Advancing Analytical Techniques in PET and rPET: Development of an ICP–MS Method for the Analysis of Trace Metals and Rare Earth Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standard Solutions
2.2. Sample Preparation
2.3. HMs and REEs Analysis by ICP-MS
2.4. Standard Solutions
2.5. Quality Assurance and Quality Control
2.6. Real Samples
3. Results and Discussion
3.1. Optimization of ICP-MS
3.2. Method Quality Assurance
3.2.1. Linearity, LOD, and LOQ
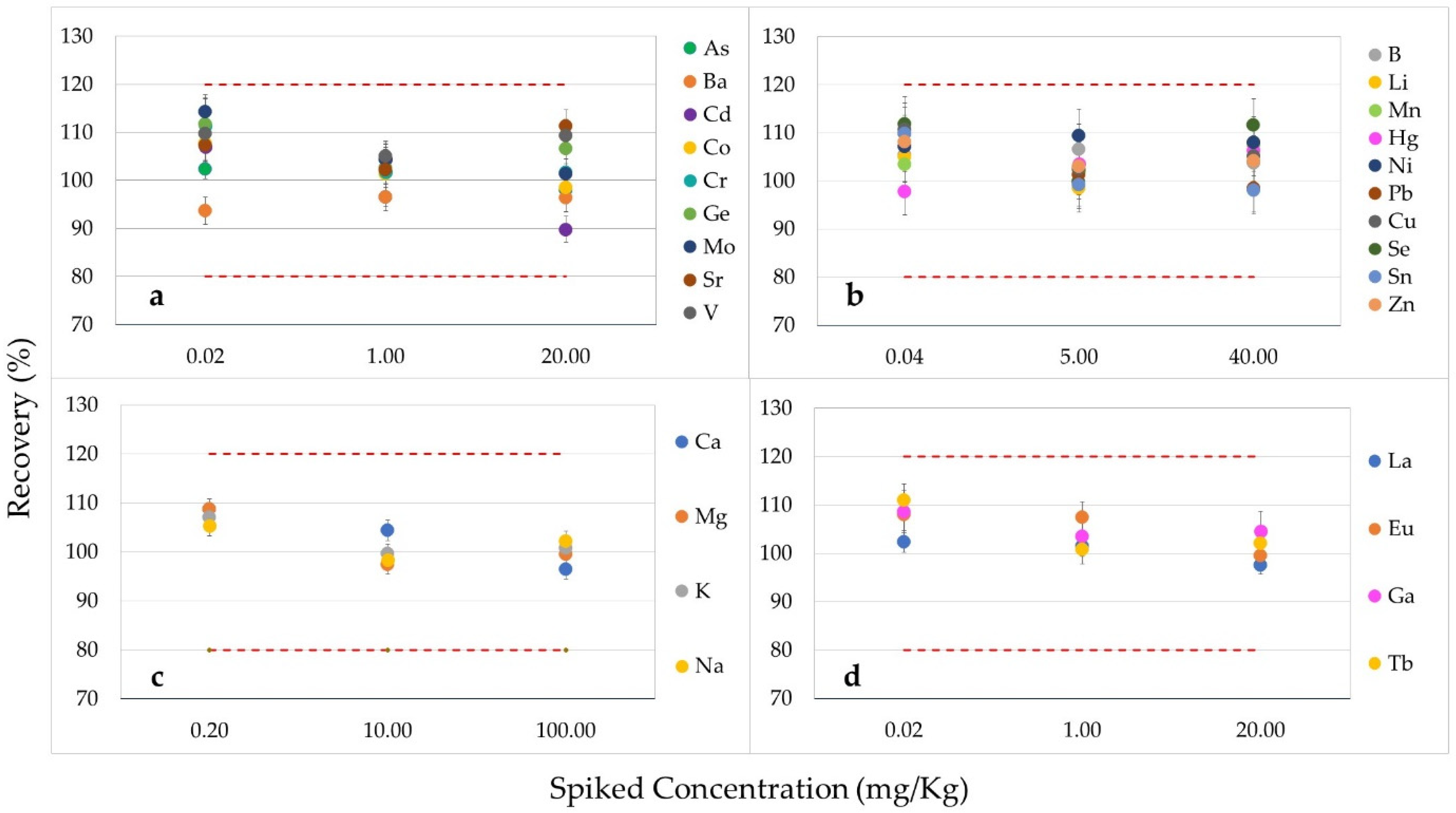
3.2.2. Accuracy and Repeatability
3.3. Application of the Method to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreier, V.N.; Appenzeller-Herzog, C.; Brüschweiler, B.J.; Geueke, B.; Wilks, M.F.; Simat, T.J.; Roth, N. Evaluating the food safety and risk assessment evidence-base of polyethylene terephthalate oligomers: Protocol for a systematic evidence map. Environ. Int. 2022, 167, 107387. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to come into Contact with Food Text with EEA Relevance OJ L 12, 15.1.2011, p. 1–89. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:012:0001:0089:en:PDF (accessed on 2 February 2024).
