Effect on the Antioxidant Properties of Native Chilean Endemic Honeys Treated with Ionizing Radiation to Remove American Foulbrood Spores
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Identification of Botanical Origin by Melissopalynological Analysis
2.3. Microbiological Detection of Spores of Paenibacillus larvae in Honey Samples
2.4. Dosimetric Assays
2.5. Preparation of Honey Solutions for Colorimetric Assays
2.6. Total Phenolic Compound Content
2.7. Determination of Antiradical Activity—DPPH Assay
2.8. Determination of Antioxidant Capacity in Honey—FRAP Assay
2.9. Statistical Analysis
3. Results
3.1. Geographical and Botanical Origin of Honey Samples
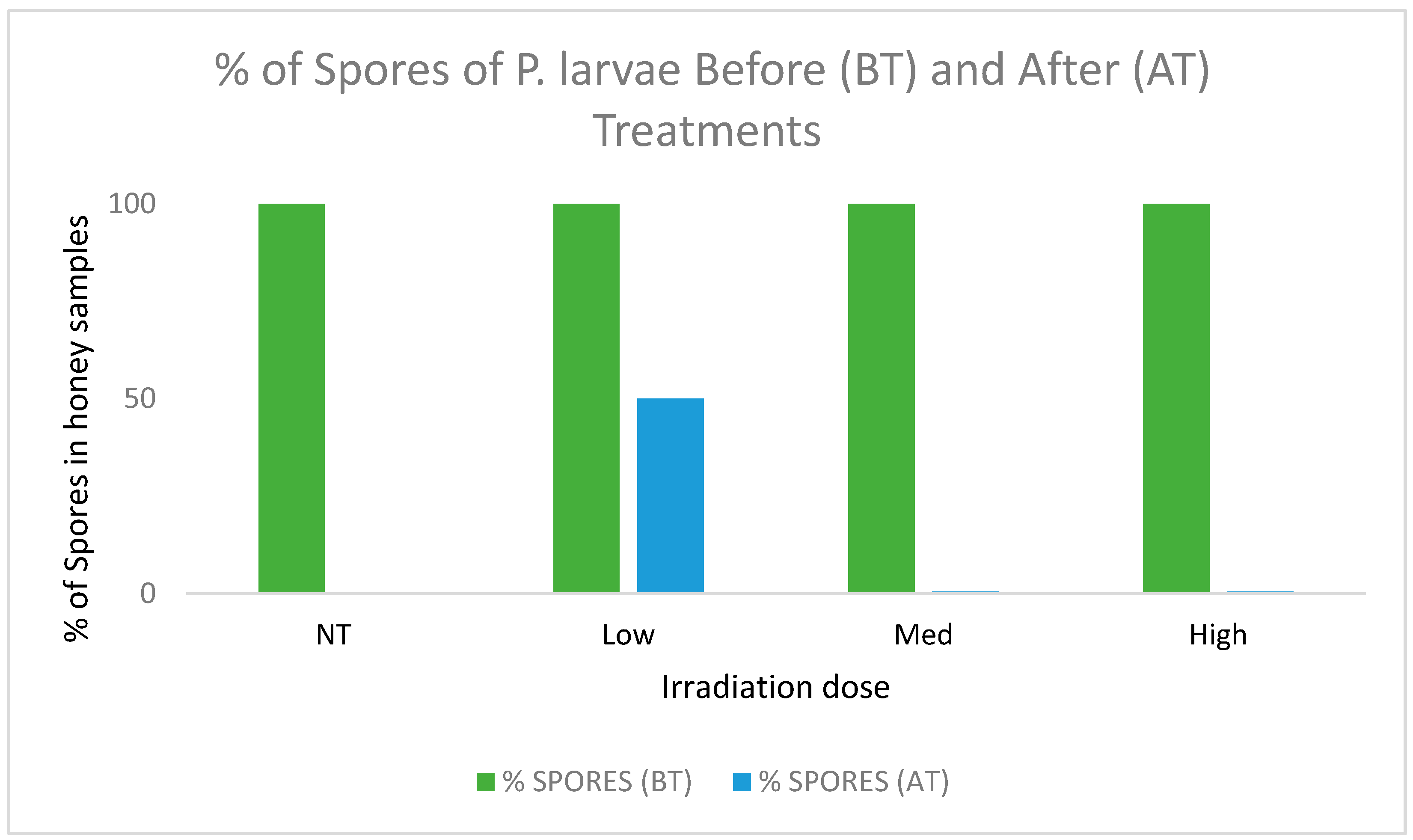
3.2. Detection of Spores of P. larvae in Honey Content
3.3. Dosimetric Assays and Selection of Radiation Dose Levels
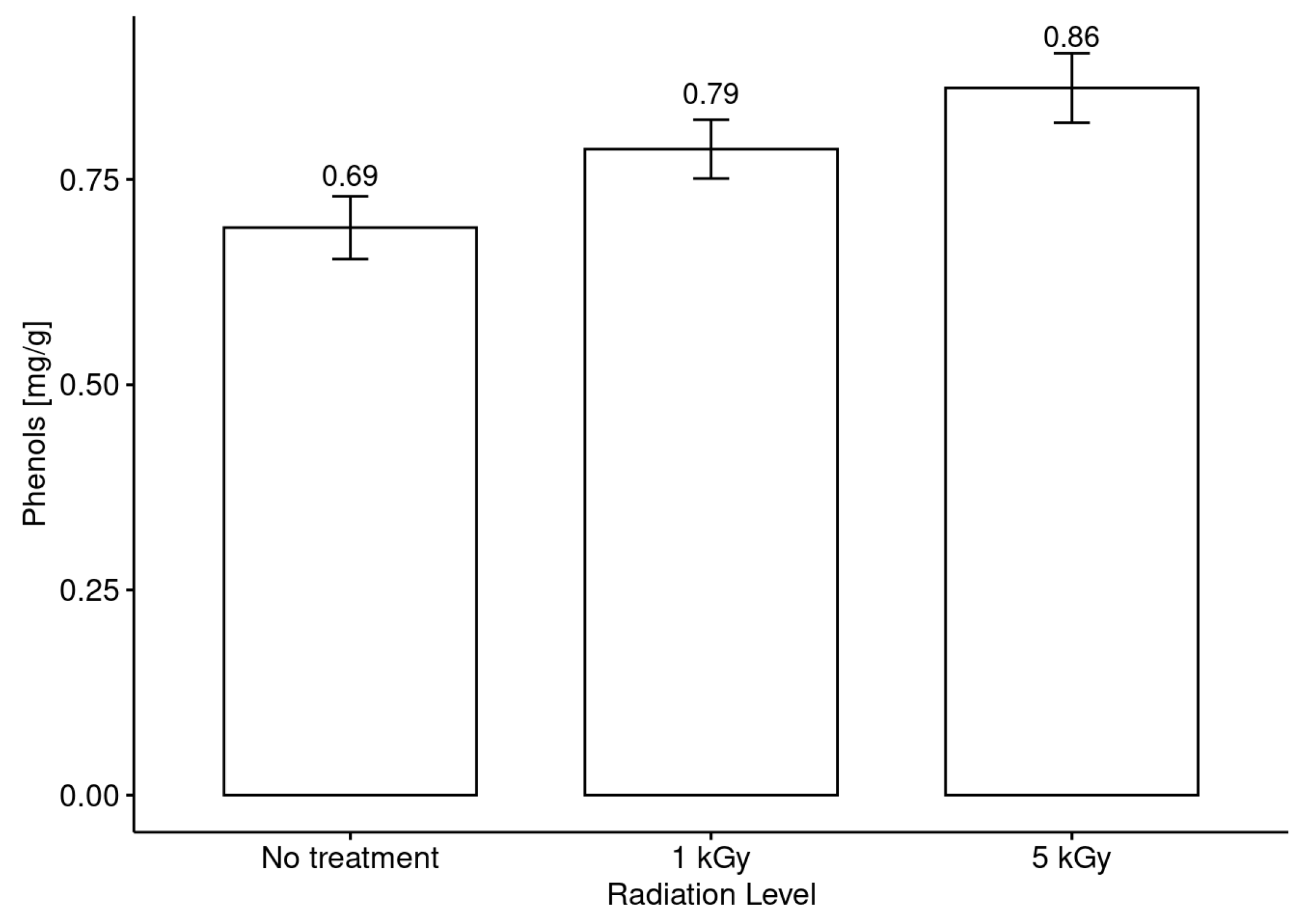
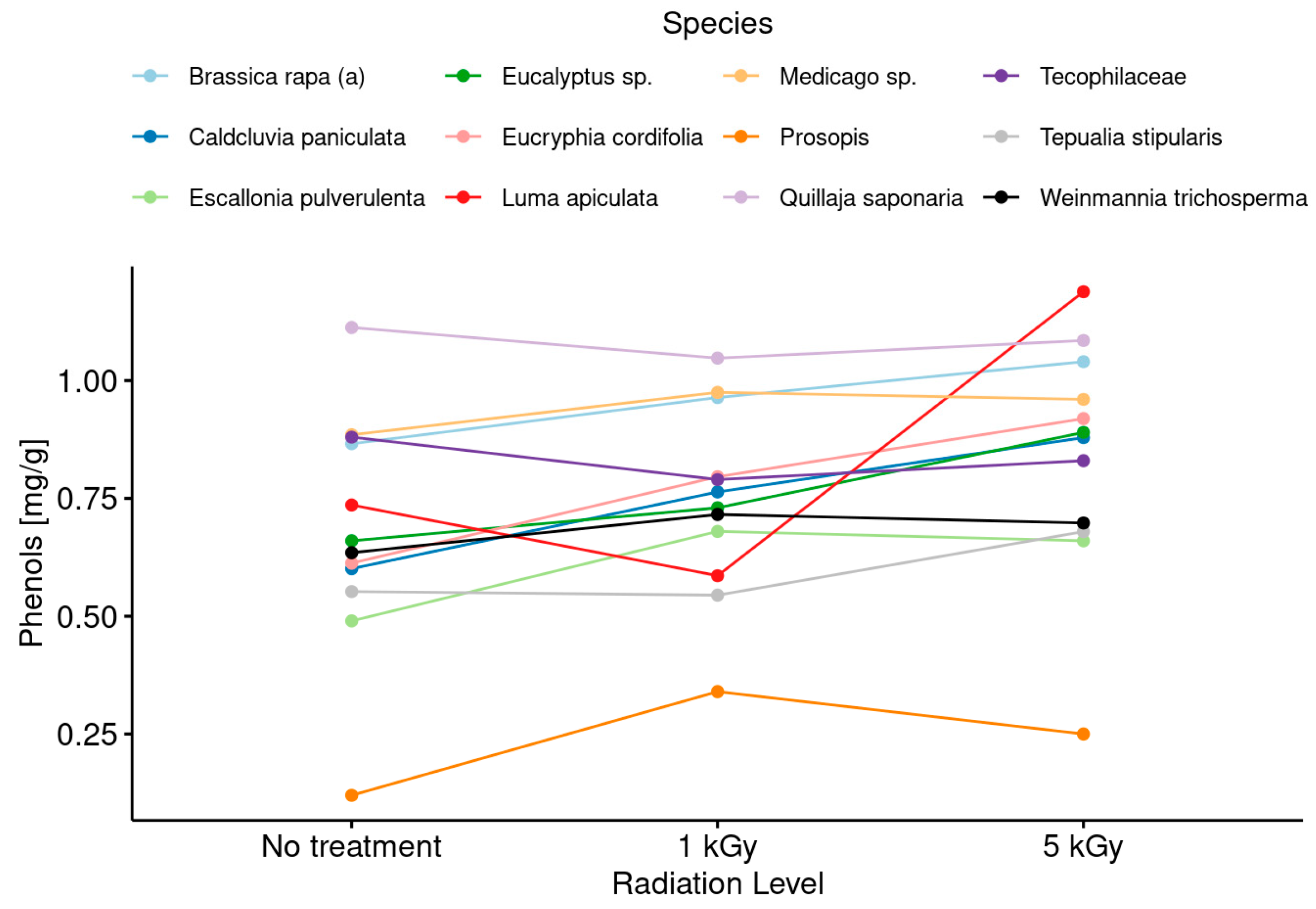
3.4. Phenolic Compound Content in Honeys before and after Irradiation Treatments
