DPP-IV Inhibitory Peptide against In Vitro Gastrointestinal Digestion Derived from Goat’s Milk Protein and Its Activity Enhancement via Amino Acid Substitution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Simulated Gastrointestinal Digestion
2.3. Detection of Milk Protein Hydrolysate by LC-MS/MS
2.4. Molecular Docking
2.5. Determination of the Optimal Concentration of DPP-IV Inhibitory Peptide Using Caco-2 Cells
2.6. Verification of DPP-IV Inhibitory Activity
2.7. Statistical Analysis
3. Results
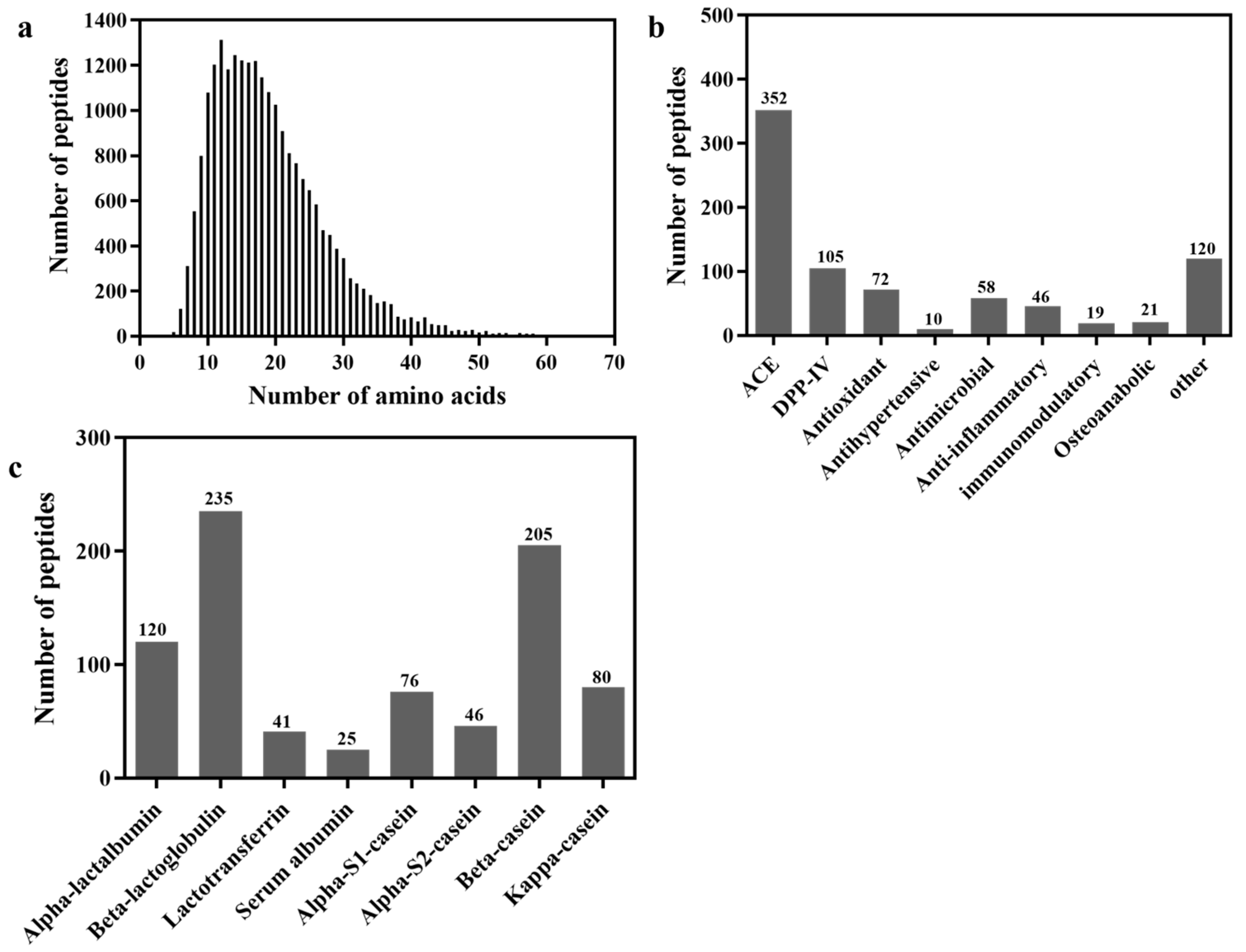
3.1. Distribution of Peptides after Gastrointestinal Digestion
3.2. Screening of DPP-IV Inhibitory Peptides
3.3. Analysis of the Nature and Structure of IL, IP, II
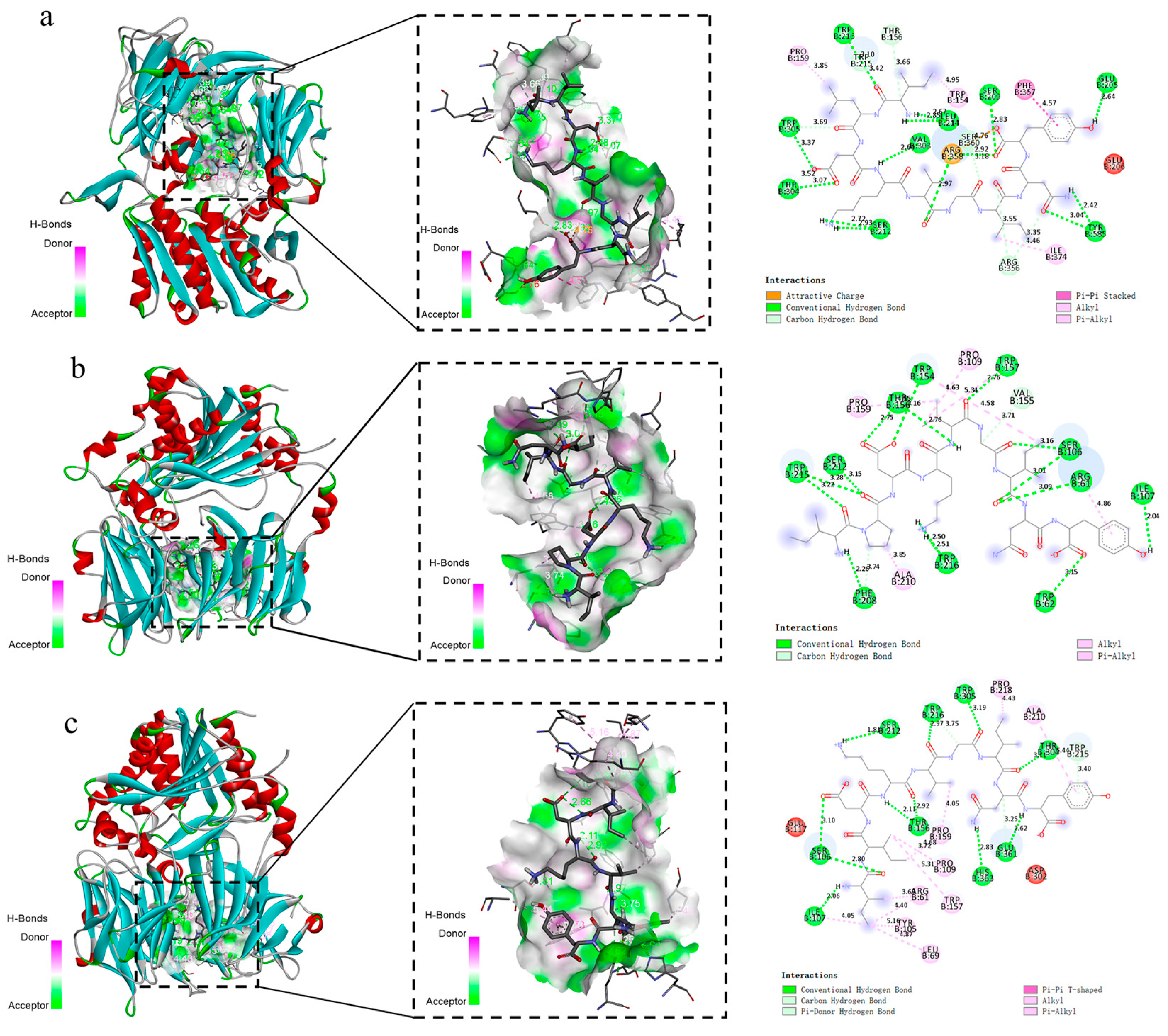
3.4. Results of Molecular Docking of Peptides with DPP-IV Enzymes
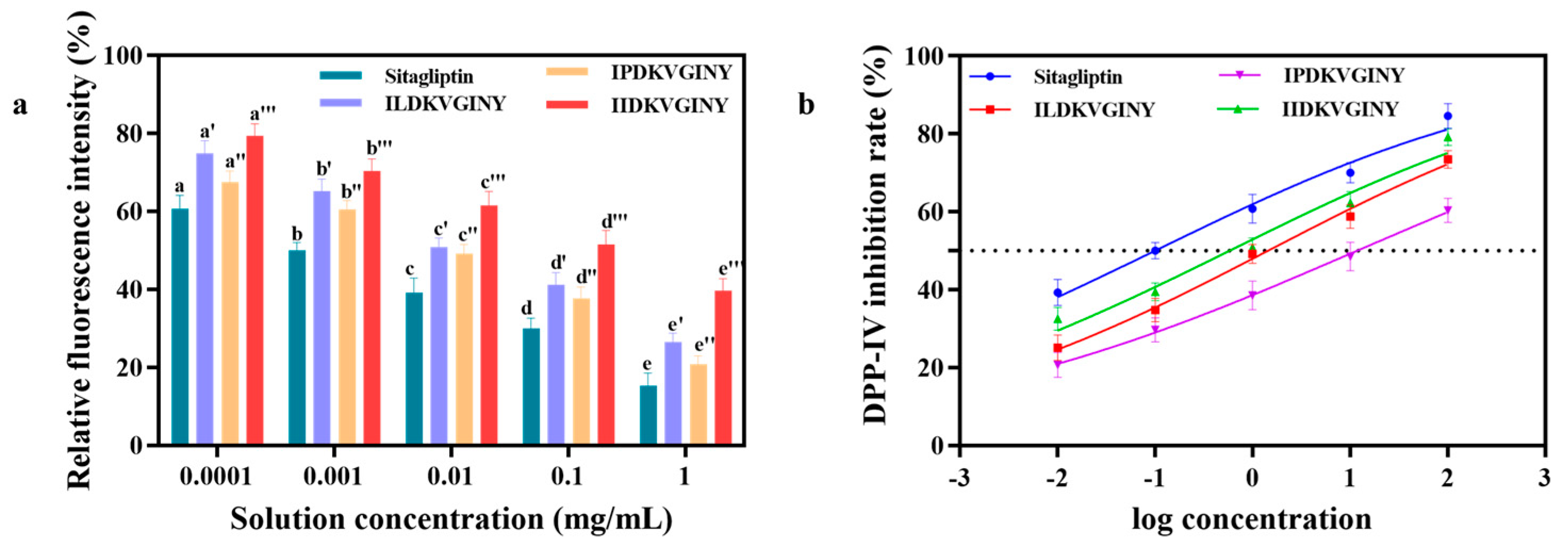
3.5. Effect of DPP-IV Inhibitory Peptides on Cell Viability of Caco-2 Cell
3.6. Results of In Vitro DPP-IV Inhibitory Activity Assay in Caco-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ceballos, L.S.; Morales, E.R.; Torre Adarve, G.d.l.; Castro, J.D.; Martinez, L.P.; Sampelayo, M.R.S. Composition of goat and cow milk produced under similar conditions and analyzed by identical methodology. J. Food Compos. Anal. 2009, 22, 322–329. [Google Scholar] [CrossRef]
- Javier Espejo-Carpio, F.; Garcia-Moreno, P.J.; Perez-Galvez, R.; Morales-Medina, R.; Guadix, A.; Guadix, E.M. Effect of digestive enzymes on the bioactive properties of goat milk protein hydrolysates. Int. Dairy J. 2016, 54, 21–28. [Google Scholar] [CrossRef]
- Sattigeri, J.A.; Sethi, S.; Davis, J.A.; Ahmed, S.; Rayasam, G.V.; Jadhav, B.G.; Chilla, S.M.; Datta, D.; Gadhave, A.; Tulasi, V.K.; et al. Approaches towards the development of chimeric DPP4/ACE inhibitors for treating metabolic syndrome. Bioorganic Med. Chem. Lett. 2017, 27, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Al-Aubaidy, H.A.; Mohammed, B.I. Glucose dependent insulinotropic polypeptide and dipeptidyl peptidase inhibitors: Their roles in management of type 2 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S170–S175. [Google Scholar] [CrossRef]
