Milk Fat Globule Membrane Is Associated with Lower Blood Lipid Levels in Adults: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
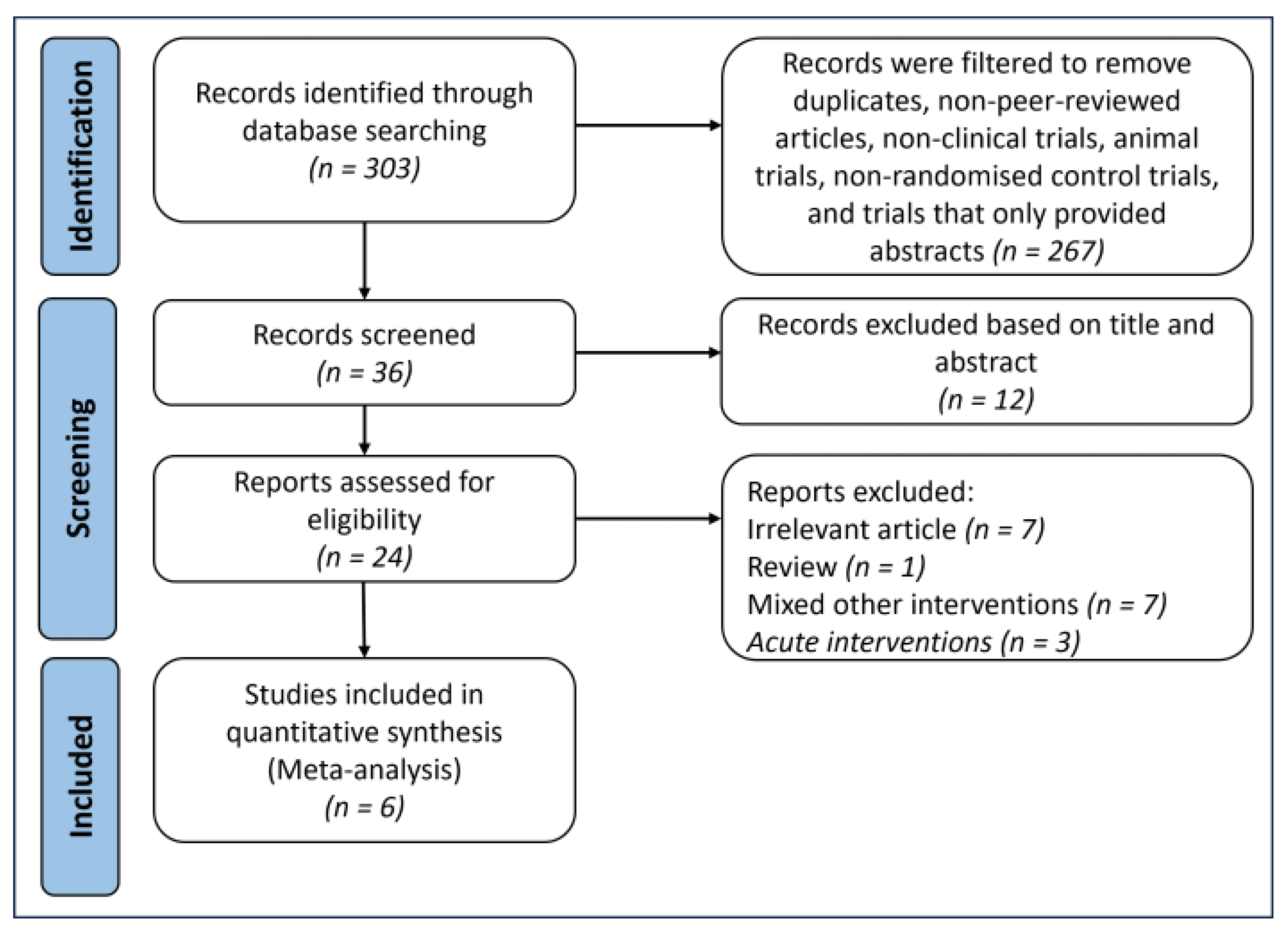
2.1. Searching and Selection Processes
- Populations: Adult human participants aged 20 years and older. No restrictions were based on ethnicity, sex, or baseline health status.
