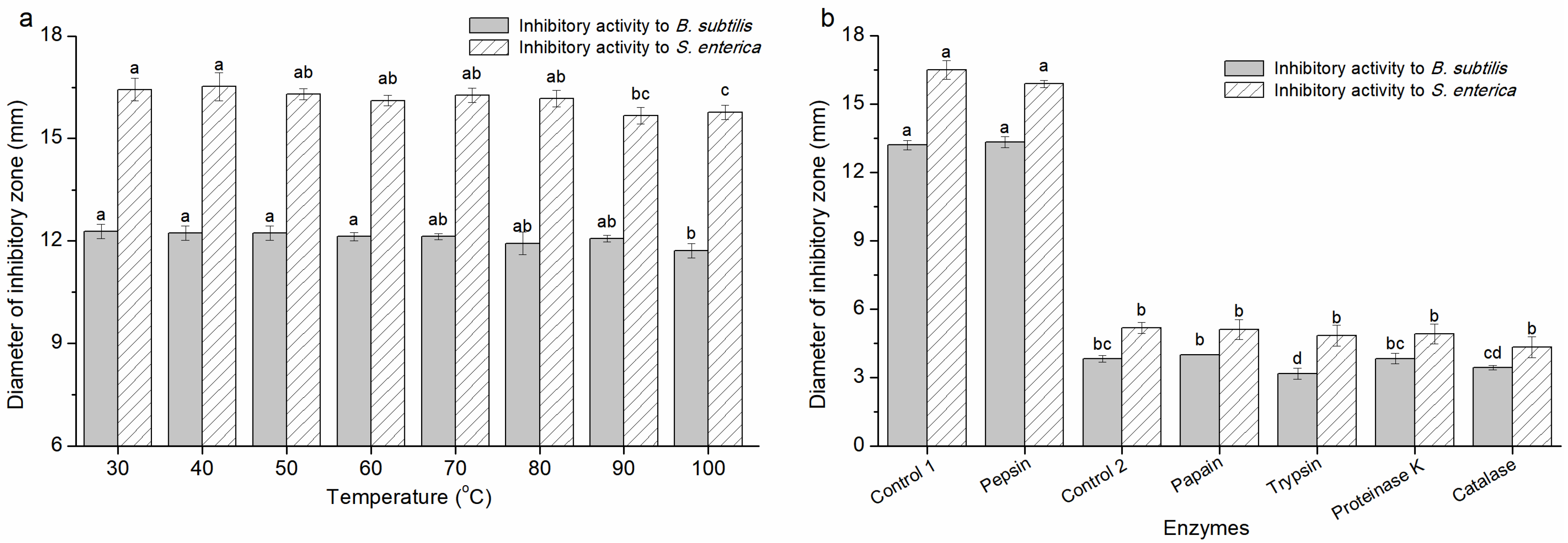
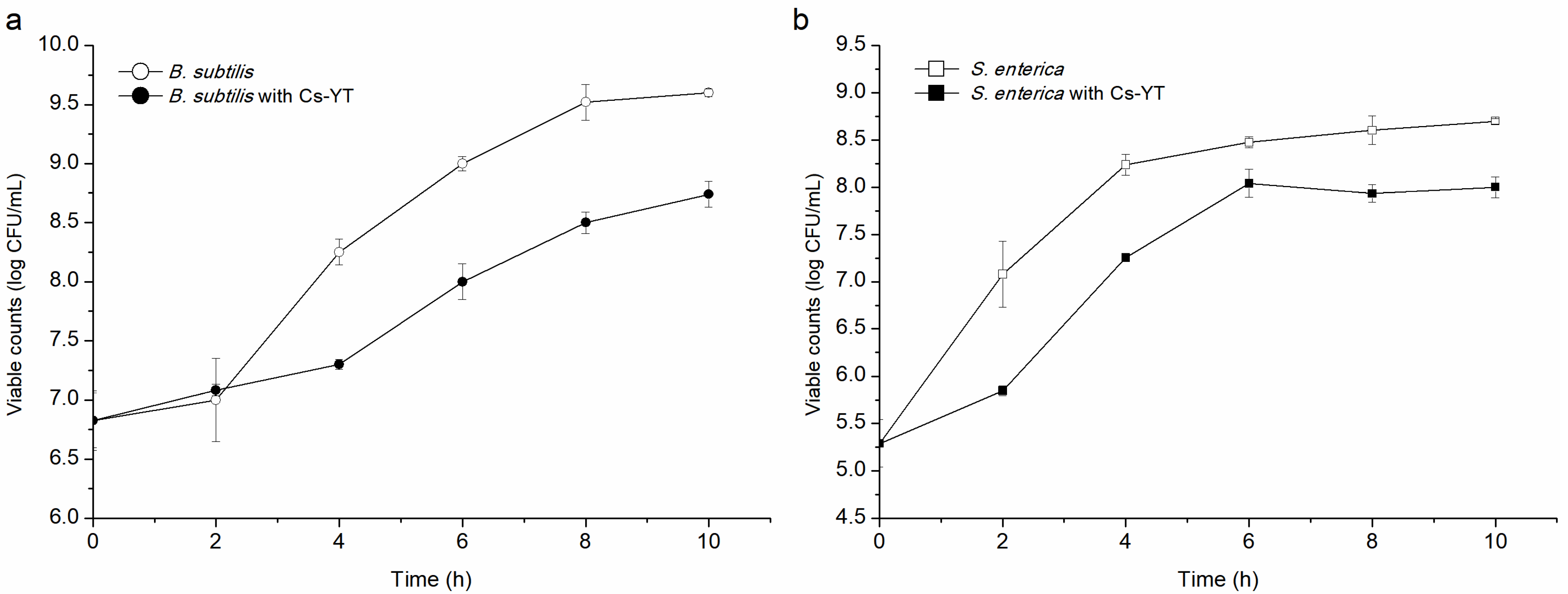
| Strain | Inhibitory Zone against Indicator Strains (mm) | |||||
|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | B. cereus | S. enterica | E. coli | P. brenneri | |
| 2 | ++ | ++ | + | + | ++ | ++ |
| 4 | ++ | + | - | +++ | +++ | +++ |
| 6 | + | ++ | - | + | + | +++ |
| 7 | ++ | +++ | +++ | +++ | +++ | +++ |
| 9 | + | ++ | + | + | +++ | +++ |
| 10 | + | + | + | + | ++ | ++ |
| 11 | ++ | +++ | + | + | +++ | +++ |
| 18 | - | - | - | - | - | - |
| 21 | ++ | ++ | +++ | ++ | ++ | ++ |
| 25 | + | ++ | + | + | ++ | ++ |
| 27 | + | + | + | + | ++ | +++ |
| 31 | + | + | + | ++ | ++ | ++ |
| 32 | - | - | - | - | - | - |
| 33 | + | + | - | + | ++ | ++ |
| 34 | - | - | - | - | - | - |
| 35 | + | + | + | + | ++ | ++ |
| 44 | ++ | ++ | ++ | + | ++ | ++ |
| 46 | + | ++ | + | + | ++ | ++ |
| 53 | - | - | - | - | - | - |
| 55 | ++++ | ++++ | +++ | +++ | ++++ | ++++ |
| 57 | - | ++ | + | + | +++ | ++ |
| 58 | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 61 | - | - | - | - | - | + |
| 65 | + | + | - | - | ++ | +++ |
| 66 | + | ++ | + | + | ++ | ++ |
| 69 | ++ | + | ++ | ++ | ++ | +++ |
| 71 | + | + | + | + | +++ | ++ |
| 74 | ++ | + | +++ | + | +++ | ++ |
| 79 | ++ | ++ | ++ | - | +++ | +++ |
| 82 | - | ++ | + | + | +++ | ++++ |
| 85 | + | ++ | ++ | + | ++ | +++ |
| 87 | ++ | ++ | + | - | +++ | +++ |
| 88 | + | - | + | + | ++ | ++ |
| 89 | + | + | + | + | ++ | +++ |
| 96 | + | +++ | ++ | + | ++ | ++++ |
| 97 | ++ | ++ | ++ | + | +++ | +++ |
| 98 | - | - | - | - | - | - |
| 99 | - | + | + | ++ | ++ | +++ |
| 103 | + | + | + | + | + | + |