- Commission Staff Working Document Evaluation of The Legislation on Food Contact Materials-Regulation (EC) No 1935/2004. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52022SC0163&qid=1706869853251 (accessed on 2 February 2024).
- Commission Regulation (EU) 2020/1245 of 2 September 2020 Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to come into Contact with Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32020R1245 (accessed on 2 February 2024).
- Aigotti, R.; Giannone, N.; Asteggiano, A.; Mecarelli, E.; Dal Bello, F.; Medana, C. Release of Selected Non-Intentionally Added Substances (NIAS) from PET Food Contact Materials: A New Online SPE-UHPLC-MS/MS Multiresidue Method. Separations 2022, 9, 188. [Google Scholar] [CrossRef]
- Plastics Europe. What are Plastics? Plastics Europe, Association of Plastics Manufacturers. 2020. Available online: https://www.plasticseurope.org/en/about-plastics/what-are-plastics (accessed on 13 February 2024).
- Sarda, P.; Hanan, J.C.; Lawrence, J.G.; Allahkarami, M. Sustainability performance of polyethylene terephthalate, clarifying challenges and opportunities. J. Polym. Sci. 2022, 60, 7–31. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Mishra, R.K.; Thomas, S. Materials recovery, direct reuse and incineration of PET bottles. In Recycling of Polyethylene Terephthalate Bottles; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 37–60. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene terephthalate (PET) bottle-to-bottle recycling for the beverage industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Dhaka, V.; Singh, S.; Anil, A.G.; Sunil Kumar Naik, T.S.; Garg, S.; Samuel, J.; Singh, J. Occurrence, toxicity and remediation of polyethylene terephthalate plastics. A review. Environ. Chem. Lett. 2022, 20, 1777–1800. [Google Scholar] [CrossRef]
- Chairat, S.; Gheewala, S.H. Life cycle assessment and circularity of polyethylene terephthalate bottles via closed and open loop recycling. Environ. Res. 2023, 236, 116788. [Google Scholar] [CrossRef]
- Radhakrishnan, S. Environmental implications of reuse and recycling of packaging. In Environmental Footprints of Packaging; Muthu, S., Ed.; Springer: Singapore, 2016; pp. 165–192. [Google Scholar] [CrossRef]
- Queiroz, F.C.B.P.; Lima, N.C.; da Silva, C.L.; Queiroz, J.V.; de Souza, G.H.S. Purchase intentions for brazilian recycled PET products—Circular economy opportunities. Recycling 2021, 6, 75. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Lopes, J.A.; Corredig, M. Chemical testing of mechanically recycled polyethylene terephthalate for food packaging in the European Union. Resour. Conserv. Recycl. 2022, 179, 106096. [Google Scholar] [CrossRef]
- Goodlaxson, B.; Curtzwiler, G.; Vorst, K. Evaluation of methods for determining heavy metal content in polyethylene terephthalate food packaging. J. Plast. Film. Sheeting 2018, 34, 119–139. [Google Scholar] [CrossRef]
- Nerín, C.; Bourdoux, S.; Faust, B.; Gude, T.; Lesueur, C.; Simat, T.; Oldring, P. Guidance in selecting analytical techniques for identification and quantification of non-intentionally added substances (NIAS) in food contact materials (FCMS). Food Addit. Contam. Part A 2022, 39, 620–643. [Google Scholar] [CrossRef]
- Gerassimidou, S.; Lanska, P.; Hahladakis, J.N.; Lovat, E.; Vanzetto, S.; Geueke, B.; Iacovidou, E. Unpacking the complexity of the PET drink bottles value chain: A chemicals perspective. J. Hazard. Mater. 2022, 430, 128410. [Google Scholar] [CrossRef]
- Geueke, B. Dossier-Non-intentionally added substances (NIAS). Food Packag. Forum 2018, 7, 1–10. [Google Scholar]
- Mao, S.; Gu, W.; Bai, J.; Dong, B.; Huang, Q.; Zhao, J.; Wang, J. Migration characteristics of heavy metals during simulated use of secondary products made from recycled e-waste plastic. J. Environ. Manag. 2020, 266, 110577. [Google Scholar] [CrossRef]
- Undas, A.K.; Groenen, M.; Peters, R.J.; van Leeuwen, S.P. Safety of recycled plastics and textiles: Review on the detection, identification and safety assessment of contaminants. Chemosphere 2023, 312, 137175. [Google Scholar] [CrossRef]
- Colombo, G.; Corredig, M.; Ünalan, I.U.; Tsochatzis, E. Untargeted screening of NIAS and cyclic oligomers migrating from virgin and recycled polyethylene terephthalate (PET) food trays. Food Packag. Shelf Life 2024, 41, 101227. [Google Scholar] [CrossRef]
