Carbon Dots-Mediated Photodynamic Treatment Reduces Postharvest Senescence and Decay of Grapes by Regulating the Antioxidant System
Abstract
:1. Introduction
2. Materials and Methods
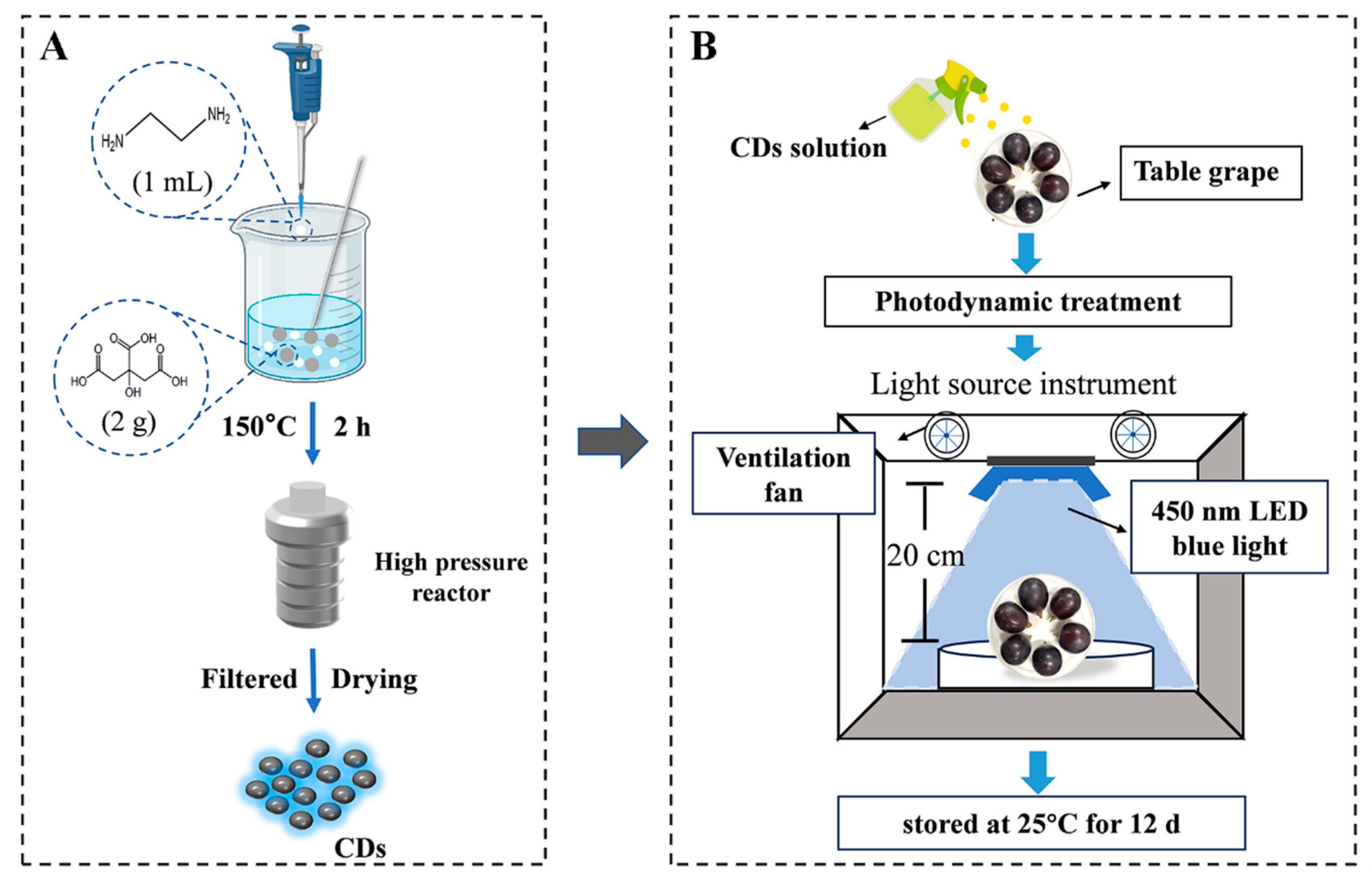
2.1. Synthesis of Nomenclature
2.2. Experimental Design and Sample Treatment
2.3. Evaluation of Decay Rate, Weight Loss, and Hardness of Grapes
2.4. Evaluation of Total Soluble Solids and Titratable Acids
2.5. Evaluation of Superoxide Anion, Hydrogen Peroxide, and Malondialdehyde Contents
2.6. Evaluation of Total Phenols, Flavonoids, and Reducing Sugars Contents
2.7. Determination of Antioxidant Enzyme Activity and Ascorbic Acid–Glutathione Circulating System Substances
2.7.1. Antioxidant Enzyme Activity Evaluation
2.7.2. Determination of Reduced Ascorbic Acid, Reduced Glutathione, Dehydroascorbic Acid, and Oxidized Glutathione
2.8. Determination of Total Antioxidant Capacity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different Treatments on the Apparent and Decay Rate of Postharvest Grapes
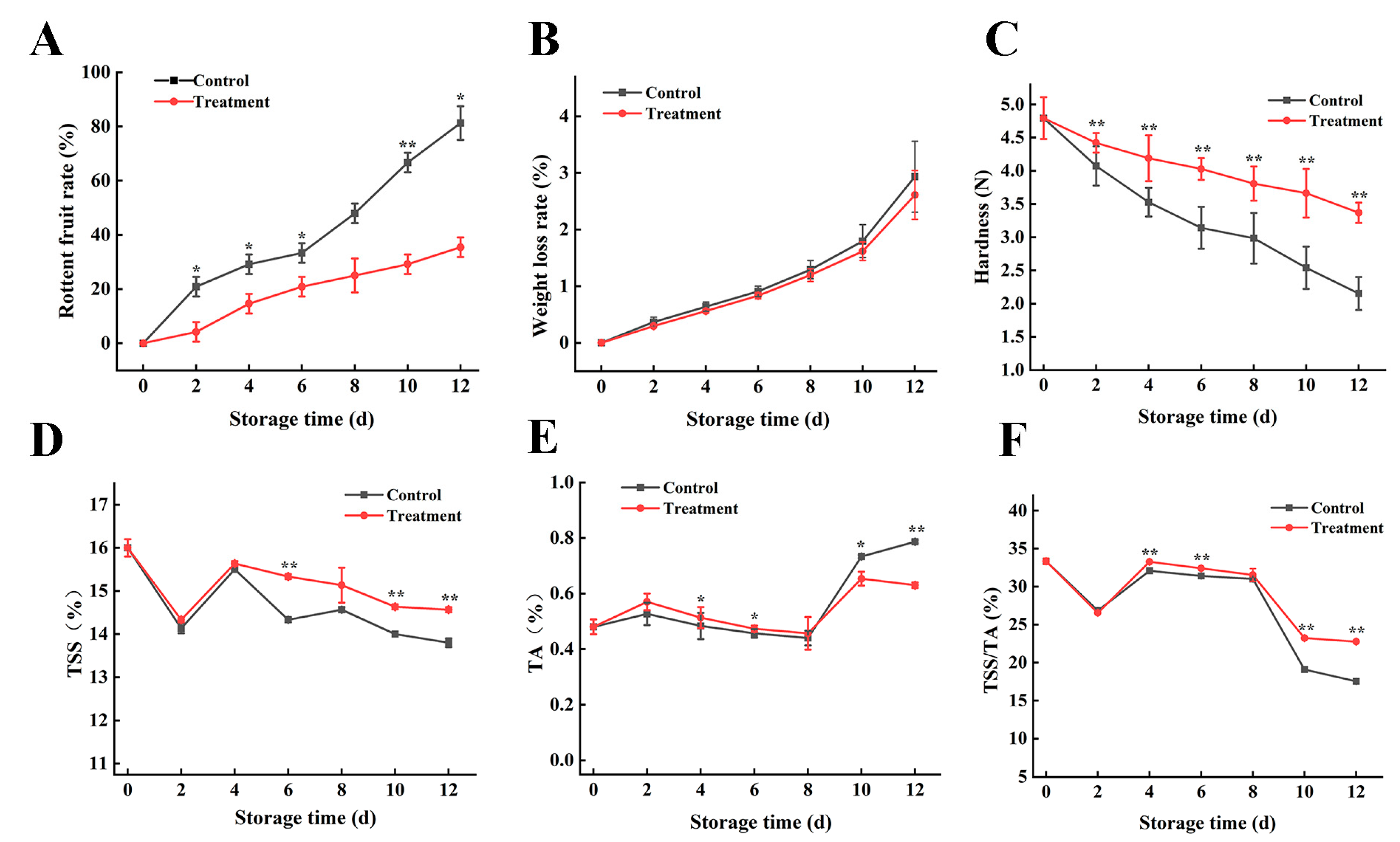
3.2. Effects of Carbon Dots Combined with Photodynamic Treatment on Decay Rate, Weight Loss Rate, and Hardness of Grape Berry during Storage
3.3. Effects on Total Soluble Solids and Titratable Acids Contents
3.4. Effect on Superoxide Anion, Hydrogen Peroxide, and Malondialdehyde Contents
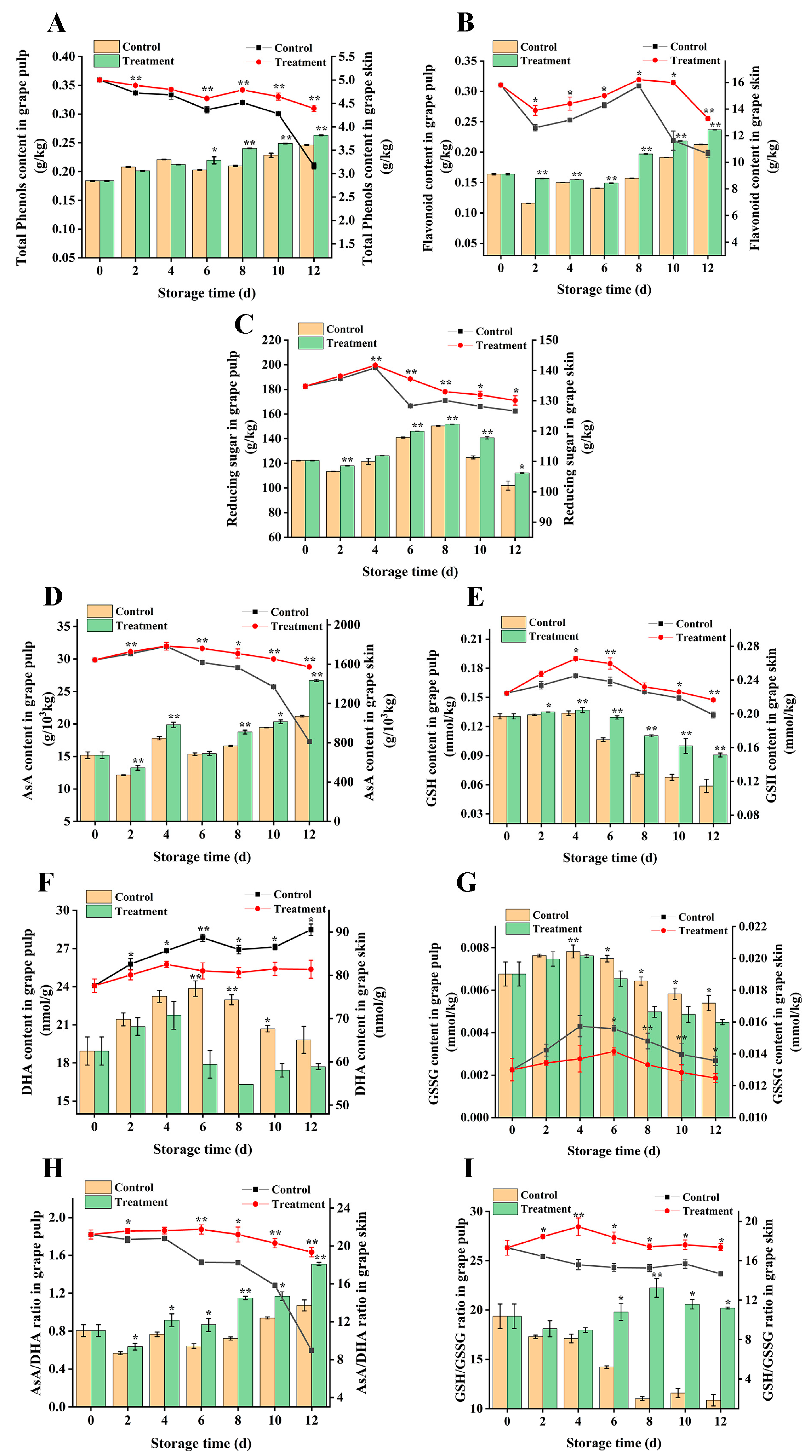
3.5. Effect on the Content of Total Phenols, Flavonoids, and Reducing Sugars
3.6. Effects on Antioxidants in the Ascorbic Acid–Glutathione Cycle in Grape Berry
3.7. Effects on the Activities of Antioxidant Enzymes in Grape Berry
3.8. Effects on the Total Antioxidant Capacity in Grape Berry
3.9. Correlation between Total Antioxidant Capacity and Quality Indexes and Antioxidant Indexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bishayee, A.; Mbimba, T.; Thoppil, R.J.; Háznagy-Radnai, E.; Sipos, P.; Darvesh, A.S.; Folkesson, H.G.; Hohmann, J. Hohmann. Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J. Nutr. Biochem. 