| 105 | + | + | + | ++ | ++ | +++ |
| 106 | ++ | ++ | ++ | + | +++ | +++ |
| 111 | ++ | ++ | ++ | + | ++ | ++ |
| 113 | +++ | ++ | ++ | + | ++++ | ++++ |
| 116 | ++ | ++ | ++ | + | + | ++ |
| 117 | +++ | +++ | + | + | +++ | +++ |
| 118 | ++ | ++ | - | ++ | ++ | +++ |
| 145 | ++ | ++ | ++ | - | ++ | ++ |
| 146 | + | + | + | ++ | ++ | ++ |
| 147 | ++ | + | + | + | +++ | +++ |
| 148 | + | + | + | + | ++ | ++ |
| 154 | ++ | +++ | ++ | + | +++ | ++ |
| 393 | + | + | + | + | ++ | + |
| p-1-3-2 | ++ | - | - | + | ++ | ++ |
| p-1-3-9 | +++ | ++ | ++ | ++ | ++++ | ++ |
| p-1-7-4 | +++ | ++ | ++ | ++ | ++++ | ++ |
| p-3-3-6 | ++ | ++ | + | ++ | +++ | ++ |
| p-11-7-6 | - | - | - | - | - | - |
| p-12-3-2 | ++++ | +++ | ++++ | ++++ | ++++ | ++++ |
| p-13-7-11 | ++ | +++ | ++ | ++ | +++ | ++ |
| p-14-3-2 | + | ++ | ++ | ++ | +++ | +++ |
| p-14-7-3 | - | + | + | ++ | ++ | +++ |
| M-4-3-1 | ++++ | ++++ | ++++ | ++++ | +++ | ++++ |
| M-14-3-1 | ++ | ++ | ++ | + | + | ++ |
| M17-6-3-2 | +++ | +++ | ++ | + | ++ | ++ |
| S-1-3-1 | ++ | +++ | ++ | + | +++ | ++ |
| S-3-3-1 | + | ++ | +++ | - | - | ++ |
| S-5-3-2 | ++ | ++ | ++ | + | ++ | ++ |
| S-11-3-1 | ++ | + | - | ++ | ++ | ++ |
| S-13-7-2 | ++ | - | ++ | ++ | ++ | ++ |
| S-14-7-5 | ++ | ++ | ++ | + | ++ | +++ |
| L-4-3-2 | ++ | + | - | ++ | ++ | +++ |
| L-11-7-2 | ++ | - | + | ++ | ++ | +++ |
| L-13-7-7 | ++ | + | + | ++ | ++ | +++ |
| L-14-7-3 | ++ | + | + | ++ | ++ | +++ |
| Y-1 | ++++ | ++++ | +++ | ++++ | ++++ | ++++ |
| Y-2 | ++ | ++ | - | ++ | ++ | +++ |
| Y-3 | ++ | + | + | ++ | ++ | ++ |
| Y-4 | + | + | + | + | ++ | ++++ |
| Y-5 | +++ | ++ | ++ | +++ | ++++ | ++++ |