- Dupont, D.; Arnout, S.; Jones, P.T.; Binnemans, K. Antimony recovery from end-of-life products and industrial process residues: A critical review. J. Sustain. Met. 2016, 2, 79–103. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Pivnenko, K.; Olsson, M.E.; Astrup, T.F. Contamination in plastic recycling: Influence of metals on the quality of reprocessed plastic. Waste Manag. 2018, 79, 595–606. [Google Scholar] [CrossRef]
- Filella, M.; Hennebert, P.; Okkenhaug, G.; Turner, A. Occurrence and fate of antimony in plastics. J. Hazard. Mater. 2020, 390, 121764. [Google Scholar] [CrossRef]
- Kishi, E.; Ozaki, A.; Ooshima, T.; Abe, Y.; Mutsuga, M.; Yamaguchi, Y.; Yamano, T. Determination of various constituent elements of polyethylene terephthalate bottles used for beverages in Japan. Packag. Technol. Sci. 2020, 33, 183–193. [Google Scholar] [CrossRef]
- Whitt, M.; Brown, W.; Danes, J.E.; Vorst, K.L. Migration of heavy metals from recycled polyethylene terephthalate during storage and microwave heating. J. Plast. Film Sheet 2016, 32, 189–207. [Google Scholar] [CrossRef]
- Puype, F.; Samsonek, J.; Knoop, J.; Egelkraut-Holtus, M.; Ortlieb, M. Evidence of waste electrical and electronic equipment (WEEE) relevant substances in polymeric food-contact articles sold on the European market. Food Addit. Contam. Part A 2015, 32, 410–426. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Cesaro, A.; Rene, E.R.; Belgiorno, V.; Lens, P.N. Bioleaching of metals from WEEE shredding dust. J. Environ. Manag. 2018, 210, 180–190. [Google Scholar] [CrossRef]
- Turner, A.; Scott, J.W.; Green, L.A. Rare earth elements in plastics. Sci. Total Environ. 2021, 774, 145405. [Google Scholar] [CrossRef] [PubMed]
- Chibwe, L.; De Silva, A.O.; Spencer, C.; Teixera, C.F.; Williamson, M.; Wang, X.; Muir, D.C. Target and nontarget screening of organic chemicals and metals in recycled plastic materials. Environ. Sci. Technol. 2023, 57, 3380–3390. [Google Scholar] [CrossRef]
- Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Brüschweiler, B.J.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); et al. Safety assessment of the substance Ln 1, 4-benzene dicarboxylic acid (with Ln= La, Eu, Gd, Tb) for use in food contact materials. EFSA J. 2018, 16, e05449. [Google Scholar] [PubMed]
- Senila, M.; Levei, E.A.; Senila, L.; Cadar, O. Validation of microwave acid digestion, diffusive gradients in thin-film preconcentration and inductively coupled plasma optical emission spectrometry methodology for the determination of REEs in natural zeolites. Anal. Methods 2024, 16, 4807–4816. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Huang, H.; Zhang, B.; Ye, Z.; Yu, X.; Shentu, X. Recent Advances in Non-Targeted Screening of Compounds in Plastic-Based/Paper-Based Food Contact Materials. Foods 2023, 12, 4135. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Linh, T.T.T.; Vo, T.K.; Nguyen, Q.H.; Van, T.K. Analytical techniques for determination of heavy metal migration from different types of locally made plastic food packaging materials using ICP-MS. Food Sci Nutr. 2023, 11, 4030–4037. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sakuma, K.; Itai, T.; Zheng, G.; Mitsunobu, S. Speciation of antimony in PET bottles produced in Japan and China by X-ray absorption fine structure spectroscopy. Environ. Sci. Technol. 2008, 42, 9045–9050. [Google Scholar] [CrossRef]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef]
- Brandão, J.; Moyo, M.; Okonkwo, J. Determination of antimony in bottled water and polyethylene terephthalate bottles: A routine laboratory quality check. Water Sci. Technol. Water Supply 2014, 14, 181–188. [Google Scholar] [CrossRef]
- Carneado, S.; Hernández-Nataren, E.; López-Sánchez, J.F.; Sahuquillo, A. Migration of antimony from polyethylene terephthalate used in mineral water bottles. Food Chem. 2015, 166, 544–550. [Google Scholar] [CrossRef]
- Kiyataka, P.H.M.; Dantas, S.T.; Albino, A.C.; Pallone, J.A.L. Antimony assessment in PET bottles for soft drink. Food Anal. Methods 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Magnusson, B. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics 2014. Available online: www.eurachem.org (accessed on 22 May 2024).