2011, 22, 1035–1046. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Z.; Yuan, Y.; Zhang, J.; Wei, J.; Wu, B. Sulfur dioxide mitigates oxidative damage by modulating hydrogen peroxide homeostasis in postharvest table grapes. Postharvest Biol. Technol. 2022, 188, 111877. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Hu, K.-D.; Song, C.-B.; Ma, R.-H.; Li, Z.-R.; Zheng, J.-L.; Fu, L.-H.; Wei, Z.-J.; Zhang, H. Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid. Med. Cell. Longev. 2016, 2016, 4715651. [Google Scholar] [CrossRef]
- Li, L.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Porat, R.; Lichter, A. The effects of 1-methylcyclopropane and ethylene on postharvest rachis browning in table grapes. Postharvest Biol. Technol. 2015, 107, 16–22. [Google Scholar] [CrossRef]
- Luesuwan, S.; Naradisorn, M.; Shiekh, K.A.; Rachtanapun, P.; Tongdeesoontorn, W. Effect of active packaging material fortified with clove essential oil on fungal growth and post-harvest quality changes in table grape during cold storage. Polymers 2021, 13, 3445. [Google Scholar] [CrossRef] [PubMed]
- Tim, M. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J. Photochem. Photobiol. B-Biol. 2015, 150, 2–10. [Google Scholar] [CrossRef]
- Luksiene, Z.; Brovko, L. Antibacterial photosensitization-based treatment for food safety. Food Eng. Rev. 2013, 5, 185–199. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon dots as potent antimicrobial agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, C. Recent development of carbon quantum dots: Biological toxicity, Antibacterial properties and Application in foods. Food Rev. Int. 2022, 38, 1513–1532. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, F.; Zhang, J.; Han, Q.; Song, L.; Meng, X. Effect of photodynamic treatments on quality and antioxidant properties of fresh-cut potatoes. Food Chem. 2021, 362, 130224. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.; Gao, J.; Jin, X.; Wang, Z.; Wang, B.; Li, K.; Li, Y. Detection and comparison of reactive oxygen species (ROS) generated by chlorophyllin metal (Fe, Mg and Cu) complexes under ultrasonic and visible-light irradiation. Ultrason. Sonochem. 2011, 18, 1028–1034. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Kohara, N.; Sato, N.; Ando, H.; Ichihashi, M. Riboflavin plays a pivotal role in the UVA-Induced cytotoxicity of fibroblasts as a key molecule in the production of H2O2 by UVA radiation in collaboration with amino acids and vitamins. Int. J. Mol. Sci. 2020, 21, 554. [Google Scholar] [CrossRef]
- Russo, M.; Rigano, F.; Arigò, A.; Dugo, P.; Mondello, L. Coumarins, psoralens and polymethoxyflavones in cold-pressed citrus essential oils: A review. J. Essent. Oil Res. 2021, 33, 221–239. [Google Scholar] [CrossRef]
- Qu, J.-H.; Wei, Q.; Sun, D.-W. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Kováčová, M.; Markovic, Z.M.; Humpolíček, P.; Mičušík, M.; Švajdlenková, H.; Kleinová, A.; Danko, M.; Kubát, P.; Vajďák, J.; Capáková, Z.; et al. Carbon quantum dots modified polyurethane nanocomposite as effective photocatalytic and antibacterial agents. ACS Biomater. Sci. Eng. 2018, 4, 3983–3993. [Google Scholar] [CrossRef]
- Jhonsi, M.A.; Ananth, D.A.; Nambirajan, G.; Sivasudha, T.; Yamini, R.; Bera, S.; Kathiravan, A. Antimicrobial activity, cytotoxicity and DNA binding studies of carbon dots. Spectrochim. Acta A 2018, 196, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Chen, S. Amphiphilic egg-derived carbon dots: Rapid plasma fabrication, pyrolysis process, and multicolor printing patterns. Angew. Chem. Int. Ed. Engl. 2012, 51, 9297–9301. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Hayriye, E.Ş.K.; Nusret, E. Quantum dots for pharmaceutical and biomedical analysis. In Spectroscopic Analyses; Eram, S., Fahmina, Z., Eds.; IntechOpen: Rijeka, Croatia, 2017; Ch. 8. [Google Scholar]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Filali, S.; Pirot, F.; Miossec, P. Biological applications and toxicity minimization of semiconductor quantum dots. Trends Biotechnol. 