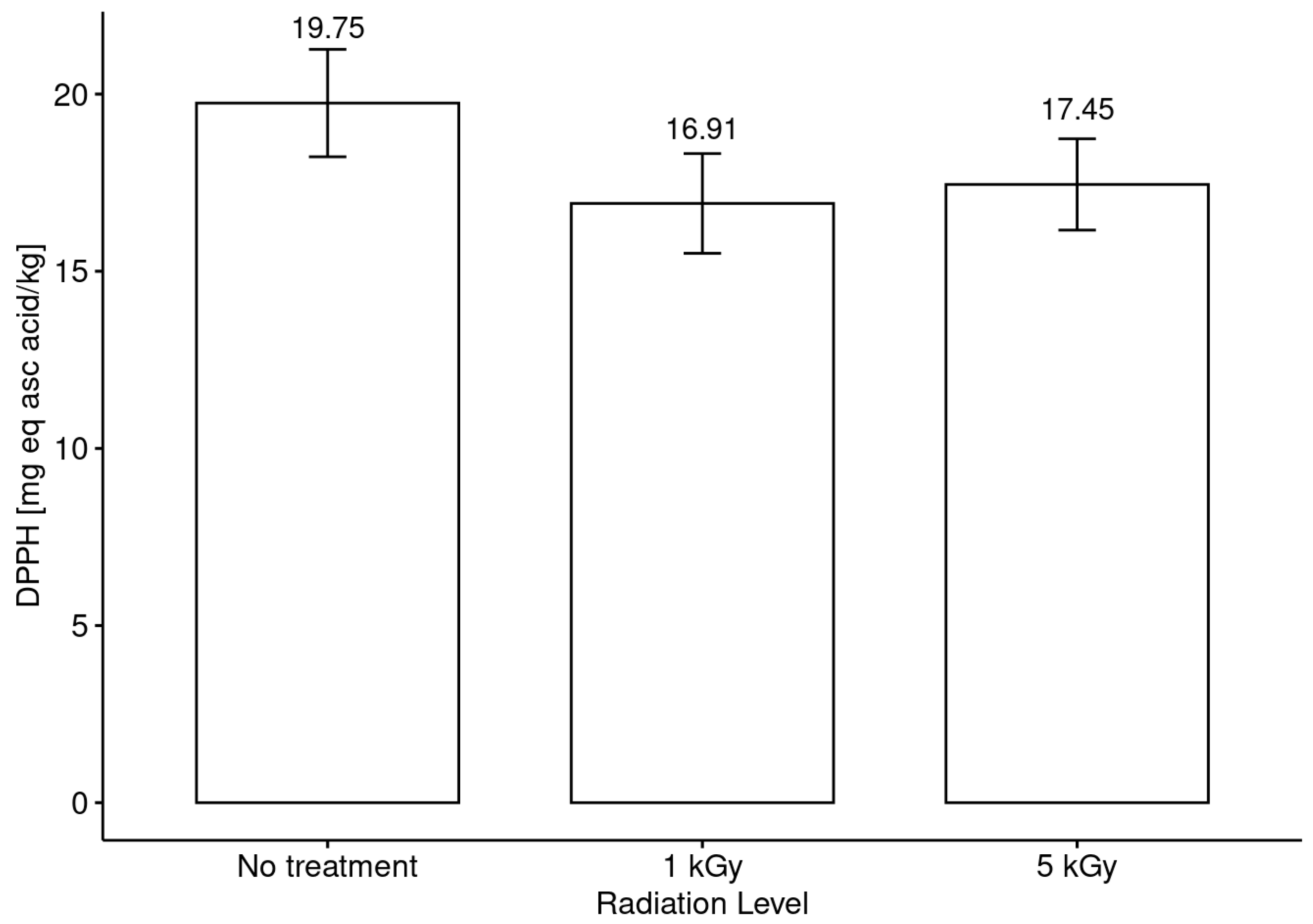
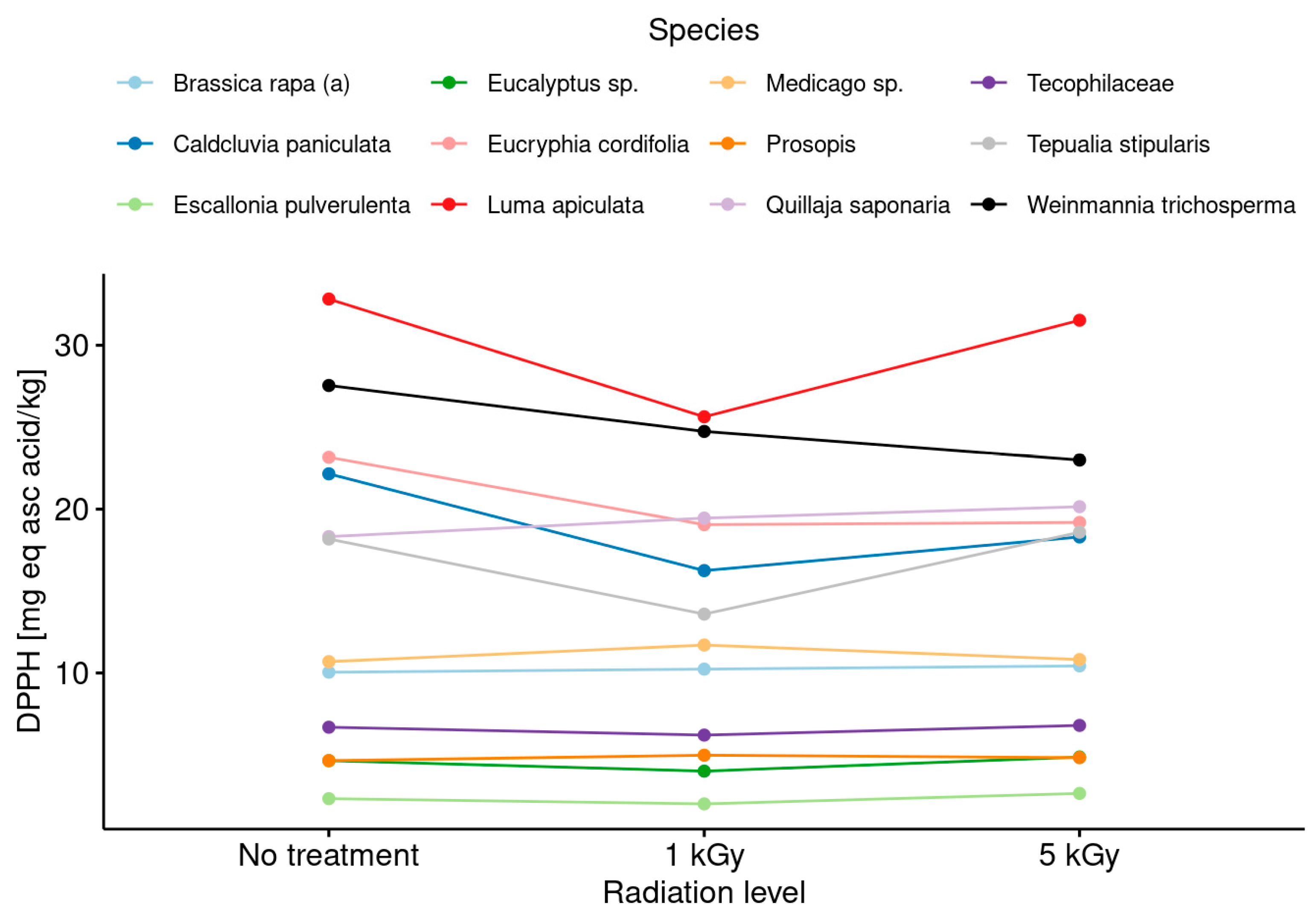
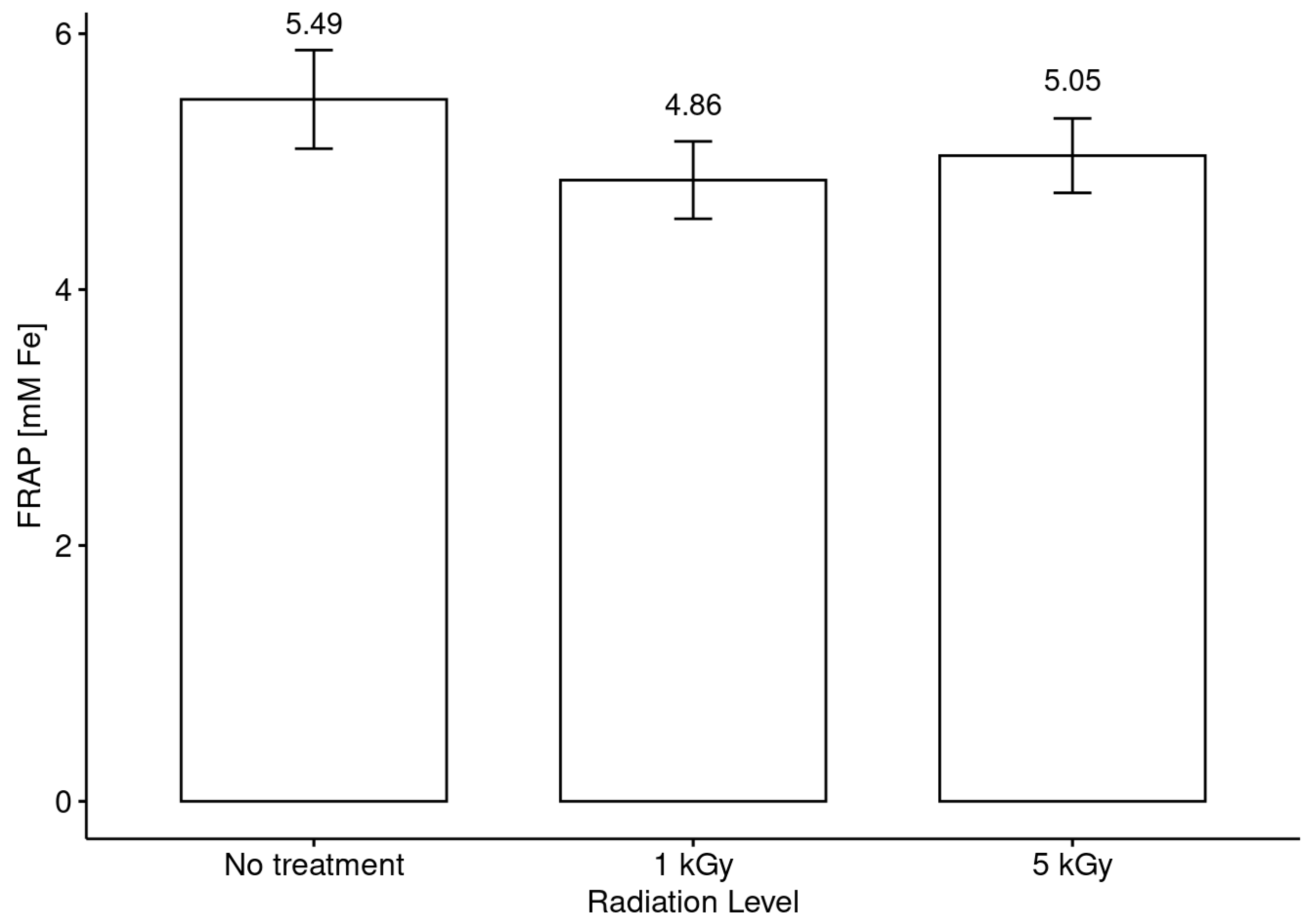
3.5. Antiradical and Antioxidant Activities in Honeys before and after Radiation Treatments
4. Discussion
5. Conclusions
- This study allowed us to analyze honey samples from an extensive range of Chilean territory. In this way, we were able to verify the presence of spores in honeys produced in areas with previous reports of American foulbrood, but it was not widely distributed throughout the country. In this sense, we can conclude that ionizing radiation was able to control the presence of American foulbrood spores (Paenibacillus larvae). The dose used in this study was in the range commonly applied to food.
- It was evidenced that the phenol content and the antiradical activity (DPPH assay) were increased with the application of both doses (low and medium) with respect to the