Technological Properties of Inulin-Enriched Doughs and Breads, Influence on Short-Term Storage and Glycemic Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Flour and Inulin
- −
- Inulin ≥ 90%;
- −
- Fructose, glucose and sucrose ≤ 10%;
- −
- The average chain length is 8–13 monomers;
- −
- Ash ≤ 0.2%.
2.2. Technological Tests
2.2.1. Water-Binding Capacity and Oil-Binding Capacity
2.2.2. Leavening Test
- V = volume measured after n minutes;
- V0 = initial volume at time 0;
- The analyses were carried out in triplicate.
2.3. Rheological Tests
2.4. Bread Production and Physical Analyses
- RHT0 = bread moisture at T0;
- RHTX = bread moisture at T2 or T4.
2.5. Color Determinations
- BI = brown index;
- a = red index;
- b = yellow index.
2.6. Determination of Bread Staling Rate
2.7. Inulin Extraction and Quantification
2.8. Predicted Glycemic Index (pGI) Evaluation
2.9. Statistical Analysis
3. Results and Discussion
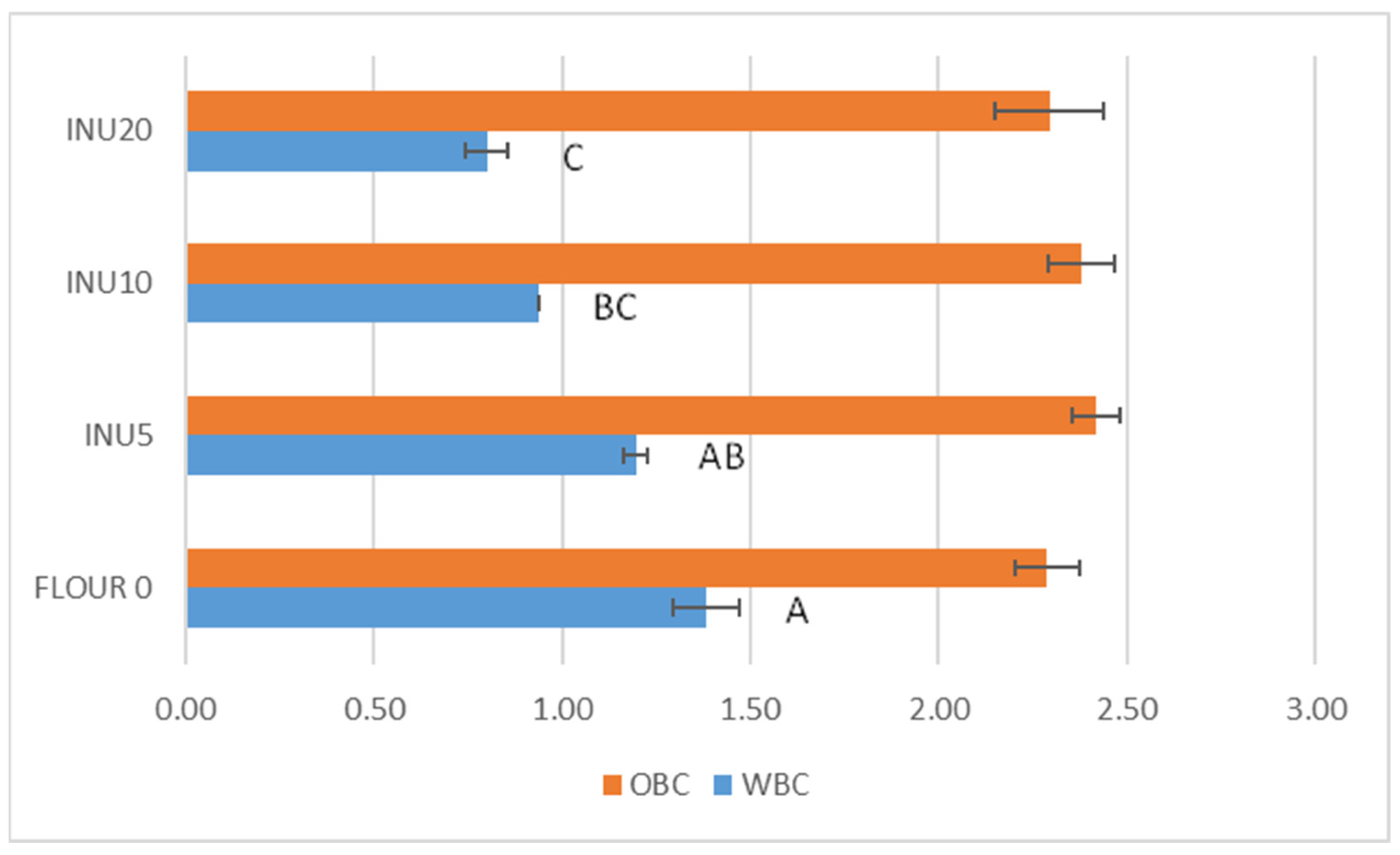
3.1. Water-Binding Capacity and Oil-Binding Capacity
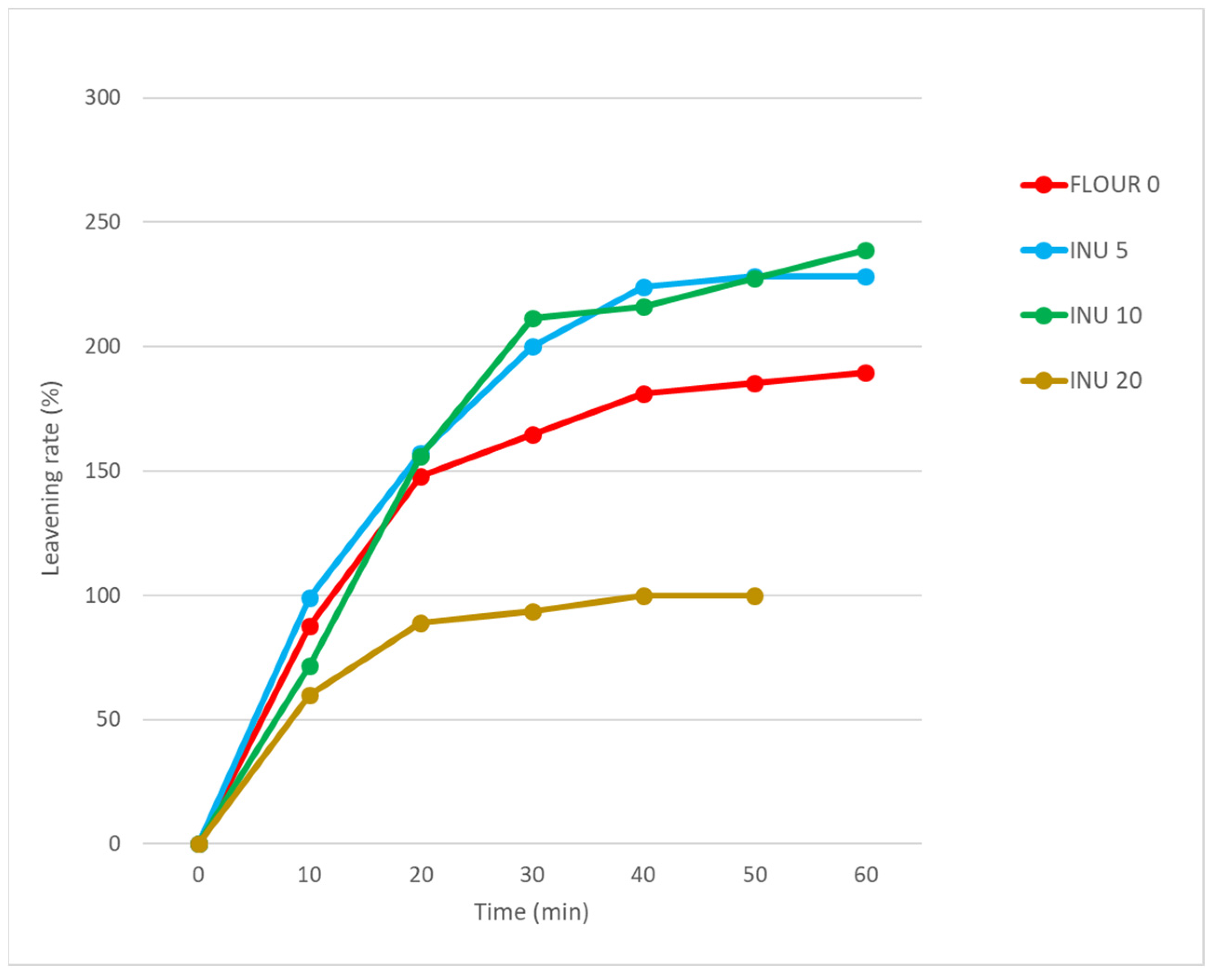
3.2. Rheological Data of Flours and Leavening Rate of the Dough
3.3. Physico-Chemical Characteristics of the Breads for Short Storage
3.4. Nutritional Characteristics of the Breads
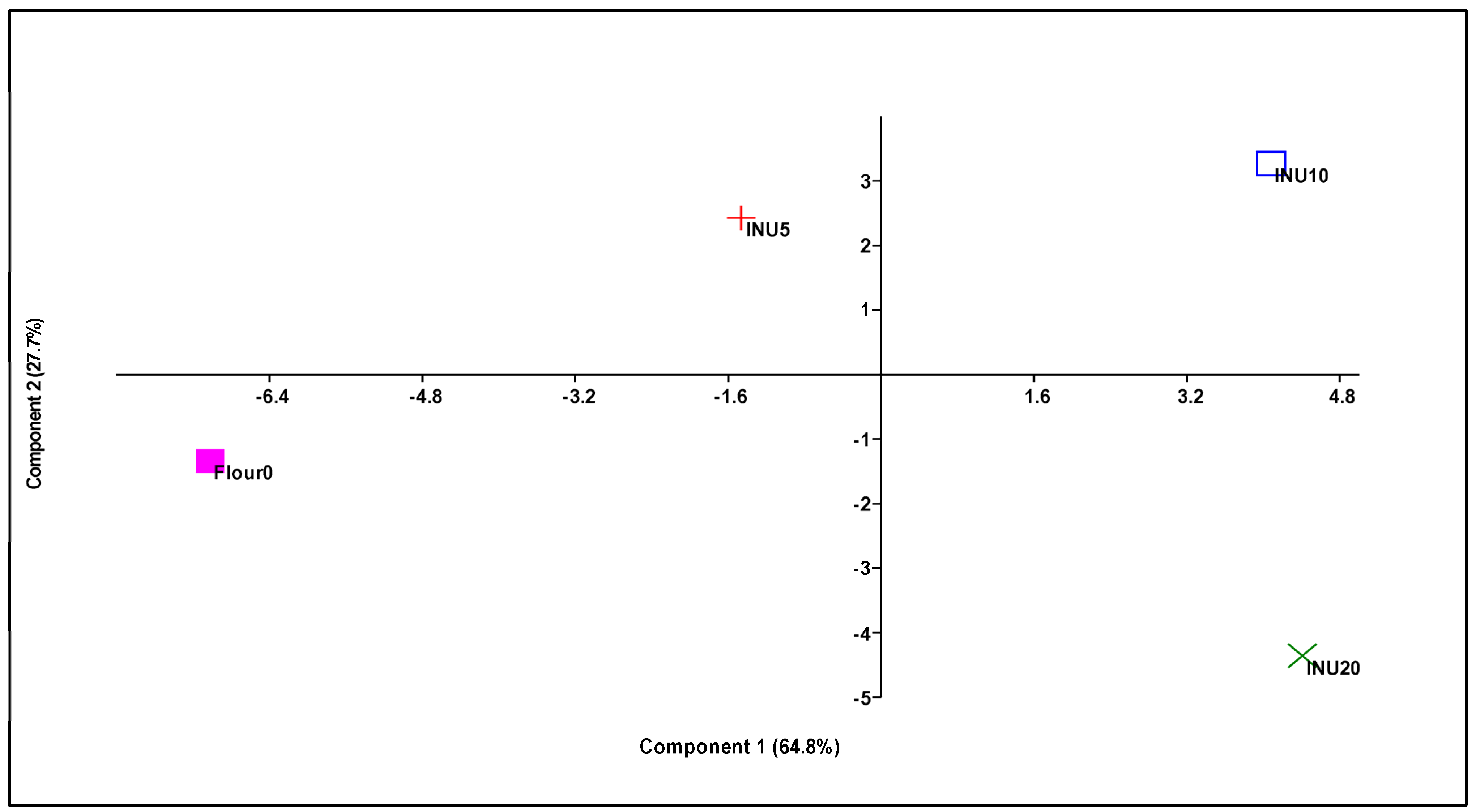
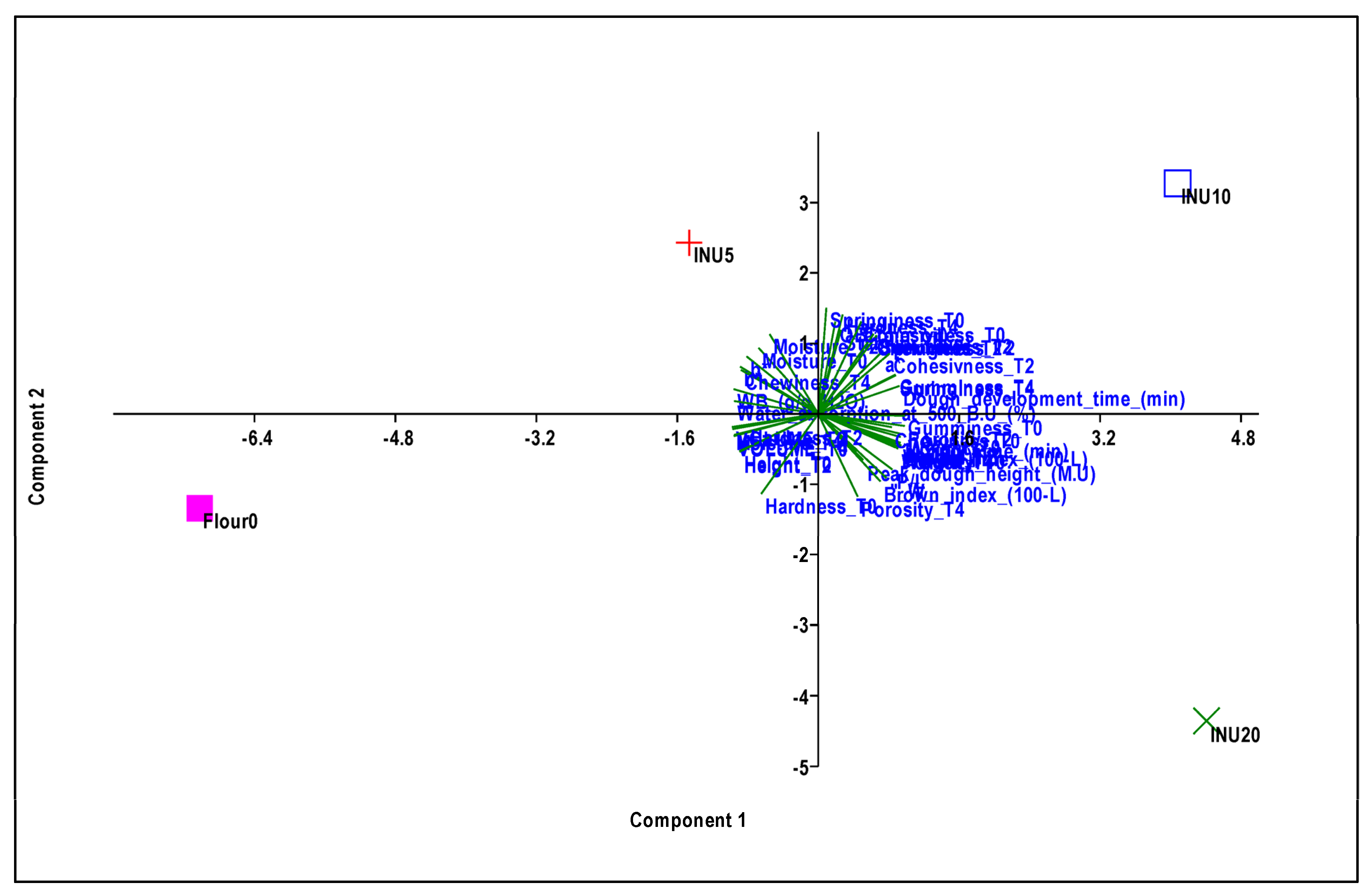