| YT | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Grx10 | ++ | ++ | ++ | + | ++ | ++ |
| Grx19 | ++ | + | + | ++ | +++ | +++ |
| V9 | ++ | ++ | ++ | ++ | - | + |
| LH100-1 | + | + | - | + | +++ | +++ |
| LV108 | +++ | + | + | ++ | ++ | ++ |
| R3 | +++ | - | + | + | + | + |
| 90-57 | ++ | + | + | ++ | +++ | +++ |
| St KY | + | + | + | + | ++ | ++ |
| S7 | ++ | + | - | + | ++ | - |
| 087-3 | + | + | + | ++ | ++ | ++ |
| 1.12.8 | ++ | + | - | ++ | ++ | ++ |
| 987-3 | - | - | - | - | - | - |
| 937-MQ | + | ++ | ++ | ++ | +++ | +++ |
| 975-1 | + | ++ | ++ | ++ | ++ | ++ |
| XPL | + | + | + | ++ | ++ | ++ |
| XPL-1-4 | - | - | - | - | - | - |
| K01-4 | ++ | ++ | ++ | +++ | +++ | +++ |
| JXR | +++ | - | + | + | + | +++ |
| 12-529 | + | + | - | + | ++ | ++ |
| LD | +++ | ++ | + | ++ | ++ | +++ |
| 5-7-7-2 | ++ | ++ | + | + | ++ | ++ |
| 9-10-1-10 | + | - | + | ++ | ++ | + |
| Strains | Initial pH of CFS | Inhibitory Zone of CFS against Indicator Strains (mm) | Inhibitory Zone of CFS with pH 5.0 against Indicator Strains (mm) | ||
|---|---|---|---|---|---|
| B. subtilis | S. enterica | B. subtilis | S. enterica | ||
| Y-1 | 4.01 ± 0.01 a | 14.50 ± 0.41 c | 16.11 ± 0.08 e | 8.20 ± 0.41 b | 8.89 ± 0.57b c |
| YT | 3.86 ± 0.03 c | 19.33 ± 0.47 a | 23.50 ± 0.41 a | 10.66 ± 0.29 a | 13.13 ± 0.19 a |
| 55 | 3.92 ± 0.02 b | 18.73 ± 0.21 a | 17.76 ± 0.20 d | 10.50 ± 0.41 a | 11.50 ± 0.41 b |
| 58 | 3.83 ± 0.03 c | 17.50 ± 0.08 b | 21.50 ± 0.41 b | 10.47 ± 0.37 a | 11.50 ± 0.40 b |
| M-4-3-1 | 3.77 ± 0.01 d | 19.17 ± 0.24 a | 19.33 ± 0.24 c | 10.34 ± 0.29 a | 9.33 ± 0.24 c |