- Mohamed, R.; Zainudin, B.H.; Yaakob, A.S. Method validation and determination of heavy metals in cocoa beans and cocoa products by microwave assisted digestion technique with inductively coupled plasma mass spectrometry. Food Chem. 2020, 303, 125392. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O.; Senila, L.; Hoaghia, A.; Miu, I. Mercury determination in natural zeolites by thermal decomposition atomic absorption spectrometry: Method validation in compliance with requirements for use as dietary supplements. Molecules 2019, 24, 4023. [Google Scholar] [CrossRef]
- Buekens, A.; Yang, J. Recycling of WEEE plastics: A review. J. Mater. Cycles Waste Manag. 2014, 16, 415–434. [Google Scholar] [CrossRef]
- Gulab, H.; Malik, S. Polyethylene terephthalate conversion into liquid fuel by its co-pyrolysis with low-and high-density polyethylene employing scrape aluminium as catalyst. Environ. Technol. 2023, 45, 3721–3735. [Google Scholar] [CrossRef]
- Damayanti, W.H.S. Strategic possibility routes of recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Brouwer, M.T.; Alvarado Chacon, F.; Thoden van Velzen, E.U. Effect of recycled content and rPET quality on the properties of PET bottles, part III: Modelling of repetitive recycling. Packag. Technol. Sci. 2020, 33, 373–383. [Google Scholar] [CrossRef]
- Duh, B. Effect of antimony catalyst on solid-state polycondensation of poly (ethylene terephthalate). Polymer 2002, 43, 3147–3154. [Google Scholar] [CrossRef]
- Whitt, M.; Vorst, K.; Brown, W.; Baker, S.; Gorman, L. Survey of heavy metal contamination in recycled polyethylene terephthalate used for food packaging. J. Plast. Film Sheet 2013, 29, 163–173. [Google Scholar] [CrossRef]
- Chapa-Martínez, C.A.; Hinojosa-Reyes, L.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.; Maya-Treviño, L.; Guzmán-Mar, J.L. An evaluation of the migration of antimony from polyethylene terephthalate (PET) plastic used for bottled drinking water. Sci. Total Environ. 2016, 565, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Curtzwiler, G.; Vorst, K.; Danes, J.E.; Auras, R.; Singh, J. Effect of recycled poly (ethylene terephthalate) content on properties of extruded poly (ethylene terephthalate) sheets. J. Plast. Film Sheet 2011, 27, 65–86. [Google Scholar] [CrossRef]
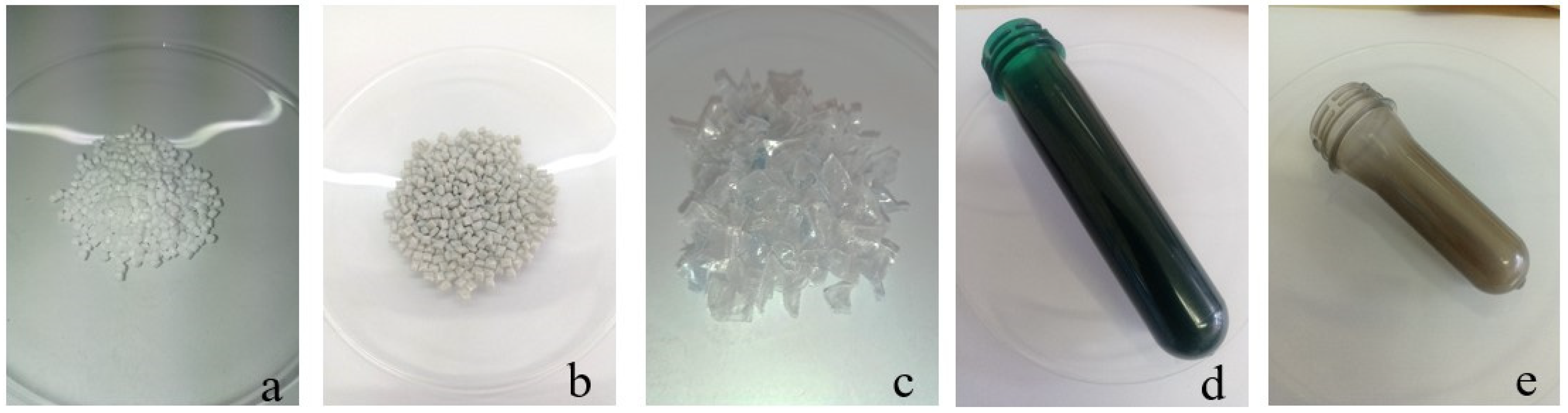




| Parameter | Value |
|---|---|