2019, 38, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. Engl. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ni, Z.-J.; Wang, W.; Thakur, K.; Ma, R.-H.; Ma, W.-P.; Wei, Z.-J. Carbon dot-mediated photodynamic treatment improves the quality attributes of post-harvest Goji Berries (Lycium barbarum L.) via regulating the antioxidant system. Foods 2024, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-M.; Elnur, E.; Wang, W.; Thakur, K.; Du, J.; Li, H.-N.; Ma, W.-P.; Liu, Y.-Q.; Ni, Z.-J.; Wei, Z.-J. Hydrogen sulfide treatment increases the antioxidant capacity of fresh Lingwu Long Jujube (Ziziphus jujuba cv. Mill) fruit during storage. Curr. Res. Food Sci. 2022, 5, 949–957. [Google Scholar] [CrossRef]
- Elam, E.; Lv, Y.-M.; Wang, W.; Thakur, K.; Ma, W.-P.; NI, Z.-J.; Wei, Z.-J. Effects of nitric oxide on postharvest storage quality of Lycium barbarum fruit. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Xiong, S.; Zhou, F.; Jiang, A.; Yang, L.; Hu, W. Ethanol vapor ameliorates chilling injury and maintains postharvest quality by increasing antioxidant capacity of hardy kiwifruit (Actinidia arguta). Sci. Hortic. 2024, 327, 112796. [Google Scholar] [CrossRef]
- Niu, X.; Liu, L.; Wang, H.; Lin, L.; Yang, Y.; Gao, Y.; Wang, X.; Sun, J.; Dong, B. Discovery of novel photosensitized nanoparticles as a preservative for the storage of strawberries and their activity against Botrytis cinerea. LWT-Food Sci. Technol. 2021, 145, 111359. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, F.; Gu, S.; Feng, K.; Hu, W.; Zhang, J.; Sun, X.; Liang, X.; Jiang, A. 1-Methylcyclopropene maintains the postharvest quality of hardy kiwifruit (Actinidia aruguta). J. Food Meas. Charact. 2021, 15, 3036–3044. [Google Scholar] [CrossRef]
- Centeno, D.C.; Osorio, S.; Nunes-Nesi, A.; Bertolo, A.L.; Carneiro, R.T.; Araújo, W.L.; Steinhauser, M.-C.; Michalska, J.; Rohrmann, J.; Geigenberger, P.; et al. Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell 2011, 23, 162–184. [Google Scholar] [CrossRef]