- Petersen, B.L.; Ward, L.S.; Bastian, E.D.; Jenkins, A.L.; Campbell, J.; Vuksan, V. A whey protein supplement decreases post-prandial glycemia. Nutr. J. 2009, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Chaoyue, M.; Dan, L.; Huifang, H.; Xiaotong, W. Identification of the DPP-IV Inhibitory Peptides from Donkey Blood and Regulatory Effect on the Gut Microbiota of Type 2 Diabetic Mice. Foods 2022, 11, 2148. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.; Yu, Z.; Zhang, T.; Liu, J. Digestion and absorption of an egg white ACE-inhibitory peptide in human intestinal Caco-2 cell monolayers. Int. J. Food Sci. Nutr. 2016, 67, 111–116. [Google Scholar] [CrossRef]
- Mundada, V.P.; Patel, M.H.; Mundada, P.K.; Sawant, K.K. Enhanced bioavailability and antihypertensive activity of nisoldipine loaded nanoemulsion: Optimization, cytotoxicity and uptake across Caco-2 cell line, pharmacokinetic and pharmacodynamic studies. Drug Dev. Ind. Pharm. 2020, 46, 376–387. [Google Scholar] [CrossRef]
- Xue, H.; Han, J.; Ma, J.; Song, H.; He, B.; Liu, X.; Yi, M.; Zhang, L. Identification of Immune-Active Peptides in Casein Hydrolysates and Its Transport Mechanism on a Caco-2 Monolayer. Foods 2023, 12, 373. [Google Scholar] [CrossRef]
- Xiaomeng, S.; Yixuan, T.; Chengyu, D.; Qingyun, W.; Qiang, C. Effects of processing on component interactions and peptide profiles of preterm infant formula based on peptidomics by Q Exactive plus analysis. LWT 2023, 189, 115429. [Google Scholar]
- Zhang, X.; Wang, R.; Cheng, C.; Zhang, Y.; Ma, Y.; Lu, W. Identification of two novel dipeptidyl peptidase-IV inhibitory peptides from sheep whey protein and inhibition mechanism revealed by molecular docking. Food Biosci. 2022, 48, 101733. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Zhu, R.; Zhang, H.; Li, D.; Li, H.; Tang, H.; Chen, L.; Peng, X.; Xu, X.; et al. Mechanistic Study of Novel Dipeptidyl Peptidase IV Inhibitory Peptides from Goat’s Milk Based on Peptidomics and In Silico Analysis. Foods 2024, 13, 1194. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides 2016, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Identification of novel dipeptidyl peptidase IV (DPP-IV) inhibitory peptides in camel milk protein hydrolysates. Food Chem. 2018, 244, 340–348. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Fitzgerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J. Funct. Foods 2013, 5, 1909–1917. [Google Scholar] [CrossRef]
- Uenishi, H.; Kabuki, T.; Seto, Y.; Serizawa, A.; Nakajima, H. Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int. Dairy J. 2011, 22, 24–30. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Susceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem. 2014, 145, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.M.; Chen, X.M.; Kitts, D.D.; Li-Chan, E.C. Investigation into the bioavailability of milk protein-derived peptides with dipeptidyl-peptidase IV inhibitory activity using Caco-2 cell monolayers. Food Funct. 2017, 8, 701–709. [Google Scholar] [CrossRef]
- Power, O.; Fernandez, A.; Norris, R.; Riera, F.A.; FitzGerald, R.J. Selective enrichment of bioactive properties during ultrafiltration of a tryptic digest of beta-lactoglobulin. J. Funct. Foods 2014, 9, 38–47. [Google Scholar] [CrossRef]
- Hayakawa, E.; Landuyt, B.; Baggerman, G.; Cuyvers, R.; Lavigne, R.; Luyten, W.; Schoofs, L. Peptidomic analysis of human reflex tear fluid. Peptides 2013, 42, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Le Maux, S.; Nongonierma, A.B.; Murray, B.; Kelly, P.M.; FitzGerald, R.J. Identification of short peptide sequences in the nanofiltration permeate of a bioactive whey protein hydrolysate. Food Res. Int. 2015, 77, 534–539. [Google Scholar] [CrossRef]
- Jia, C.-L.; Hussain, N.; Ujiroghene, O.J.; Pang, X.-Y.; Zhang, S.-W.; Lu, J.; Liu, L.; Lv, J.-P. Generation and characterization of dipeptidyl peptidase-IV inhibitory peptides from trypsin-hydrolyzed α-lactalbumin-rich whey proteins. Food Chem. 2020, 318, 126333. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martinez-Maqueda, D.; Recio, I.; Hernandez-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in p-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.M.E.; Meng, G.; Cheung, I.W.Y.; Li-Chan, E.C.Y. Do whey protein-derived peptides have dual dipeptidyl-peptidase IV and angiotensin I-converting enzyme inhibitory activities? J. Funct. Foods 2016, 21, 87–96. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, J.; Huang, J.; Li, Q.; Zhang, X. Lys-C/Arg-C, a More Specific and Efficient Digestion Approach for Proteomics Studies. Anal. Chem. 2018, 90, 9700–9707. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Prospects for the management of type 2 diabetes using food protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Curr. Opin. Food Sci. 2016, 8, 19–24. [Google Scholar] [CrossRef]
- Jadhav, P.B.; Jadhav, S.B.; Zehravi, M.; Mubarak, M.S.; Islam, F.; Jeandet, P.; Khan, S.L.; Hossain, N.; Rashid, S.; Ming, L.C.; et al. Virtual Screening, Synthesis, and Biological Evaluation of Some Carbohydrazide Derivatives as Potential DPP-IV Inhibitors. Molecules 2022, 28, 149. [Google Scholar] [CrossRef]
- Hassanzadeh-Rostami, Z.; Abbasi, A.; Faghih, S. Effects of biscuit fortified with whey protein isolate and wheat bran on weight loss, energy intake, appetite score, and appetite regulating hormones among overweight or obese adults. J. Funct. Foods 2020, 70, 103743. [Google Scholar] [CrossRef]
- Chungchunlam, S.M.S.; Henare, S.J.; Ganesh, S.; Moughan, P.J. Dietary whey protein influences plasma satiety-related hormones and plasma amino acids in normal-weight adult women. Eur. J. Clin. Nutr. 2015, 69, 179–186. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. An in silico model to predict the potential of dietary proteins as sources of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Chem. 2014, 165, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xu, Q.; Lin, L.; Zeng, X.-A.; Sun, B.; Zhao, M. In Vitro Metabolic Stability of a Casein-Derived Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptide VPYPQ and Its Controlled Release from Casein by Enzymatic Hydrolysis. J. Agric. Food Chem. 2019, 67, 10604–10613. [Google Scholar] [CrossRef] [PubMed]