untreated honey, but no clear trend in this modification between the phenol content and the botanical origin of the monofloral honey samples was observed.
- Our results suggest the strategic need to define territories according to botanical, geographical and ecological criteria for the characterization of honeys and, therefore, to increase their value in terms of bioactive compounds and absence of bee diseases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014, 153, 35–43. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ölmez, Ç. Some qualitative properties of different monofloral honeys. Food Chem. 2014, 163, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, S.; Jessica Gunnoo, J.; Marques Passos, T.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, P.; Montenegro, G.; Leyton, F.; Ascar, L.; Ramirez, O.; Giordano, A. Bioactive compounds and antibacterial properties of monofloral Ulmo honey. CyTA J. Food 2019, 18, 11–19. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Tischer-Seraglio, S.K.; Schulz, M.; Brugnerotto, P.; Silva, B.; Gonzaga, L.V.; Fett, R.; Oliveira-Costa, A.C. Quality, composition and health-protective properties of citrus honey: A review. Food Res. Int. 2021, 143, 110268. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Guerra-Rodríguez, M.E.; Vázquez, M. Analytical Methods Used in the Quality Control of Honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Kivima, E.; Tanilas, K.; Martverk, K.; Rosenvald, S.; Timberg, L.; Laos, K. The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys. Foods 2021, 10, 511. [Google Scholar] [CrossRef]
- Oficina de Estudios y Políticas Agrarias; Ministerio de Agricultura de Chile ODEPA. Apicultura Chilena: Principales Cifras y Desafíos Futuros. 2021. Available online: https://bibliotecadigital.odepa.gob.cl/bitstream/handle/20.500.12650/71017/ArtApiculturaChilena092021.pdf (accessed on 17 July 2024).