3.5. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spina, A.; Guarnaccia, P.; Canale, M.; Sanfilippo, R.; Bizzini, M.; Blangiforti, S.; Zingale, S.; Lo Piero, A.R.; Allegra, M.; Sicilia, A.; et al. Sicilian Rivet Wheat Landraces: Grain Characteristics and Technological Quality of Flour and Bread. Plants 2023, 12, 2641. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Summo, C.; Timpanaro, N.; Canale, M.; Sanfilippo, R.; Amenta, M.; Strano, M.C.; Allegra, M.; Papa, M.; Pasqualone, A. Lupin as ingredient in durum wheat breadmaking: Physicochemical properties of flour blends and bread quality. Foods 2024, 13, 807. [Google Scholar] [CrossRef]
- Spina, A.; Saletti, R.; Fabroni, S.; Natalello, A.; Cunsolo, V.; Scarangella, M.; Rapisarda, P.; Canale, M.; Muccilli, V. Multielemental, Nutritional, and Proteomic Characterization of Different Lupinus spp. Genotypes: A Source of Nutrients for Dietary Use. Molecules 2022, 27, 8771. [Google Scholar] [CrossRef] [PubMed]
- Ficco, D.B.M.; Canale, M.; Giannone, V.; Strano, M.C.; Allegra, M.; Zingale, S.; Spina, A. Durum wheat bread with a potentially high health value through the addition of durum wheat thin bran or barley flour. Plants 2023, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, A.; Sutar, P.P.; Mishra, M. Inulin-A polysaccharide: Review on its functional and prebiotic efficacy. J. Food Biochem. 2022, 46, e14386. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Bustos, M.C.; Vignola, M.B.; Paesani, C.; Salinas, C.N.; Perez, G.T. A study on fibre addition to gluten free bread: Its effects on bread quality and in vitro digestibility. J. Food Sci. Tech. 2017, 54, 244–252. [Google Scholar] [CrossRef]