| Pump Speed | 30 rpm |
| RF Power | 1550 W |
| Nebulizer Gas Flow rate | 1.17 L·min−1 |
| Plasma Gas Flow | 4.8 mL min−1 |
| QCell Bias | −18 V |
| Quadrupole Bias | −21 V |
| Scan Settings | 0.01–0.3 s dwell time per analyte, 10 sweeps |
| Analyte | IS | Linearity Range (µg/L) | R2 | LOD (mg/kg) | LOQ (mg/kg) | |
|---|---|---|---|---|---|---|
| HMs | Al | 89Y | 0.50–200 | 0.992 | 0.030 | 0.10 |
| Sb | 115In | 0.10–500 | 0.997 | 0.006 | 0.02 | |
| As | 89Y | 0.10–100 | 0.995 | 0.006 | 0.02 | |
| Ba | 115In | 0.10–100 | 0.995 | 0.006 | 0.02 | |
| B | 89Y | 0.20–200 | 0.991 | 0.012 | 0.04 | |
| Cd | 115In | 0.10–50 | 0.998 | 0.006 | 0.02 | |
| Ca | 89Y | 1.00–500 | 0.996 | 0.060 | 0.20 | |
| Co | 89Y | 0.10–100 | 0.998 | 0.006 | 0.02 | |
| Cr | 89Y | 0.10–100 | 0.998 | 0.006 | 0.02 | |
| Fe | 89Y | 0.50–200 | 0.993 | 0.030 | 0.10 | |
| Ge | 89Y | 0.10–100 | 0.995 | 0.006 | 0.02 | |
| Li | 89Y | 0.20–200 | 0.994 | 0.012 | 0.04 | |
| Mg | 89Y | 1.00–500 | 0.996 | 0.060 | 0.20 | |
| Mn | 89Y | 0.20–200 | 0.992 | 0.012 | 0.04 | |
| Hg | 115In | 0.20–200 | 0.995 | 0.012 | 0.04 | |
| Mo | 89Y | 0.10–100 | 0.994 | 0.006 | 0.02 | |
| Ni | 89Y | 0.20–200 | 0.992 | 0.012 | 0.04 | |
| Pb | 115In | 0.20–200 | 0.998 | 0.012 | 0.04 | |
| K | 89Y | 1.00–500 | 0.993 | 0.060 | 0.20 | |
| Cu | 89Y | 0.20–200 | 0.991 | 0.012 | 0.04 | |
| Se | 89Y | 0.20–200 | 0.999 | 0.012 | 0.04 | |
| Na | 89Y | 1.00–500 | 0.994 | 0.060 | 0.20 | |
| Sn | 115In | 0.20–200 | 0.993 | 0.012 | 0.04 | |
| Sr | 115In | 0.10–100 | 0.995 | 0.006 | 0.02 | |
| V | 89Y | 0.10–100 | 0.997 | 0.006 | 0.02 | |
| Zn | 193Ir | 0.20–200 | 0.992 | 0.012 | 0.04 | |
| REEs | La | 115In | 0.10–100 | 0.995 | 0.006 | 0.02 |
| Eu | 115In | 0.10–100 | 0.997 | 0.006 | 0.02 | |
| Gd | 115In | 0.10–100 | 0.998 | 0.006 | 0.02 | |
| Tb | 115In | 0.10–100 | 0.994 | 0.006 | 0.02 | |
| Analyte | Spiked Value (mg/kg) | Calculated Analyte Concentration (mg/kg) | SD (mg/kg) | RSD (%) |
|---|---|---|---|---|
| Al | 0.10 | 0.105 | 0.017 | 15.9 |
| 5.00 | 4.43 | 0.23 | 5.3 | |
| 40.00 | 38.09 | 3.48 | 9.1 | |
| Fe | 0.10 | 0.097 | 0.019 | 19.6 |
| 5.00 | 4.68 | 0.61 | 13.1 | |
| 40.00 | 38.23 | 3.03 | 7.9 | |
| Sb | 0.02 | 0.018 | 0.003 | 15.6 |
| 10.00 | 8.69 | 0.17 | 2.0 | |
| 100.00 | 101.66 | 3.90 | 3.8 | |
| As | 0.02 | 0.019 | 0.003 | 14.9 |
| 1.00 | 0.90 | 0.07 | 7.2 | |
| 20.00 | 20.09 | 3.01 | 15.0 | |