- Servicio Agrícola y Ganadero–División de Protección Pecuaria. Boletin Apícola N°9. 2024. Available online: https://www.sag.gob.cl/sites/default/files/BOLET%C3%8DN%20AP%C3%8DCOLA%20N%C2%B09.pdf (accessed on 17 July 2024).
- Lobos, I.; Silva, M.; Ulloa, P.; Pavez, P. Mineral and Botanical Composition of Honey Produced in Chile’s Central-Southern Region. Foods 2022, 11, 251. [Google Scholar] [CrossRef]
- Montenegro, G. Dimensión productiva: Diagnóstico del sector apícola variables relevantes para una estrategia de diferenciación de mieles chilenas. In Mieles Chilenas para el Mundo—Atributos, Propiedades e Innovación; Food and Agriculture Organization of the United Nations—FAO: Rome, Italy, 2023; pp. 151–163. [Google Scholar]
- Oficina de Estudios y Políticas Agrarias; Ministerio de Agricultura de Chile ODEPA. 2024. Available online: https://www.odepa.gob.cl/publicaciones/noticias/agro-en-la-prensa/comparado-con-igual-periodo-del-ano-anterior-exportaciones-de-mieles-crecieron-en-volumen-66-en-enero-junio-de-2024 (accessed on 9 August 2024).
- Oficina de Estudios y Políticas Agrarias; Ministerio de Agricultura de Chile ODEPA. Temporada Apícola 2022 23. 2023. Available online: https://bibliotecadigital.odepa.gob.cl/bitstream/handle/20.500.12650/72984/Art_TemporadaApicola2022_23.pdf (accessed on 9 August 2024).
- Lazarus, M.; Lovaković, B.T.; Orct, T.; Sekovanić, A.; Bilandžić, N.; Đokić, M.; Kolanović, B.S.; Varenina, I.; Jurič, A.; Lugomer, M.D.; et al. Difference in pesticides, trace metal(loid)s and drug residues between certified organic and conventional honeys from Croatia. Chemosphere 2021, 266, 128954. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Murcia-Morales, M.; Gómez-Ramos, M.J.; Parrilla-Vázquez, P.; Diaz-Galiano, F.J.; Garcia-Valverde, M.; Gámiz-Lopez, V.; Flores, J.M.; Fernandez-Alba, A.R. Distribution of chemical residues in the beehive compartments and their transfer to the honeybee brood. Sci. Total Environ. 2020, 710, 136288. [Google Scholar] [CrossRef] [PubMed]
- El Agrebi, N.; Traynor, K.; Wilmart, O.; Tosi, S.; Leinartz, L.; Danneels, E.; de Graaf, D.C.; Saegerman, C. Pesticide and veterinary drug residues in Belgian beeswax: Occurrence, toxicity, and risk to honey bees. Sci. Total Environ. 2020, 745, 141036. [Google Scholar] [CrossRef] [PubMed]
- Willmer, P. Climate Change: Bees and Orchids Lose Touch. Curr. Biol. 2014, 24, R1133–R1135. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.C.; Shirey, V.; Ponisio, L.C.; Gelhaus, J.K. Responses from bees, butterflies, and ground beetles to different fire and site characteristics: A global meta-analysis. Biol. Conserv. 2021, 261, 109265. [Google Scholar] [CrossRef]
- Flores, J.M.; Gil-Lebrero, S.; Gámiz, V.; Rodríguez, M.I.; Ortiz, M.A.; Quiles, F.J. Effect of the climate change on honey bee colonies in a temperate Mediterranean zone assessed through remote hive weight monitoring system in conjunction with exhaustive colonies assessment. Sci. Total Environ. 2019, 653, 1111–1119. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Toth, A.L. Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 2018, 26, 114–119. [Google Scholar] [CrossRef]
- Mejias, E. American Foulbrood and the Risk in the Use of Antibiotics as a Treatment. In Modern Beekeeping Bases for Sustainable Production, 1st ed.; Rebolledo, R., Ed.; IntechOpen: London, UK, 2020; pp. 97–110. [Google Scholar] [CrossRef]
- Poppinga, L.; Genersch, E. Molecular pathogenesis of American Foulbrood: How Paenibacillus larvae kills honey bee larvae. Curr. Opin. Insect Sci. 2015, 10, 29–36. [Google Scholar] [CrossRef]
- Human, H.; Pirk, C.W.W.; Crewe, R.M.; Dietemann, V. The honeybee disease American foulbrood—An African perspective. Afr. Èntomol. 2011, 19, 551–557. [Google Scholar] [CrossRef][Green Version]
- Lindström, A.; Korpela, S.; Fries, I. The distribution of Paenibacillus larvae spores in adult bees and honey and larval mortality, following the addition of American foulbrood diseased brood or spore-contaminated honey in honey bee (Apis mellifera) colonies. J. Invertebr. Pathol. 2008, 99, 82–86. [Google Scholar] [CrossRef]
- El-Meihy, R.; Hassan, E.O.; Alamoudi, S.A.; Negm, S.; Al-Hoshani, N.; Al-Ghamdi, M.S.; Nowar, E.E.; El-Meihy, R.M.; Hassan, E.O.; Alamoudi, S.A.; et al. Probing the interaction of Paenibacillus larvae bacteriophage as a biological agent to control the American foulbrood disease in honeybee. Saudi J. Biol. Sci. 2024, 31, 104002. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Anido, M.; Branchiccela, B.; Harriet, J.; Campá, J.; Zunino, P. American Foulbrood in Uruguay: Twelve years from its first report. J. Invertebr. Pathol. 2012, 110, 129–131. [Google Scholar] [CrossRef]
- Servicio Agrícola y Ganadero–División de Protección Pecuaria–Loque Americana. 2024. Available online: https://www.sag.gob.cl/ambitos-de-accion/loque-americana-la (accessed on 9 August 2024).
- Servicio Agrícola y Ganadero–División de Protección Pecuaria, Establecimientos Exportadores Pecuarios: Normativa Repú-blica Popular China. 2024. Available online: https://www2.sag.gob.cl/pecuaria/establecimientos_habilitados_exportar/normativa/china/normativa_china.html (accessed on 9 August 2024).
- Obodovskiy, I. Nuclei and Nuclear Radiations. In Radiation: Fundamentals, Applications, Risks, and Safety; Elsevier Science: Amsterdam, The Netherlands, 2019; pp. 41–62. [Google Scholar] [CrossRef]
- Rossi, L.; Watson, D.; Escandarani, S.; Miranda, A.; Troncoso, A. Radiation on the dining table. Rev. Chil. Infectol. 2009, 26, 318–330. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration—FDA. Food Irradiation: What You Need to Know. 2016. Available online: https://www.fda.gov/food/buy-store-serve-safe-food/food-irradiation-what-you-need-know (accessed on 9 August 2024).