- Arp, C.G.; Correa, M.J.; Ferrero, C. Kinetic study of staling in breads with high-amylose resistant starch. Food Hydrocoll. 2020, 106, 105879. [Google Scholar] [CrossRef]
- Mohammadi, F.; Shiri, A.; Tahmouzi, S.; Mollakhalili-Meybodi, N.; Nematollahi, A. Application of inulin in bread: A review of technological properties and factors affecting its stability. Food Sci. Nutr. 2023, 11, 639–650. [Google Scholar] [CrossRef]
- Lightowler, H.; Thondre, S.; Holz, A.; Theis, S. Replacement of glycaemic carbohydrates by inulin-type fructans from chicory (oligofructose, inulin) reduces the postprandial blood glucose and insulin response to foods: Report of two double-blind, randomized, controlled trials. Eur. J. Nutr. 2018, 57, 1259–1268. [Google Scholar] [CrossRef]
- Singh, V.; San Yeoh, B.; Chassaing, B.; Xiao, X.; Saha, P.; Olvera, R.A.; Vijay-Kumar, M. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 2018, 175, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Cheng, X.; Qian, L.; Huo, A.; Chen, J.; Sun, Y. Extraction, physicochemical properties, functional activities and applications of inulin polysaccharide: A review. Plant Foods Hum. Nutr. 2023, 78, 243–252. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocch, G.; Cicala, M. Mechanisms of action of prebiotics and their effects on gastro-intestinal disorders in adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Jayasena, D.D.; Wimalasiri, K.M.; Ranadheera, C.S.; Ajlouni, S. Inulin fructans–food applications and alternative plant sources: A review. Int. J. Food. Sci. Tech. 2022, 57, 5764–5780. [Google Scholar] [CrossRef]