- José, O.M.M.; Aleix, G.; Sarah, T.H.; Adrià, C.M.; Raúl, B.D.; Cristina, V.; Miquel, M.; Gerard, P.; Santiago, G.V. Activity and selectivity cliffs for DPP-IV inhibitors: Lessons we can learn from SAR studies and their application to virtual screening. Med. Res. Rev. 2018, 38, 1874–1915. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, L.; Zhang, Y.; Sheng, N.-J.; Wang, Z.-K.; Wu, T.-Z.; Wang, X.-Z.; Wu, H. Rapid Identification of Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptides from Ruditapes philippinarum Hydrolysate. Molecules 2017, 22, 1714. [Google Scholar] [CrossRef]
- Metzler, W.J.; Yanchunas, J.; Weigelt, C.; Kish, K.; Klei, H.E.; Xie, D.; Zhang, Y.; Corbett, M.; Tamura, J.K.; He, B.; et al. Involvement of DPP-IV catalytic residues in enzyme-saxagliptin complex formation. Protein Sci. A Publ. Protein Soc. 2008, 17, 240–250. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J. Funct. Foods 2012, 4, 403–422. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mazzocchi, C.; Paolella, S.; FitzGerald, R.J. Release of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from milk protein isolate (MPI) during enzymatic hydrolysis. Food Res. Int. 2017, 94, 79–89. [Google Scholar] [CrossRef]


| Source Peptide Fragment | Active Peptide Fragment | Source Protein | Serial Number |
|---|---|---|---|
| YPVEPFTESQS | YPVEPF [14,15] | Beta-casein | 129–134 |
| EPVLGPVR | VLGP [16] | Beta-casein | 212–215 |
| VLPVPQKVVP | LPVPQ [14] | Beta-casein | 186–190 |
| NSLPQNILPLT | LPQ [17] | Beta-casein | 85–87 |
| LHLPLPLV | LPLPL [14,18] | Beta-casein | 150–154 |
| LHLPLPLV | LPL [18] | Beta-casein | 150–152 |
| FLQPEIMGVP | FLQP [16] | Beta-casein | 102–105 |
| VLPVPQKVVP | LPVP | Beta-casein | 186–189 |
| FPQYLQYPYQ | YPY [18] | Kappa-casein | 79–81 |
| FLPYPYYAKPI | LPYPY [18,19] | Kappa-casein | 77–81 |
| YIPIQYVLSR | IPIQ [18,19] | Kappa-casein | 47–51 |
| YIPIQYVLSR | IPI [16,19,20] | Kappa-casein | 47–49 |
| FKNWVK | WV [21] | Alpha-lactalbumin | 45–46 |
| WLPAEYEDGL | WL [22] | Alpha-lactalbumin | 123–124 |
| ILDKVGINY | ILDKVGINY | Alpha-lactalbumin | 114–122 |
| VGINYWLAHK | VGINYWLAHK [23] | Alpha-lactalbumin | 118–127 |