- Duan, X.; Liu, T.; Zhang, D.; Su, X.; Lin, H.; Jiang, Y. Effect of pure oxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Int. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Yu, R.; Wu, C.; Fan, G.; Li, T. Effects of postharvest application of methyl jasmonate on physicochemical characteristics and antioxidant system of the blueberry fruit. Sci. Hortic. 2019, 258, 108785. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Chen, Y.; Wei, J.; Wu, B. Nitric oxide treatment maintains postharvest quality of table grapes by mitigation of oxidative damage. Postharvest Biol. Technol. 2019, 152, 9–18. [Google Scholar] [CrossRef]
- Mirdehghan, S.; Rahimi, S. Pre-harvest application of polyamines enhances antioxidants and table grape (Vitis vinifera L.) quality during postharvest period. Food Chem. 2016, 196, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Miyazaki, K.; Ose, K.; Imahori, Y. Hot water treatment to alleviate chilling injury and enhance ascorbate-glutathione cycle in sweet pepper fruit during postharvest cold storage. Sci. Hortic. 2019, 257, 108715. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S.; Fard, J.R.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hortic. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Anjum, N.A.; Ahmad, I.; Mohmood, I.; Pacheco, M.; Duarte, A.C.; Pereira, E.; Umar, S.; Ahmad, A.; Khan, N.A.; Iqbal, M.; et al. Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids-A review. Environ. Exp. Bot. 2012, 75, 307–324. [Google Scholar] [CrossRef]
- Chumyam, A.; Shank, L.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Effects of chlorine dioxide fumigation on redox balancing potential of antioxidative ascorbate-glutathione cycle in ‘Daw’ longan fruit during storage. Sci. Hortic. 2017, 222, 76–83. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Dai, X.; Yu, M.; Yu, Z. Effect of methyl jasmonate on the quality and antioxidant capacity by modulating ascorbate-glutathione cycle in peach fruit. Sci. Hortic. 