- Brasil, J.A.; Silveira, K.C.D.; Salgado, S.M.; Livera, A.V.S.; Faro, Z.P.D.; Guerra, N.B. Effect of the addition of inulin on the nutritional, physical and sensory parameters of bread. Braz. J. Pharm. Sci. 2011, 47, 185–191. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Piłat, B.; Starowicz, M.; Jeliński, T.; Szmatowicz, B. High-quality gluten-free sponge cakes without sucrose: Inulin-type fructans as sugar alternatives. Foods 2020, 9, 1735. [Google Scholar] [CrossRef]
- Teferra, T.F. Possible actions of inulin as prebiotic polysaccharide: A review. Food Front. 2021, 2, 407–416. [Google Scholar] [CrossRef]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002, 87, S287–S291. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, R.; Canale, M.; Dugo, G.; Oliveri, C.; Scarangella, M.; Strano, M.C.; Amenta, M.; Crupi, A.; Spina, A. Effects of Partial Replacement of Durum Wheat Re-Milled Semolina with Bean Flour on Physico-Chemical and Techno-logical Features of Doughs and Breads during Storage. Plants 2022, 12, 1125. [Google Scholar] [CrossRef]
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from artichoke processing industry: Reuse in bread-making and evaluation of the physico-chemical characteristics of the final product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis, 11th ed.; The American Association of Cereal Chemists: Saint Paul, MN, USA, 2000. [Google Scholar]
- UNI 10783; Wheat Flour-Determination of Rheological Properties Using an Alveograph. ISO: Geneva, Switzerland, 1999.