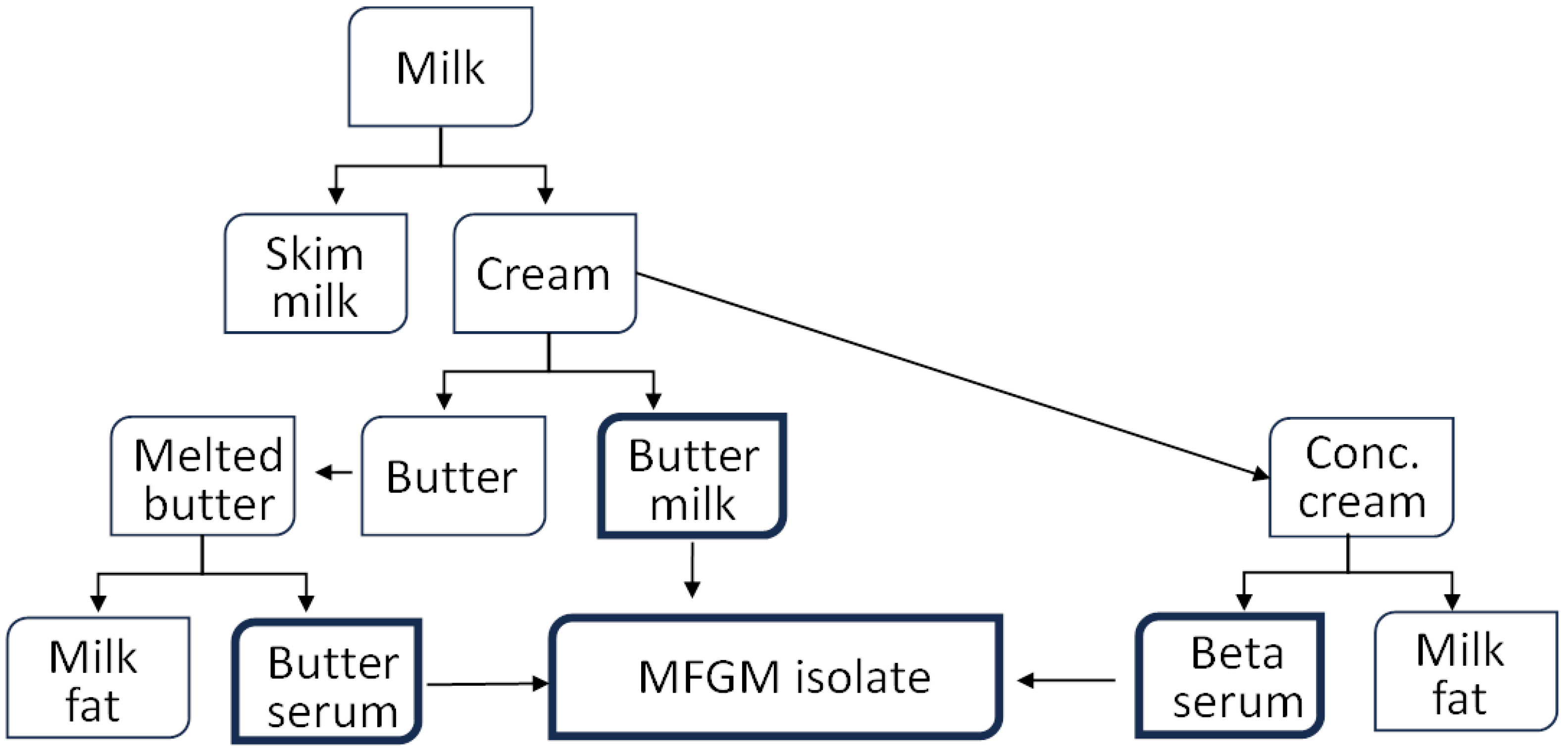
- Intervention: MFGM derived from bovine dairy products, such as cream or buttermilk. Studies involving other forms of dairy or non-dairy polar lipids were excluded.
- Comparator: A control group that received either a placebo or no supplementation. Studies without a clear control group or matched polar lipid amount were excluded.
- Outcome: Changes in blood lipid levels, specifically, total cholesterol (TC), LDL, HDL, and TG. Studies that did not measure at least one of these outcomes were excluded.