| Ba | 0.02 | 0.019 | 0.004 | 19.2 |
| 1.00 | 0.94 | 0.10 | 10.7 | |
| 20.00 | 19.35 | 3.17 | 16.4 | |
| Cd | 0.02 | 0.017 | 0.001 | 3.0 |
| 1.00 | 0.91 | 0.12 | 13.3 | |
| 20.00 | 17.71 | 0.62 | 3.5 | |
| Co | 0.02 | 0.017 | 0.001 | 4.9 |
| 1.00 | 0.94 | 0.11 | 11.7 | |
| 20.00 | 22.43 | 1.12 | 5.0 | |
| Cr | 0.02 | 0.021 | 0.003 | 12.4 |
| 1.00 | 1.10 | 0.12 | 11.0 | |
| 20.00 | 22.48 | 1.48 | 6.6 | |
| Ge | 0.02 | 0.018 | 0.003 | 14.9 |
| 1.00 | 1.16 | 0.05 | 4.7 | |
| 20.00 | 17.36 | 0.89 | 5.1 | |
| Mo | 0.02 | 0.017 | 0.002 | 11.1 |
| 1.00 | 1.13 | 0.21 | 18.9 | |
| 20.00 | 22.03 | 2.11 | 9.6 | |
| Sr | 0.02 | 0.019 | 0.003 | 13.7 |
| 1.00 | 0.98 | 0.17 | 17.1 | |
| 20.00 | 22.71 | 1.45 | 6.4 | |
| V | 0.02 | 0.016 | 0.001 | 7.9 |
| 1.00 | 1.04 | 0.12 | 11.4 | |
| 20.00 | 21.03 | 2.68 | 12.8 | |
| B | 0.04 | 0.034 | 0.002 | 4.6 |
| 5.00 | 5.22 | 0.84 | 16.2 | |
| 40.00 | 37.49 | 3.53 | 9.4 | |
| Li | 0.04 | 0.037 | 0.006 | 17.1 |
| 5.00 | 4.63 | 0.77 | 16.6 | |
| 40.00 | 35.39 | 1.09 | 3.1 | |
| Mn | 0.04 | 0.035 | 0.003 | 7.8 |
| 5.00 | 4.28 | 0.42 | 9.7 | |
| 40.00 | 41.02 | 2.61 | 6.4 | |
| Hg | 0.04 | 0.037 | 0.006 | 15.8 |
| 5.00 | 5.30 | 0.73 | 13.7 | |
| 40.00 | 37.66 | 3.70 | 9.8 | |
| Ni | 0.04 | 0.038 | 0.007 | 18.5 |
| 5.00 | 4.75 | 0.89 | 18.7 | |
| 40.00 | 41.22 | 1.19 | 2.9 | |
| Pb | 0.04 | 0.037 | 0.006 | 15.9 |
| 5.00 | 4.57 | 0.66 | 14.4 | |
| 40.00 | 36.58 | 2.02 | 5.5 | |
| Cu | 0.04 | 0.037 | 0.006 | 16.7 |
| 5.00 | 5.17 | 0.59 | 11.4 | |
| 40.00 | 44.75 | 1.60 | 3.6 | |
| Se | 0.04 | 0.041 | 0.004 | 9.0 |
| 5.00 | 5.40 | 0.56 | 10.4 | |
| 40.00 | 42.71 | 0.75 | 1.8 | |
| Sn | 0.04 | 0.034 | 0.001 | 3.9 |
| 5.00 | 4.31 | 0.26 | 6.1 | |
| 40.00 | 35.53 | 2.07 | 5.8 | |
| Zn | 0.04 | 0.034 | 0.003 | 9.1 |
| 5.00 | 5.58 | 0.33 | 5.9 | |
| 40.00 | 43.79 | 2.31 | 5.3 | |
| Ca | 0.20 | 0.189 | 0.026 | 13.6 |
| 10.00 | 11.39 | 0.70 | 6.2 | |
| 100.00 | 109.93 | 6.82 | 6.2 | |
| Mg | 0.20 | 0.216 | 0.030 | 13.7 |
| 10.00 | 9.13 | 0.61 | 6.7 | |
| 100.00 | 107.14 | 1.37 | 1.3 | |
| K | 0.20 | 0.192 | 0.022 | 11.3 |
| 10.00 | 10.31 | 1.35 | 13.1 | |
| 100.00 | 98.81 | 2.17 | 2.2 | |
| Na | 0.20 | 0.188 | 0.021 | 11.2 |
| 10.00 | 9.81 | 0.93 | 9.5 | |
| 100.00 | 101.18 | 5.01 | 5.0 | |
| La | 0.02 | 0.018 | 0.003 | 15.1 |
| 1.00 | 1.03 | 0.05 | 4.5 | |
| 20.00 | 22.75 | 1.35 | 5.9 | |