- Simone-Finstrom, M.; Aronstein, K.; Goblirsch, M.; Rinkevich, F.; de Guzman, L. Gamma irradiation inactivates honey bee fungal, microsporidian, and viral pathogens and parasites. J. Invertebr. Pathol. 2018, 153, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.A.; Razzak, M.; Hossain, M.A.; Hossain, A.; Islam, M.; Shahjalal, M.; Khan, R.A.; Huque, R. Gamma radiation processing of honey of Mustard, Black seed and Lychee flower: Measurement of antioxidant, antimicrobial, and Fourier transform infrared (FT-IR) spectra. Meas. Food 2022, 6, 100026. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Hailu, D.; Belay, A. Melissopalynology and antioxidant properties used to differentiate Schefflera abyssinica and polyfloral honey. PLoS ONE 2020, 15, e0240868. [Google Scholar] [CrossRef]
- De Graaf, D.C.; Alippi, A.M.; Antúnez, K.; Aronstein, K.A.; Budge, G.; De Koker, D.; De Smet, L.; Dingman, D.W.; Evans, J.D.; Foster, L.J.; et al. Standard methods for American foulbrood research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- ISO/ASTM 51607: 2013; Standard Practice for Use of an Alanine-EPR Dosimetry System. International Organization for Standardization ASTM International: West Conshohocken, PA, USA, 2013. Available online: https://www.iso.org/obp/ui?utm_source=google&utm_medium=ppc_paid_social&utm_campaign=am24-registration&gad_sour-ce=1&gclid=CjwKCAjw1920BhA3EiwAJT3lSTsbch0IsSgF83aBMdcOD6lE4S1HYefy-vSqF2EyVUrx290bnv9jtxoC88IQAvD_BwE#iso:std:iso-astm:51607:ed-3:v1:en (accessed on 17 July 2024).
- Mejías, E.; Montenegro, G. The Antioxidant Activity of Chilean Honey and Bee Pollen Produced in the Llaima Volcano’s Zones. J. Food Qual. 2012, 35, 315–322. [Google Scholar] [CrossRef]
- Buratti, S.; Benedetti, S.; Cosio, M.S. Evaluation of the antioxidant power of honey, propolis and royal jelly by amperometric flow injection analysis. Talanta 2007, 71, 1387–1392. [Google Scholar] [CrossRef]
- Mejías, E.; Gómez, C.; Garrido, T. Suitable Areas for Apiculture Expansion Determined by Antioxidant Power, Chemical Profiles, and Pesticide Residues in Caldcluvia paniculata Honey and Beeswax Samples. Insects 2022, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Biblioteca del Congreso Nacional de Chile BCN Resolución 636 Exenta: Establece Dosis Máximas de Irradiación de Alimentos. Ministerio de Salud de Chile; Subsecretaría de Salud Pública. 2014. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1066251 (accessed on 9 August 2024).
- Locke, B.; Low, M.; Forsgren, E. An integrated management strategy to prevent outbreaks and eliminate infection pressure of American foulbrood disease in a commercial beekeeping operation. Prev. Vet. Med. 2019, 167, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Brady, T.S.; Merrill, B.D.; Hilton, J.A.; Payne, A.M.; Stephenson, M.B.; Hope, S. Bacteriophages as an alternative to conventional antibiotic use for the prevention or treatment of Paenibacillus larvae in honeybee hives. J. Invertebr. Pathol. 2017, 150, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, I.; Elekonich, M.M.; Abel-Santos, E.; Wing, H.J. Comparison of in vitro methods for the production of Paenibacillus larvae endospores. J. Microbiol. Methods 2015, 116, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Marche, M.G.; Satta, A.; Floris, I.; Lazzeri, A.M.; Ruiu, L. Inhibition of Paenibacillus larvae by an extracellular protein fraction from a honeybee-borne Brevibacillus laterosporus strain. Microbiol. Res. 2019, 227, 126303. [Google Scholar] [CrossRef]
- Gagnaire, B.; Bonnet, M.; Tchamitchian, S.; Cavalié, I.; Della-Vedova, C.; Dubourg, N.; Adam-Guillermin, C.; Brunet, J.-L.; Belzunces, L.P. Physiological effects of gamma irradiation in the honeybee, Apis mellifera. Ecotoxicol. Environ. Saf. 2019, 174, 153–163. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration—FDA. Overview of Irradiation of Food and Packaging; 2004; Chapter 1, pp. 1–11. Available online: https://www.fda.gov/food/irradiation-food-packaging/overview-irradiation-food-and-packaging (accessed on 17 July 2024).
- Shamsudin, S.; Selamat, J.; Sanny, M.; Bahari, S.; Jambari, N.N.; Khatib, A. A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules 2019, 24, 3898. [Google Scholar] [CrossRef]
- Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423. [Google Scholar] [CrossRef]
- De Guzman, Z.M.; Cervancia, C.R.; Dimasuay, K.G.B.; Tolentino, M.M.; Abrera, G.B.; Cobar, M.L.C.; Fajardo, A.C.; Sabino, N.G.; Manila-Fajardo, A.C.; Feliciano, C.P. Radiation inactivation of Paenibacillus larvae and sterilization of American Foul Brood (AFB) infected hives using Co-60 gamma rays. Appl. Radiat. Isot. 2011, 69, 1374–1379. [Google Scholar] [CrossRef]
- Borsdorf, A.; Marchant, C.; Rovira, A.; Sánchez, R. Chile Cambiando. Revisitando la Geografía Regional de Wolfgang Weischet; Serie GEO libros N°36; Instituto de Geografía, Pontificia Universidad Católica de Chile/Instituto de Ciencias Ambientales y Evolutivas, Facultad de Ciencias; Universidad Austral de Chile: Santiago, Chile, 2020; pp. 1–973. [Google Scholar]