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Appraisal
2.4. Statistical Analysis
3. Results
3.1. Results of the Search and Study Characteristics
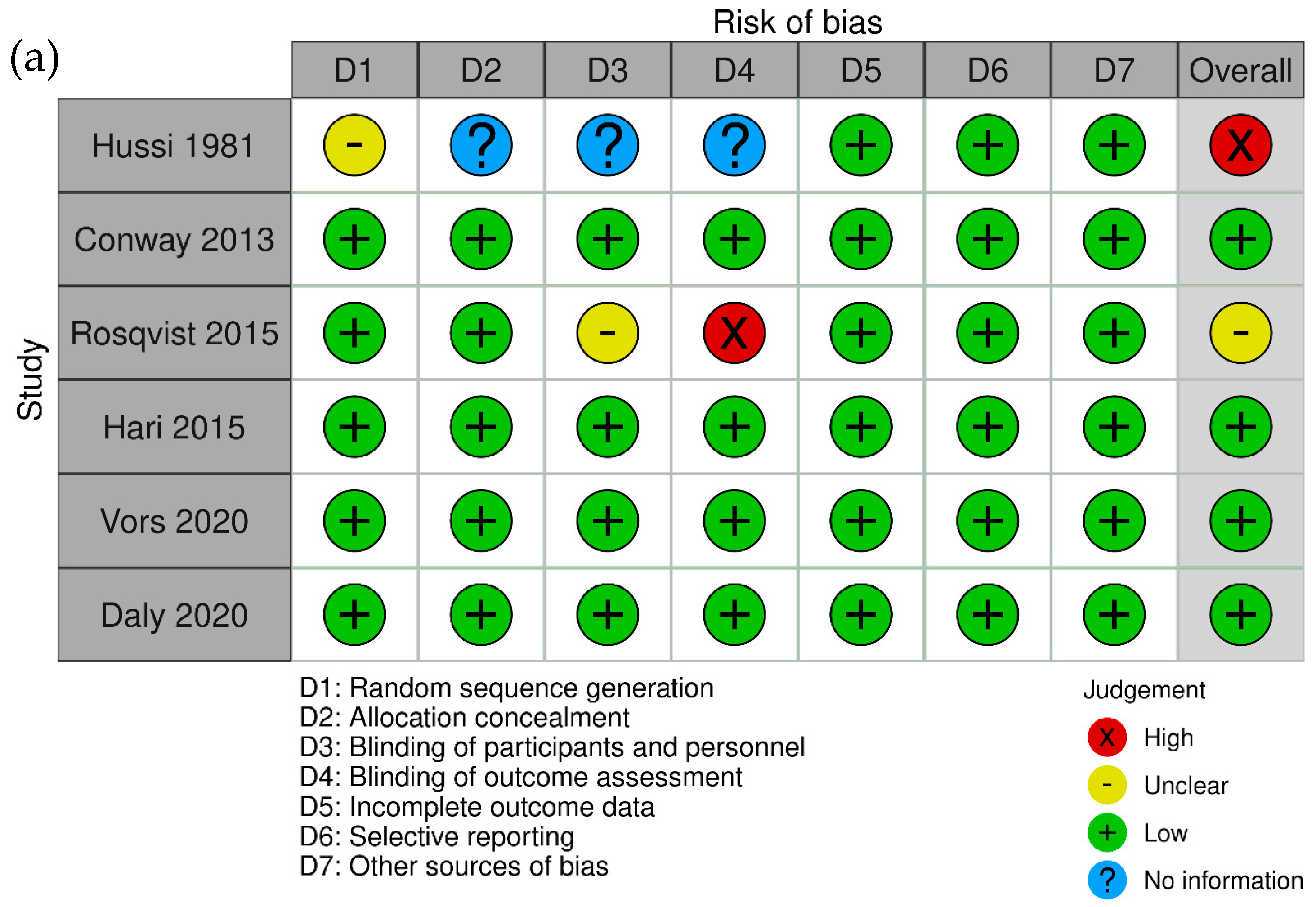
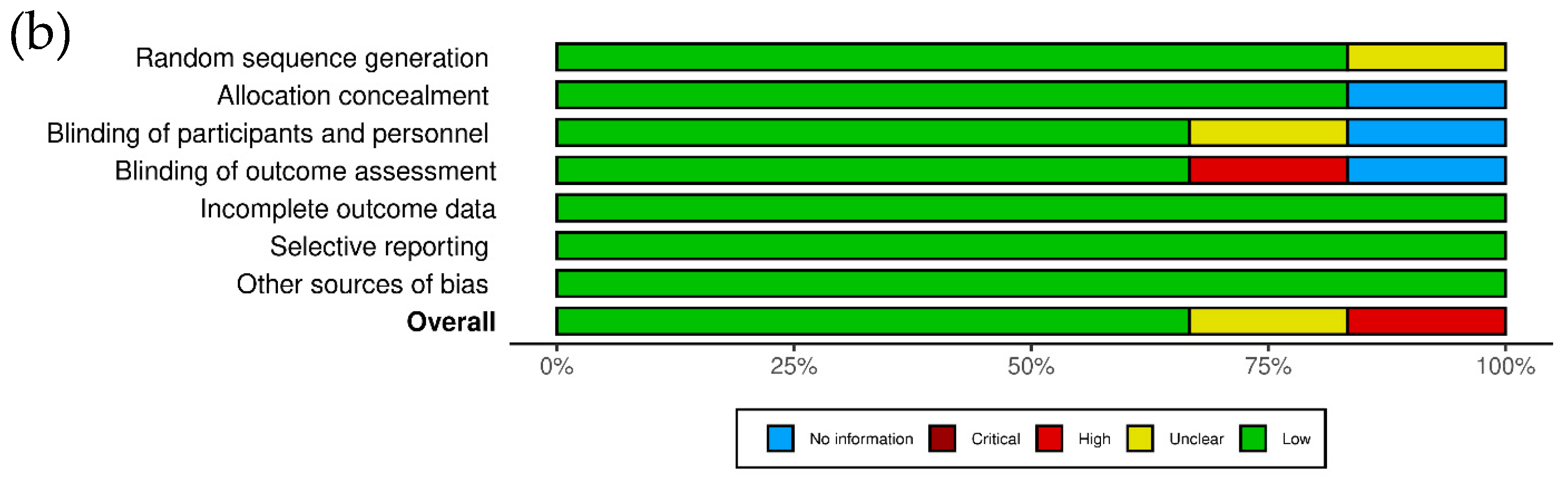