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Rockville, MD, USA, 2011. [Google Scholar]
- Dallmann, H. Porentabelle, 4th ed.; Verlag Moritz Schäfer: Detmold, Germany, 1981. [Google Scholar]
- Bot, B.; Sánchez, H.; de la Torre, M.; Osella, C. Mother dough in bread making. Food Sci. Nutr. 2014, 2, 24–29. [Google Scholar] [CrossRef][Green Version]
- Rózylo, R.; Laskowski, J. Predicting bread quality (bread loaf volume and crumb texture). Pol. J. Food Nutr. Sci. 2011, 61, 61–67. [Google Scholar] [CrossRef]
- Bavaro, A.R.; Di Biase, M.; Linsalata, V.; D’Antuono, I.; Di Stefano, V.; Lonigro, S.L.; Garbetta, A.; Valerio, F.; Melilli, M.G.; Cardinali, A. Potential Prebiotic Effect of Inulin-Enriched Pasta after In Vitro Gastrointestinal Digestion and Simulated Gut Fermentation. Foods 2024, 13, 1815. [Google Scholar] [CrossRef]
- Steegmans, M.; Iliaens, S.; Hoebregs, H. Enzymatic, spectrophotometric determination of glucose, fructose, sucrose, and inulin/oligofructose in foods. J. AOAC Int. 2004, 87, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Garbetta, A.; D’Antuono, I.; Melilli, M.G.; Sillitti, C.; Linsalata, V.; Scandurra, S.; Cardinali, A. Inulin Enriched Durum Wheat Spaghetti: Effect of Polymerization Degree on Technological and Nutritional Characteristics. J. Funct. Foods 2020, 71, 104004. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Salinas, M.V.; Zuleta, A.; Ronayne, P.; Puppo, M.C. Wheat flour enriched with calcium and inulin: A study of hydration and rheological properties of dough. Food Bioproc. Tech. 2012, 5, 3129–3141. [Google Scholar] [CrossRef]
- Liu, Y.; Leng, Y.; Xiao, S.; Zhang, Y.; Ding, W.; Ding, B.; Wu, Y.; Wang, X.; Fu, Y. Effect of inulin with different degrees of polymerization on dough rheology, gelatinization, texture and protein composition properties of extruded flour products. LWT 2022, 159, 113225. [Google Scholar] [CrossRef]
- Luo, D.; Kou, X.; Zhang, T.; Nie, Y.; Xu, B.; Li, P.; Li, X.; Han, S.; Liu, J. Effect of inulin on rheological properties of soft and strong wheat dough. Int. J. Food Sci. Technol. 2018, 53, 1648–1656. [Google Scholar] [CrossRef]
- Brennan, C.S.; Kuri, V.; Tudorica, C.M. Inulin-enriched pasta: Effects on textural properties and starch degradation. Food Chem. 2004, 86, 189–193. [Google Scholar] [CrossRef]
- Wang, J.; Rosell, C.M.; de Barber, C.B. Effect of the addition of different fibres on wheat dough performance and bread quality. Food Chem. 2002, 79, 221–226. [Google Scholar] [CrossRef]
- Peressini, D.; Sensidoni, A. Effect of soluble dietary fibre addition on rheological and breadmaking properties of wheat doughs. J. Cereal Sci. 2009, 49, 190–201. [Google Scholar] [CrossRef]
- O’brien, C.M.; Mueller, A.; Scannell, A.G.M.; Arendt, E.K. Evaluation of the effects of fat replacers on the quality of wheat bread. J. Food Eng. 2003, 56, 265–267. [Google Scholar] [CrossRef]
- Sirbu, A.; Arghire, C. Functional bread: Effect of inulin-type products addition on dough rheology and bread quality. J. Cereal Sci. 2017, 75, 220–227. [Google Scholar] [CrossRef]
- Singh, K.K.; Norton, R.S. Metabolic changes induced during adaptation of Saccharomyces cerevisiae to a water stress. Arch. Microbiol. 1991, 156, 38–42. [Google Scholar] [CrossRef]
- Edgley, M.; Brown, A.D. Yeast water relations: Physiological changes induced by solute stress in Saccharomyces cerevisiae and Saccharomyces rouxii. Microbiology 1983, 129, 3453–3463. [Google Scholar] [CrossRef][Green Version]