- Viteri, R.; Zacconi, F.; Montenegro, G.; Giordano, A. Bioactive compounds in Apis mellifera monofloral honeys. J. Food Sci. 2020, 86, 1552–1582. [Google Scholar] [CrossRef] [PubMed]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Almeida-Muradian, L.B.; Sabato, S.F. Effect of gamma radiation on honey quality control. Radiat. Phys. Chem. 2009, 78, 583–584. [Google Scholar] [CrossRef]
- Carhuapoma, M.; Bonilla, P.; Suarez, S.; Villa, R.; López, G.S. Estudio de la composición química y actividad antioxidante del aceite esencial de Luma chequen (Moliná) A. Gray “arrayán”. Cienc. Investig. 2005, 8, 73–79. [Google Scholar] [CrossRef]
- Moncada, X.; Plaza, D.; Stoll, A.; Payacan, C.; Seelenfreund, D.; Martínez, E.; Bertin, A.; Squeo, F. Genetic diversity and structure of the vulnerable species Prosopis chilensis (Molina) Stuntz in the Coquimbo Region, Chile. Gayana Bot 2019, 76, 91–104. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.; Adnan, N.A.; Eddie-Tan, T.T. Antioxidant Activity of Three Honey Samples in relation with Their Biochemical Components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Le Conte, Y.; Navajas, M. Climate change: Impact on honey bee populations and diseases. Rev. Sci. Tech. 2008, 27, 499–510. [Google Scholar]


| Sample | Región of Chile | Species 1 | Grains of Pollen (%) | Species 2 | Grains of Pollen (%) | Species 3 | Grains of Pollen (%) | Total Grains of Pollen | Presence of Spores of P. larvae |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Coquimbo | Escallonia pulverulenta | 67.49 | Olea/Citrus | 8.78 | Eucalyptus sp. | 6.29 | 683 | Yes |
| 2 | Coquimbo | Brassica rapa (a) | 33.22 | Eucalyptus sp. | 12.58 | Trifolium repens (a) | 8.71 | 620 | Yes |
| 3 | Coquimbo | Brassica rapa (a) | 74.13 | Eucalyptus sp. | 5.9 | Trifolium repens (a) | 4.54 | 661 | Yes |
| 4 | Coquimbo | Tecophilaceae | 33.39 | Quillaja saponaria | 28.87 | Lotus corniculatus | 7.9 | 620 | Yes |
| 5 | Coquimbo | Quillaja saponaria | 77.18 | Medicago sp | 6.25 | Trifolium repens (a) | 3.92 | 688 | Yes |
| 6 | Coquimbo | Prosopis | 69.09 | Brassica rapa | 10.86 | Tecophilaceae | 4.34 | 783 | Yes |
| 7 | Metropolitana | Medicago sp. | 15.95 | Luma chequen | 10.4 | Eucalyptus sp. | 9.11 | 702 | No |
| 8 | Metropolitana | Quillaja saponaria | 78.28 | Medicago sp. | 4.87 | Lotus corniculatus | 3.11 | 677 | Yes |
| 9 | Metropolitana | Brassica rapa (a) | 23.12 | Quillaja saponaria | 16.85 | Luma chequen | 13.65 | 713 | No |
| 10 | Metropolitana | Medicago sp. | 25.25 | Quillaja saponaria | 21.66 | Robinia pseudoacacia (a) | 11.47 | 697 | No |
| 11 | Metropolitana | Brassica rapa (a) | 22.73 | Tecophilaceae | 15.39 | Schinus polygamus | 11.36 | 695 | No |
| 12 | Metropolitana | Quillaja saponaria | 57.58 | Medicago sp. | 10.67 | Brassica rapa (a) | 4.21 | 712 | No |
| 13 | Metropolitana | Eucalyptus sp. | 21.25 | Robinia pseudoacacia (a) | 20.43 | Luma chequen | 18.39 | 734 | No |
| 14 | Metropolitana | Quillaja saponaria | 47.89 | Luma chequen | 19.34 | Medicago sp. | 12.45 | 697 | No |
| 15 | Metropolitana | Brassica rapa (a) | 39.67 | Quillaja saponaria | 15.34 | Schinus polygamus | 9.67 | 654 | No |
| 16 | O´Higgins | Galega officinalis (a) | 52.64 | Lotus corniculatus | 20.16 | Aristotelia chilensis | 5.12 | 625 | No |
| 17 | O´Higgins | Medicago sp. (a) | 31.58 | Lotus corniculatus | 25.27 | Galega officinalis (a) | 1001 | 649 | No |
| 18 | Biobio | Medicago sp. (a) | 38.76 | Lotus corniculatus | 12.16 | Aristotelia chilensis | 10.32 | 707 | No |
| 19 | Biobio | Castanea sativa | 55.52 | Caldcluvia paniculata | 12.42 | Medicago sp. | 7.52 | 652 | No |
| 20 | Araucania | Cladcluvia paniculata | 28.71 | Lotus pedunculatus | 23.76 | Escallonia rubra | 19.93 | 627 | No |
| 21 | Los Rios | Caldcluvia paniculata | 38.89 | Lotus pedunculatus | 26.58 | Buddleja globosa | 10.85 | 617 | No |
| 22 | Los Rios | Eucryphia cordifolia | 96.06 | Lotus pedunculatus | 1.57 | Buddleja globosa | 0.94 | 636 | No |
| 23 | Los Rios | Eucryphia cordifolia | 96.91 | Gevuina avellana | 1.21 | Luma apiculata | 1.07 | 744 | No |
| 24 | Los Rios | Caldcluvia paniculata | 77.11 | Lotus pedunculatus | 6.96 | Luma apiculata | 6.69 | 747 | No |
| 25 | Los Rios | Aristotelia chilensis | 49.84 | Lotus pedunculatus | 9.96 | Weinmannia trichosperma | 7.52 | 612 | No |
| 26 | Los Rios | Eucryphia cordifolia | 53.32 | Weinmannia trichosperma | 9.82 | Buddleja globosa | 8.61 | 662 | No |
| 27 | Los Rios | Caldcluvia paniculata | 45.43 | Buddleja globosa | 11.12 | Weinmannia trichosperma | 10.21 | 656 | No |
| 28 | Los Rios | Eucryphia cordifolia | 78.03 | Escallonia rubra | 3.23 | Weinmannia trichosperma | 3.07 | 651 | No |
| 29 | Los Rios | Weinmannia trichosperma | 87.26 | Caldcluvia paniculata | 9.22 | Buddleja globosa | 2.19 | 683 | No |
| 30 | Los Rios | Weinmannia trichosperma | 59.89 | Caldcluvia paniculata | 30.57 | Trifolium pratense (a) | 2.49 | 723 | No |
| 31 | Los Rios | Weinmannia trichosperma | 89.31 | Caldcluvia paniculata | 6.72 | Aristotelia chilensis | 2.14 | 655 | No |
| 32 | Los Rios | Weinmannia trichosperma | 88.13 | Aristotelia chilensis | 10.71 | Lotus pedunculatus | 0.43 | 691 | No |