| ILDKVGINYWLAHK | ILDKVGINYWLAHK [23] | Alpha-lactalbumin | 114–127 |
| VAGTWYSLA | VAGTWY [20] | Beta-lactoglobulin | 31–36 |
| TKIPAVFK | IPAVFK [20,24] | Beta-lactoglobulin | 94–99 |
| VLVLDTDYK | VLVLDTDYK [20] | Beta-lactoglobulin | 108–116 |
| VLVLDTDYK | VLDTDY [24,25] | Beta-lactoglobulin | 110–115 |
| LDIQKVAGTW | IQKVAGTW [25] | Beta-lactoglobulin | 28–35 |
| IPAVFKIDALN | IPAVFKIDA [23] | Beta-lactoglobulin | 94–102 |
| TKIPAVFK | IPAVF [24] | Beta-lactoglobulin | 94–98 |
| TKIPAVFK | IPA [6,24] | Beta-lactoglobulin | 94–96 |
| QEPVLGPVR | VR [16] | Milk Serum albumin | 432–433 |
| VLPVPQKVVP | LP [16] | Milk Serum albumin | 136–137 |
| TKIPAVFK | IP [16] | Milk Serum albumin | 321–322 |
| FYPQLFR | YP [16,18] | Lactotransferrin | 185–186 |
| TPDNIDIWIGG | WI [22] | Lactotransferrin | 144–145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Lian, Y.; Xue, H.; Zhou, Y.; Wei, Y.; Ma, J.; Tan, Y.; Wu, Y. DPP-IV Inhibitory Peptide against In Vitro Gastrointestinal Digestion Derived from Goat’s Milk Protein and Its Activity Enhancement via Amino Acid Substitution. Foods 2024, 13, 2721. https://doi.org/10.3390/foods13172721
He B, Lian Y, Xue H, Zhou Y, Wei Y, Ma J, Tan Y, Wu Y. DPP-IV Inhibitory Peptide against In Vitro Gastrointestinal Digestion Derived from Goat’s Milk Protein and Its Activity Enhancement via Amino Acid Substitution. Foods. 2024; 13(17):2721. https://doi.org/10.3390/foods13172721
Chicago/Turabian StyleHe, Baoyuan, Yanhui Lian, Haiyan Xue, Yan Zhou, Yi Wei, Jun Ma, Yalin Tan, and Yawen Wu. 2024. "DPP-IV Inhibitory Peptide against In Vitro Gastrointestinal Digestion Derived from Goat’s Milk Protein and Its Activity Enhancement via Amino Acid Substitution" Foods 13, no. 17: 2721. https://doi.org/10.3390/foods13172721
APA StyleHe, B., Lian, Y., Xue, H., Zhou, Y., Wei, Y., Ma, J., Tan, Y., & Wu, Y. (2024). DPP-IV Inhibitory Peptide against In Vitro Gastrointestinal Digestion Derived from Goat’s Milk Protein and Its Activity Enhancement via Amino Acid Substitution. Foods, 13(17), 2721. https://doi.org/10.3390/foods13172721