- Cavella, S.; Romano, A.; Giancone, T.; Masi, P. The influence of dietary fibres on bubble development during bread making. In Bubbles in Food 2; AACC International Press: Saint Paul, MN, USA, 2008; pp. 311–321. [Google Scholar]
- Ronda, F.; Quilez, J.; Pando, V.; Roos, Y.H. Fermentation time and fiber effects on recrystallization of starch components and staling of bread from frozen part-baked bread. J. Food Eng. 2014, 131, 116–123. [Google Scholar] [CrossRef]
- Barros, J.H.; Franco, C.M. Changes in rheology, quality, and staling of white breads enriched with medium-polymerized inulin. Food Sci. Technol. Int. 2022, 28, 32–39. [Google Scholar] [CrossRef]
- Mandala, I.; Polaki, A.; Yanniotis, S. Influence of frozen storage on bread enriched with different ingredients. J. Food Eng. 2009, 92, 137–145. [Google Scholar] [CrossRef]
- Rudel, H.W. A Composition of Flours Containing Vital Gluten and Soluble Oat Dietary Fiber and a Baked Product Produced Therefrom. U.S. Patent Application No. 4,961,937, 9 October 1990. [Google Scholar]
- Pomeranz, Y.; Shogren, M.D.; Finney, K.F.; Bechtel, D.B. Fiber in breadmaking—Effects on functional properties. Cereal Chem. 1977, 54, 25–41. [Google Scholar]
- Anil, M. Using of hazelnut testa as a source of dietary fiber in breadmaking. J. Food Eng. 2007, 80, 61–67. [Google Scholar] [CrossRef]
- Juszczak, L.; Witczak, T.; Ziobro, R.; Korus, J.; Cieślik, E.; Witczak, M. Effect of inulin on rheological and thermal properties of gluten-free dough. Carbohydr. Polym. 2012, 90, 353–360. [Google Scholar] [CrossRef]
- Ziobro, R.; Korus, J.; Juszczak, L.; Witczak, T. Influence of inulin on physical characteristics and staling rate of gluten-free bread. J. Food Eng. 2013, 116, 21–27. [Google Scholar] [CrossRef]
- Kou, X.; Luo, D.; Zhang, K.; Xu, W.; Li, X.; Xu, B.; Li, P.; Han, S.; Liu, J. Textural and staling characteristics of steamed bread prepared from soft flour added with inulin. Food Chem. 2019, 301, 125272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Staling of Chinese steamed bread: Quantification and control. Trends Food Sci. Technol. 2016, 55, 118–127. [Google Scholar] [CrossRef]
- Fois, S.; Sanna, M.; Stara, G.; Roggio, T.; Catzeddu, P. Rheological properties and baking quality of commercial durum wheat meals used to make flat crispy bread. Eur. Food Res. Technol. 2011, 232, 713–722. [Google Scholar] [CrossRef]
- Hager, A.S.; Ryan, L.A.; Schwab, C.; Gänzle, M.G.; O’Doherty, J.V.; Arendt, E.K. Influence of the soluble fibres inulin and oat β-glucan on quality of dough and bread. Eur. Food Res. Technol. 2011, 232, 405–413. [Google Scholar] [CrossRef]
- Capriles, V.D.; Arêas, J.A. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013, 4, 104–110. [Google Scholar] [CrossRef]
- Mohammadi, F.; Ehrampoush, M.H.; Shamsi, F.; Ardakani, S.A.Y.; Mollakhalili-Meybodi, N. Inulin enriched wheat bread: Interaction of polymerization degree and fermentation type. J. Food Meas. Charact. 2021, 15, 5408–5417. [Google Scholar] [CrossRef]