| Eu | 0.02 | 0.019 | 0.003 | 15.5 |
| 1.00 | 1.05 | 0.09 | 8.1 | |
| 20.00 | 22.90 | 1.10 | 4.8 | |
| Ga | 0.02 | 0.023 | 0.001 | 4.9 |
| 1.00 | 1.10 | 0.05 | 4.6 | |
| 20.00 | 22.75 | 1.55 | 6.8 | |
| Tb | 0.02 | 0.023 | 0.001 | 5.8 |
| 1.00 | 1.05 | 0.17 | 15.9 | |
| 20.00 | 21.77 | 2.55 | 11.7 |
| Analyte | Spiked Value (mg/kg) | Calculated Analyte Concentration (mg/kg) | SD (mg/kg) | RSD (%) |
|---|---|---|---|---|
| Al | 0.10 | 0.108 | 0.012 | 11.1 |
| 5.00 | 4.81 | 0.29 | 6.1 | |
| 40.00 | 43.08 | 4.79 | 11.1 | |
| Fe | 0.10 | 0.103 | 0.016 | 15.6 |
| 5.00 | 4.71 | 0.41 | 8.6 | |
| 40.00 | 39.59 | 3.58 | 9.1 | |
| Sb | 0.02 | 0.021 | 0.002 | 7.2 |
| 10.00 | 9.29 | 1.11 | 12.0 | |
| 100.00 | 99.49 | 5.58 | 5.6 | |
| As | 0.02 | 0.020 | 0.003 | 13.1 |
| 1.00 | 0.97 | 0.12 | 12.5 | |
| 20.00 | 19.59 | 1.49 | 7.6 | |
| Ba | 0.02 | 0.019 | 0.002 | 8.9 |
| 1.00 | 0.97 | 0.06 | 6.3 | |
| 20.00 | 19.27 | 1.80 | 9.3 | |
| Cd | 0.02 | 0.021 | 0.002 | 10.8 |
| 1.00 | 1.04 | 0.06 | 5.8 | |
| 20.00 | 17.97 | 0.79 | 4.4 | |
| Co | 0.02 | 0.022 | 0.003 | 11.9 |
| 1.00 | 1.01 | 0.07 | 7.3 | |
| 20.00 | 19.70 | 1.70 | 8.6 | |
| Cr | 0.02 | 0.022 | 0.001 | 4.4 |
| 1.00 | 1.02 | 0.06 | 5.7 | |
| 20.00 | 20.35 | 1.79 | 8.8 | |
| Ge | 0.02 | 0.022 | 0.001 | 6.1 |
| 1.00 | 1.03 | 0.07 | 6.5 | |
| 20.00 | 21.31 | 3.34 | 15.7 | |
| Mo | 0.02 | 0.023 | 0.001 | 4.4 |
| 1.00 | 1.04 | 0.05 | 4.3 | |
| 20.00 | 20.28 | 1.26 | 6.2 | |
| Sr | 0.02 | 0.021 | 0.002 | 7.8 |
| 1.00 | 1.02 | 0.11 | 10.4 | |
| 20.00 | 22.26 | 2.54 | 11.4 | |
| V | 0.02 | 0.022 | 0.001 | 5.0 |
| 1.00 | 1.05 | 0.10 | 9.1 | |
| 20.00 | 21.87 | 1.74 | 7.9 | |
| B | 0.04 | 0.042 | 0.005 | 12.8 |
| 5.00 | 5.32 | 0.65 | 12.2 | |
| 40.00 | 41.47 | 4.14 | 10.0 | |
| Li | 0.04 | 0.042 | 0.002 | 5.4 |
| 5.00 | 4.92 | 0.60 | 12.3 | |
| 40.00 | 43.21 | 2.59 | 6.0 | |
| Mn | 0.04 | 0.041 | 0.004 | 10.0 |
| 5.00 | 5.11 | 0.57 | 11.2 | |
| 40.00 | 42.97 | 2.35 | 5.5 | |
| Hg | 0.04 | 0.039 | 0.005 | 12.2 |
| 5.00 | 5.18 | 0.59 | 11.4 | |
| 40.00 | 42.52 | 3.95 | 9.3 | |
| Ni | 0.04 | 0.043 | 0.002 | 5.5 |
| 5.00 | 5.47 | 0.32 | 5.9 | |
| 40.00 | 43.17 | 2.72 | 6.3 | |
| Pb | 0.04 | 0.044 | 0.004 | 8.1 |
| 5.00 | 5.06 | 0.58 | 11.5 | |
| 40.00 | 39.38 | 6.06 | 15.4 | |
| Cu | 0.04 | 0.044 | 0.003 | 6.9 |
| 5.00 | 5.12 | 0.52 | 10.1 | |
| 40.00 | 42.03 | 5.10 | 12.1 | |
| Se | 0.04 | 0.045 | 0.003 | 5.6 |