| p-12-3-2 | 3.86 ± 0.03 c | 18.50 ± 0.41 a | 17.77 ± 0.21 d | 10.07 ± 0.09 a | 11.33 ± 0.27 b |
| Strain | Inhibitory Zone (mm) | |
|---|---|---|
| B. subtilis | S. enterica | |
| Y-1 | - | - |
| YT | 12.00 ± 0.25 a | 12.50 ± 0.25 a |
| 55 | - | - |
| 58 | - | 10.12 ± 0.03 b |
| M-4-3-1 | - | - |
| p-12-3-2 | - | - |
| pH | Diameter of Inhibitory Zone (mm) | |
|---|---|---|
| B. subtilis | S. enterica | |
| 3.0 | 18.56 ± 0.08 a | 22.50 ± 0.41 a |
| 4.0 | 10.10 ± 0.29 b | 12.49 ± 0.15 b |
| 5.0 | 3.72 ± 0.21 c | 5.87 ± 0.27 c |
| 6.0 | - | - |
| 7.0 | - | - |
| 8.0 | - | - |
| 9.0 | - | - |
| Indicator Strains | Diameter of Inhibitory Zone (mm) |
|---|---|
| B. subtilis | 7.63 ± 0.85 b |
| S. aureus | 8.00 ± 0.82 ab |
| B. cereus | 8.13 ± 1.31 ab |
| S. enterica | 9.13 ± 1.65 ab |
| E. coli | 8.38 ± 0.95 ab |
| P. brenneri | 9.88 ± 1.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, C.; Li, F.; Yu, P.; Chen, X.; Yin, Y.; Chen, D.; Gu, R.; Zhang, C.; Pang, B. Isolation, Identification and Antibacterial Characteristics of Lacticaseibacillus rhamnosus YT. Foods 2024, 13, 2706. https://doi.org/10.3390/foods13172706
Guan C, Li F, Yu P, Chen X, Yin Y, Chen D, Gu R, Zhang C, Pang B. Isolation, Identification and Antibacterial Characteristics of Lacticaseibacillus rhamnosus YT. Foods. 2024; 13(17):2706. https://doi.org/10.3390/foods13172706
Chicago/Turabian StyleGuan, Chengran, Feng Li, Peng Yu, Xuan Chen, Yongqi Yin, Dawei Chen, Ruixia Gu, Chenchen Zhang, and Bo Pang. 2024. "Isolation, Identification and Antibacterial Characteristics of Lacticaseibacillus rhamnosus YT" Foods 13, no. 17: 2706. https://doi.org/10.3390/foods13172706