| 33 | Los Rios | Weinmannia trichosperma | 95.65 | Aristotelia chilensis | 2.39 | Cissus striata | 0.9 | 667 | No |
| 34 | Los Rios | Weinmannia trichosperma | 55.83 | Caldcluvia paniculata | 33.83 | Buddleja globosa | 5.42 | 609 | No |
| 35 | Los Rios | Weinmannia trichosperma | 77.98 | Caldcluvia paniculata | 12.66 | Buddleja globosa | 3.58 | 727 | No |
| 36 | Los Rios | Caldcluvia paniculata | 77.46 | Azara microphylla | 10.67 | Lotus pedunculatus | 5.11 | 684 | No |
| 37 | Los Rios | Eucryphia cordifolia | 82.66 | Azara microphylla | 14.22 | Weinmannia trichosperma | 0.94 | 640 | No |
| 38 | Los Rios | Eucryphia cordifolia | 60.32 | Azara microphylla | 32.67 | Lotus pedunculatus | 3.73 | 698 | No |
| 39 | Los Rios | Weinmannia trichosperma | 86.34 | Caldcluvia paniculata | 6.19 | Aristotelia chilensis | 3.78 | 639 | No |
| 40 | Los Lagos | Tepualia stipularis | 52.42 | Lotus pedunculatus | 17.79 | Weinmannia trichosperma | 12.48 | 641 | No |
| 41 | Los Lagos | Tepualia stipularis | 58.23 | Lotus pedunculatus | 23.61 | Caldcluvia paniculata | 7.58 | 699 | No |
| 42 | Los Lagos | Trifolium repens | 43.08 | Medicago sp. | 14.41 | Lomatia sp. | 12.69 | 701 | No |
| 43 | Los Lagos | Caldcluvia paniculata | 90.32 | Lotus pedunculatus | 3.99 | Tepualia stipularis | 3.84 | 651 | No |
| 44 | Los Lagos | Luma apiculata | 59.38 | Trifolium repens | 18.32 | Tepualia stipularis | 5.91 | 677 | No |
| 45 | Los Lagos | Eucryphia cordifolia | 91.61 | Tepualia stipularis | 4.28 | Lotus pedunculatus | 3.67 | 655 | No |
| 46 | Los Lagos | Caldcluvia paniculata | 94.01 | Tepualia stipularis | 1.89 | Luma apiculata | 1.61 | 751 | No |
| 47 | Los Lagos | Eucryphia cordifolia | 89.74 | Tepualia stipularis | 4.24 | Weinmannia trichosperma | 2.61 | 614 | No |
| 48 | Los Lagos | Eucryphia cordifolia | 96.74 | Weinmannia trichosperma | 1.71 | Tepualia stipularis | 0.93 | 645 | No |
| 49 | Los Lagos | Eucryphia cordifola | 96.05 | Lotus pedunculatus | 1.11 | Trifolium pratense | 0.79 | 633 | No |
| 50 | Los Lagos | Tepualia stipularis | 31.53 | Luma apiculata | 26.72 | Caldcluvia paniculata | 24.82 | 685 | No |
| 51 | Los Lagos | Eucryphia cordifolia | 35.48 | Tepualia stipularis | 34.98 | Lotus pedunculatus | 11.39 | 606 | No |
| 52 | Los Lagos | Caldcluvia paniculata | 81.54 | Lotus pedunculatus | 6.27 | Tepualia stipularis | 5.96 | 665 | No |
| 53 | Los Lagos | Eucryphia cordifolia | 92.36 | Tepualia stipularis | 1.98 | Weinmannia trichosperma | 1.53 | 632 | No |
| 54 | Los Lagos | Eucryphia cordifolia | 96.74 | Weinmannia trichosperma | 1.71 | Tepualia stipularis | 0.93 | 681 | No |
| Sample | Region of Chile | Presence of Spores of P. larvae |
|---|---|---|
| 1 | Coquimbo | Yes |
| 2 | Coquimbo | Yes |
| 3 | Coquimbo | Yes |
| 4 | Coquimbo | Yes |
| 5 | Coquimbo | Yes |
| 6 | Coquimbo | Yes |
| 7 | Metropolitana | No |
| 8 | Metropolitana | Yes |
| 9 | Metropolitana | No |
| 10 | Metropolitana | No |
| 11 | Metropolitana | No |
| 12 | Metropolitana | No |
| 13 | Metropolitana | No |
| 14 | Metropolitana | No |
| 15 | Metropolitana | No |
| 16 | O´Higgins | No |
| 17 | O´Higgins | No |
| 18 | Biobio | No |
| 19 | Biobio | No |
| 20 | Araucania | No |
| 21 | Los Rios | No |
| 22 | Los Rios | No |
| 23 | Los Rios | No |
| 24 | Los Rios | No |
| 25 | Los Rios | No |
| 26 | Los Rios | No |
| 27 | Los Rios | No |
| 28 | Los Rios | No |
| 29 | Los Rios | No |
| 30 | Los Rios | No |
| 31 | Los Rios | No |
| 32 | Los Rios | No |
| 33 | Los Rios | No |
| 34 | Los Rios | No |
| 35 | Los Rios | No |
| 36 | Los Rios | No |
| 37 | Los Rios | No |
| 38 | Los Rios | No |
| 39 | Los Rios | No |
| 40 | Los Lagos | No |
| 41 | Los Lagos | No |
| 42 | Los Lagos | No |
| 43 | Los Lagos | No |
| 44 | Los Lagos | No |
| 45 | Los Lagos | No |
| 46 | Los Lagos | No |
| 47 | Los Lagos | No |
| 48 | Los Lagos | No |
| 49 | Los Lagos | No |
| 50 | Los Lagos | No |
| 51 | Los Lagos | No |
| 52 | Los Lagos | No |
| 53 | Los Lagos | No |
| 54 | Los Lagos | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejías, E.; Gomez, C.; Garrido, T. Effect on the Antioxidant Properties of Native Chilean Endemic Honeys Treated with Ionizing Radiation to Remove American Foulbrood Spores. Foods 2024, 13, 2710. https://doi.org/10.3390/foods13172710
Mejías E, Gomez C, Garrido T. Effect on the Antioxidant Properties of Native Chilean Endemic Honeys Treated with Ionizing Radiation to Remove American Foulbrood Spores. Foods. 2024; 13(17):2710. https://doi.org/10.3390/foods13172710
Chicago/Turabian StyleMejías, Enrique, Carlos Gomez, and Tatiana Garrido. 2024. "Effect on the Antioxidant Properties of Native Chilean Endemic Honeys Treated with Ionizing Radiation to Remove American Foulbrood Spores" Foods 13, no. 17: 2710. https://doi.org/10.3390/foods13172710
APA StyleMejías, E., Gomez, C., & Garrido, T. (2024). Effect on the Antioxidant Properties of Native Chilean Endemic Honeys Treated with Ionizing Radiation to Remove American Foulbrood Spores. Foods, 13(17), 2710. https://doi.org/10.3390/foods13172710