- Morreale, F.; Benavent-Gil, Y.; Rosell, C.M. Inulin enrichment of gluten free breads: Interaction between inulin and yeast. Food Chem. 2019, 278, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Verspreet, J.; Hemdane, S.; Dornez, E.; Cuyvers, S.; Delcour, J.A.; Courtin, C.M. Maximizing the concentrations of wheat grain fructans in bread by exploring strategies to prevent their yeast (Saccharomyces cerevisiae)-mediated degradation. J. Agric. Food Chem. 2013, 61, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Rao, V. The prebiotic properties of oligofructose at low intake levels. Nutr. Res. 2001, 21, 843–848. [Google Scholar] [CrossRef]
- Mollakhalili-meybodi, N.; Ehrampoush, M.H.; Hajimohammadi, B.; Mosaddegh, M.H. Formulation optimization of functional wheat bread with low glycemic index from technological and nutritional perspective. Food Sci. Nutr. 2023, 11, 284–294. [Google Scholar] [CrossRef] [PubMed]


| Bread Type | Flour (g) | Commercial Inulin (g) | Commercial Yeast (g) | NaCl (g) | Water (g) |
|---|---|---|---|---|---|
| Flour 0 | 100 | - | 1.5 | 2.2 | 58 |
| INU 5 | 95 | 5 | 1.5 | 2.2 | 48 |
| INU 10 | 90 | 10 | 1.5 | 2.2 | 45 |
| INU 20 | 80 | 20 | 1.5 | 2.2 | 41 |
| Sample | Farinograph | Mixograph | Alveograph | ||||
|---|---|---|---|---|---|---|---|
| Dough Development Time (min) | Stability (min) | Water Absorption at 500 B.U. * (%) | Mixing Time (min) | Peak Dough Height (M.U.) ** | W (10−4 × J) | P/L | |
| Flour 0 | 1.80 ± 0.28 b | 8.15 ± 0.35 b | 57.50 ± 0.28 a | 4.61 ± 0.08 d | 5.78 ± 0.11 | 154 ± 8.49 b | 0.82 ± 0.02 c |
| INU5 | 14.80 ± 0.85 a | 8.20 ± 0.42 b | 48.25 ± 0.21 b | 6.90 ± 0.37 c | 5.48 ± 0.04 | 150 ± 2.83 b | 2.45 ± 0.17 bc |
| INU10 | 18.35 ± 0.07 a | 13.10 ± 0.28 a | 44.85 ± 1.06 bc | 7.32 ± 0.02 b | 5.93 ± 0.11 | 175 ± 2.83 ab | 3.21 ± 0.20 ab |
| INU20 | 16.65 ± 0.07 a | 6.35 ± 0.21 b | 40.75 ± 0.21 c | 8.70 ± 0.04 a | 5.95 ± 0.35 | 203 ± 1.41 a | 4.55 ± 0.04 a |
| Days | Type of Bread | Moisture (g/100 g) | ΔRH (%) | Weight (g) | Volume (cm3) | Height (cm) | Porosity (1–8) * |
|---|---|---|---|---|---|---|---|
| T0 | Flour 0 | 35.72 ± 0.01 a | - | 153.99 ± 0.89 c | 773.33 ± 10.41 a | 9.23 ± 0.06 a | 4.33 ± 0.29 b |
| INU5 | 33.50 ± 0.01 a | - | 156.70 ± 0.76 ab | 574.17 ± 10.10 b | 8.39 ± 0.03 ab | 4.50 ± 0.25 b | |
| INU10 | 32.15 ± 0.01 ab | - | 159.44 ± 0.74 ab | 449.17 ± 1.44 c | 6.54 ± 0.25 c | 7.07 ± 0.38 a | |
| INU20 | 27.06 ± 0.01 b | - | 160.92 ± 1.81 a | 465.00 ± 9.01 c | 7.32 ± 0.17 b | 7.53 ± 0.35 a | |
| T2 | Flour 0 | 31.67 ± 0.02 a | 11.3 | 142.70 ± 1.09 c | 746.67 ± 20.21 a | 8.35 ± 0.06 a | 4.33 ± 0.29 b |
| INU5 | 29.81 ± 0.01 ab | 11.0 | 148.01 ± 0.75 bc | 541.67 ± 12.58 b | 7.71 ± 0.03 ab | 4.50 ± 0.25 b | |
| INU10 | 29.87 ± 0.00 ab | 7.1 | 153.02 ± 0.40 ab | 449.17 ± 6.61 c | 6.33 ± 0.25 c | 7.07 ± 0.38 a | |
| INU20 | 25.73 ± 0.01 b | 4.9 | 156.19 ± 1.86 a | 460.83 ± 9.46 c | 7.20 ± 0.17 b | 7.53 ± 0.35 a | |
| T4 | Flour 0 | 27.80 ± 0.01 a | 22.2 | 131.32 ± 1.09 c | 615.00 ± 7.07 a | 8.00 ± 0.14 a | 5.13 ± 0.18 c |
| INU5 | 27.21 ± 0.01 a | 18.8 | 141.29 ± 0.57 bc | 520.50 ± 7.78 ab | 7.53 ± 0.15 ab | 6.13 ± 0.18 bc | |
| INU10 | 27.05 ± 0.00 ab | 15.9 | 144.79 ± 0.47 ab | 422.50 ± 3.54 ab | 6.00 ± 0.10 c | 7.38 ± 0.18 b | |
| INU20 | 24.32 ± 0.00 b | 10.1 | 153.20 ± 1.46 a | 443.75 ± 8.84 b | 6.77 ± 0.32 b | 7.53 ± 0.35 a |
| Days after Baking | Type of Bread | Hardness (N) | Springiness | Gumminess (N) | Chewiness (N × mm) | Cohesiveness |
|---|---|---|---|---|---|---|
| T0 | Flour 0 | 8.59 ± 0.45 b | 0.95 ± 0.01 | 3.13 ± 0.02 d | 3.01 ± 0.05 d | 0.90 ± 0.01 a |