2022, 303, 111216. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Ge, Y.; Duan, B.; Li, C.; Tang, Q.; Li, X.; Wei, M.; Chen, Y.; Li, J. γ-Aminobutyric acid delays senescence of blueberry fruit by regulation of reactive oxygen species metabolism and phenylpropanoid pathway. Sci. Hortic. 2018, 240, 303–309. [Google Scholar] [CrossRef]
- Niazi, Z.; Razavi, F.; Khademi, O.; Aghdam, M.S. Exogenous application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, cv. Karaj) during cold storage. Sci. Hortic. 2021, 285, 110198. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Z.; Pan, J.; Li, J.; Li, X.; Khoo, H.E.; Dong, X. Melatonin treatment improves postharvest quality and regulates reactive oxygen species metabolism in “Feizixiao” litchi based on principal component analysis. Front. Plant Sci. 2022, 13, 965345. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Li, J.; Ji, Y.; Han, C.; Jin, P.; Zheng, Y. Responses of fresh-cut strawberries to ethanol vapor pretreatment: Improved quality maintenance and associated antioxidant metabolism in gene expression and enzyme activity levels. J. Agric. Food Chem. 2018, 66, 8382–8390. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Z.-J.; Xue, Y.; Wang, W.; Du, J.; Thakur, K.; Ma, W.-P.; Wei, Z.-J. Carbon Dots-Mediated Photodynamic Treatment Reduces Postharvest Senescence and Decay of Grapes by Regulating the Antioxidant System. Foods 2024, 13, 2717. https://doi.org/10.3390/foods13172717
Ni Z-J, Xue Y, Wang W, Du J, Thakur K, Ma W-P, Wei Z-J. Carbon Dots-Mediated Photodynamic Treatment Reduces Postharvest Senescence and Decay of Grapes by Regulating the Antioxidant System. Foods. 2024; 13(17):2717. https://doi.org/10.3390/foods13172717
Chicago/Turabian StyleNi, Zhi-Jing, Ying Xue, Wei Wang, Juan Du, Kiran Thakur, Wen-Ping Ma, and Zhao-Jun Wei. 2024. "Carbon Dots-Mediated Photodynamic Treatment Reduces Postharvest Senescence and Decay of Grapes by Regulating the Antioxidant System" Foods 13, no. 17: 2717. https://doi.org/10.3390/foods13172717