| 5.00 | 4.99 | 0.48 | 9.6 | |
| 40.00 | 44.59 | 2.71 | 6.1 | |
| Sn | 0.04 | 0.044 | 0.003 | 7.5 |
| 5.00 | 4.96 | 0.41 | 8.3 | |
| 40.00 | 39.19 | 2.14 | 5.5 | |
| Zn | 0.04 | 0.043 | 0.005 | 11.9 |
| 5.00 | 5.15 | 0.50 | 9.8 | |
| 40.00 | 41.67 | 4.56 | 10.9 | |
| Ca | 0.20 | 0.217 | 0.015 | 7.0 |
| 10.00 | 10.44 | 0.58 | 5.5 | |
| 100.00 | 96.37 | 4.95 | 5.1 | |
| Mg | 0.20 | 0.217 | 0.015 | 6.9 |
| 10.00 | 9.75 | 0.90 | 9.3 | |
| 100.00 | 99.52 | 4.61 | 4.6 | |
| K | 0.20 | 0.214 | 0.013 | 6.1 |
| 10.00 | 9.96 | 0.98 | 9.9 | |
| 100.00 | 100.72 | 5.19 | 5.2 | |
| Na | 0.20 | 0.211 | 0.022 | 10.5 |
| 10.00 | 9.83 | 0.91 | 9.3 | |
| 100.00 | 102.22 | 9.61 | 9.4 | |
| La | 0.02 | 0.020 | 0.003 | 13.2 |
| 1.00 | 1.01 | 0.06 | 6.0 | |
| 20.00 | 19.52 | 2.68 | 13.7 | |
| Eu | 0.02 | 0.022 | 0.002 | 9.1 |
| 1.00 | 1.07 | 0.14 | 12.7 | |
| 20.00 | 19.91 | 2.37 | 11.9 | |
| Ga | 0.02 | 0.022 | 0.002 | 6.9 |
| 1.00 | 1.04 | 0.10 | 10.0 | |
| 20.00 | 20.91 | 1.38 | 6.6 | |
| Tb | 0.02 | 0.022 | 0.002 | 7.7 |
| 1.00 | 1.01 | 0.09 | 8.7 | |
| 20.00 | 20.41 | 1.81 | 8.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Duca, F.; Montuori, P.; De Rosa, E.; De Simone, B.; Scippa, S.; Dadà, G.; Triassi, M. Advancing Analytical Techniques in PET and rPET: Development of an ICP–MS Method for the Analysis of Trace Metals and Rare Earth Elements. Foods 2024, 13, 2716. https://doi.org/10.3390/foods13172716
Di Duca F, Montuori P, De Rosa E, De Simone B, Scippa S, Dadà G, Triassi M. Advancing Analytical Techniques in PET and rPET: Development of an ICP–MS Method for the Analysis of Trace Metals and Rare Earth Elements. Foods. 2024; 13(17):2716. https://doi.org/10.3390/foods13172716
Chicago/Turabian StyleDi Duca, Fabiana, Paolo Montuori, Elvira De Rosa, Bruna De Simone, Stefano Scippa, Giuseppe Dadà, and Maria Triassi. 2024. "Advancing Analytical Techniques in PET and rPET: Development of an ICP–MS Method for the Analysis of Trace Metals and Rare Earth Elements" Foods 13, no. 17: 2716. https://doi.org/10.3390/foods13172716
APA StyleDi Duca, F., Montuori, P., De Rosa, E., De Simone, B., Scippa, S., Dadà, G., & Triassi, M. (2024). Advancing Analytical Techniques in PET and rPET: Development of an ICP–MS Method for the Analysis of Trace Metals and Rare Earth Elements. Foods, 13(17), 2716. https://doi.org/10.3390/foods13172716