| INU5 | 4.19 ± 0.88 c | 0.95 ± 0.02 | 7.25 ± 0.01 c | 6.97 ± 0.08 c | 0.90 ± 0.01 a | |
| INU10 | 8.66 ± 0.53 b | 0.96 ± 0.01 | 15.81 ± 0.03 a | 15.23 ± 0.19 a | 0.81 ± 0.04 ab | |
| INU20 | 12.63 ± 0.90 a | 0.94 ± 0.02 | 10.30 ± 0.16 b | 9.78 ± 0.08 b | 0.76 ± 0.05 b | |
| T2 | Flour 0 | 17.67 ± 0.06 a | 0.81 ± 0.01 b | 11.63 ± 0.01 d | 9.50 ± 0.13 c | 0.65 ± 0.05 a |
| INU5 | 14.70 ± 1.55 ab | 0.90 ± 0.02 a | 27.76 ± 0.11 c | 5.09 ± 0.07 d | 0.69 ± 0.03 a | |
| INU10 | 13.37 ± 1.08 b | 0.94 ± 0.03 a | 39.00 ± 0.17 b | 36.60 ± 0.05 a | 0.71 ± 0.02 a | |
| INU20 | 16.17 ± 1.87 ab | 0.76 ± 0.01 b | 41.74 ± 0.17 a | 32.17 ± 0.04 | 0.64 ± 0.01 a | |
| T4 | Flour 0 | 22.96 ± 3.09 a | 0.75 ± 0.01 c | 21.26 ± 0.47 d | 15.01 ± 1.03 c | 0.34 ± 0.01 c |
| INU5 | 20.20 ± 0.87 b | 0.86 ± 0.01 b | 104.14 ± 1.32 b | 85.18 ± 5.40 b | 0.64 ± 0.02 ab | |
| INU10 | 21.87 ± 0.70 ab | 0.95 ± 0.01 a | 200.59 ± 2.81 a | 190.44 ± 2.93 a | 0.78 ± 0.01 a | |
| INU20 | 21.34 ± 2.02 b | 0.81 ± 0.01 bc | 62.15 ± 2.84 c | 49.88 ± 2.36 bc | 0.58 ± 0.01 b |
| Sample | Crust | Crumb | ||||||
|---|---|---|---|---|---|---|---|---|
| Brown Index (100 − L*) | a* | b* | ΔE | Brown index | a* | b* | ΔE | |
| Flour 0 | 29.45 ± 0.43 c | 4.00 ± 1.05 d | 26.77 ± 2.40 b | 0.0 | 38.39 ± 1.75 a | −1.27 ± 0.03 b | 11.77 ± 1.14 c | 0.0 |
| INU5 | 31.34 ± 1.70 bc | 8.33 ± 0.61 c | 32.40 ± 0.92 ab | 27.0 | 35.29 ± 0.79 ab | −1.00 ± 0.20 b | 15.40 ± 0.35 ab | 11.4 |
| INU10 | 39.92 ± 2.36 b | 12.36 ± 0.25 b | 33.97 ± 1.53 a | 115.7 | 30.98 ± 0.69 b | −0.91 ± 0.06 b | 16.68 ± 0.11 a | 39.6 |
| INU20 | 51.67 ± 2.31 a | 16.48 ± 0.78 a | 30.13 ± 1.73 ab | 330.4 | 23.47 ± 0.89 c | 0.05 ± 0.06 a | 13.05 ± 0.27 bc | 113.0 |
| Bread | ||||
|---|---|---|---|---|
| Sample | Flour 0 | INU5 | INU10 | INU20 |
| Inulin (g/100 g) | 0.18 ± 0.02 d | 3.53 ± 0.160 c | 6.32 ± 0.21 b | 14.42 ± 0.07 a |
| Hydrolysis Index (HI) | 99.83 ± 1.39 a | 99.26 ± 1.34 a | 95.48 ± 1.49 b | 90.48 ± 0.52 c |
| Predicted Glycemic Index (pGI) | 94.52 ± 0.76 a | 94.20 ± 0.74 a | 92.13 ± 0.82 b | 89.39 ± 0.28 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canale, M.; Sanfilippo, R.; Strano, M.C.; Bavaro, A.R.; Amenta, M.; Bizzini, M.; Allegra, M.; Blangiforti, S.; Spina, A. Technological Properties of Inulin-Enriched Doughs and Breads, Influence on Short-Term Storage and Glycemic Response. Foods 2024, 13, 2711. https://doi.org/10.3390/foods13172711
Canale M, Sanfilippo R, Strano MC, Bavaro AR, Amenta M, Bizzini M, Allegra M, Blangiforti S, Spina A. Technological Properties of Inulin-Enriched Doughs and Breads, Influence on Short-Term Storage and Glycemic Response. Foods. 2024; 13(17):2711. https://doi.org/10.3390/foods13172711
Chicago/Turabian StyleCanale, Michele, Rosalia Sanfilippo, Maria Concetta Strano, Anna Rita Bavaro, Margherita Amenta, Michele Bizzini, Maria Allegra, Sebastiano Blangiforti, and Alfio Spina. 2024. "Technological Properties of Inulin-Enriched Doughs and Breads, Influence on Short-Term Storage and Glycemic Response" Foods 13, no. 17: 2711. https://doi.org/10.3390/foods13172711
APA StyleCanale, M., Sanfilippo, R., Strano, M. C., Bavaro, A. R., Amenta, M., Bizzini, M., Allegra, M., Blangiforti, S., & Spina, A. (2024). Technological Properties of Inulin-Enriched Doughs and Breads, Influence on Short-Term Storage and Glycemic Response. Foods, 13(17), 2711. https://doi.org/10.3390/foods13172711