3.2. Risk of Bias Assessment
3.3. Total Cholesterol
3.4. Low-Density Lipoprotein Cholesterol
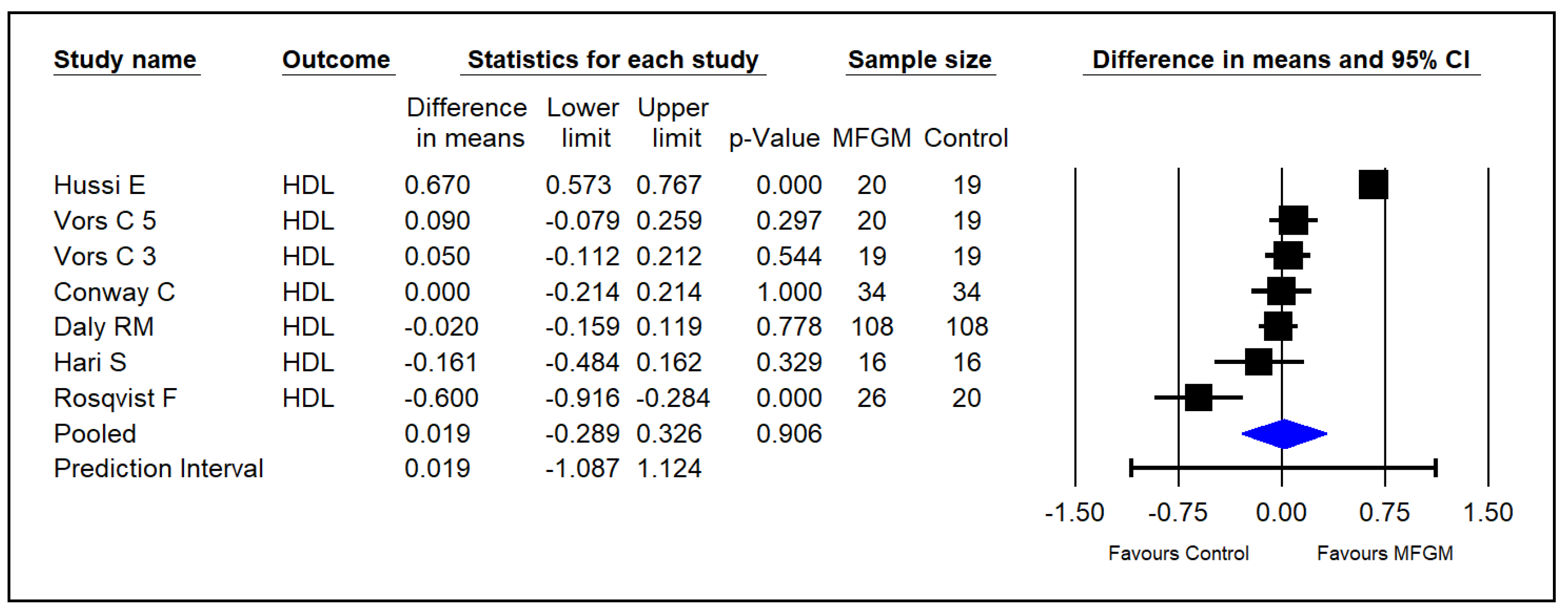
3.5. High-Density Lipoprotein Cholesterol
3.6. Triglycerides
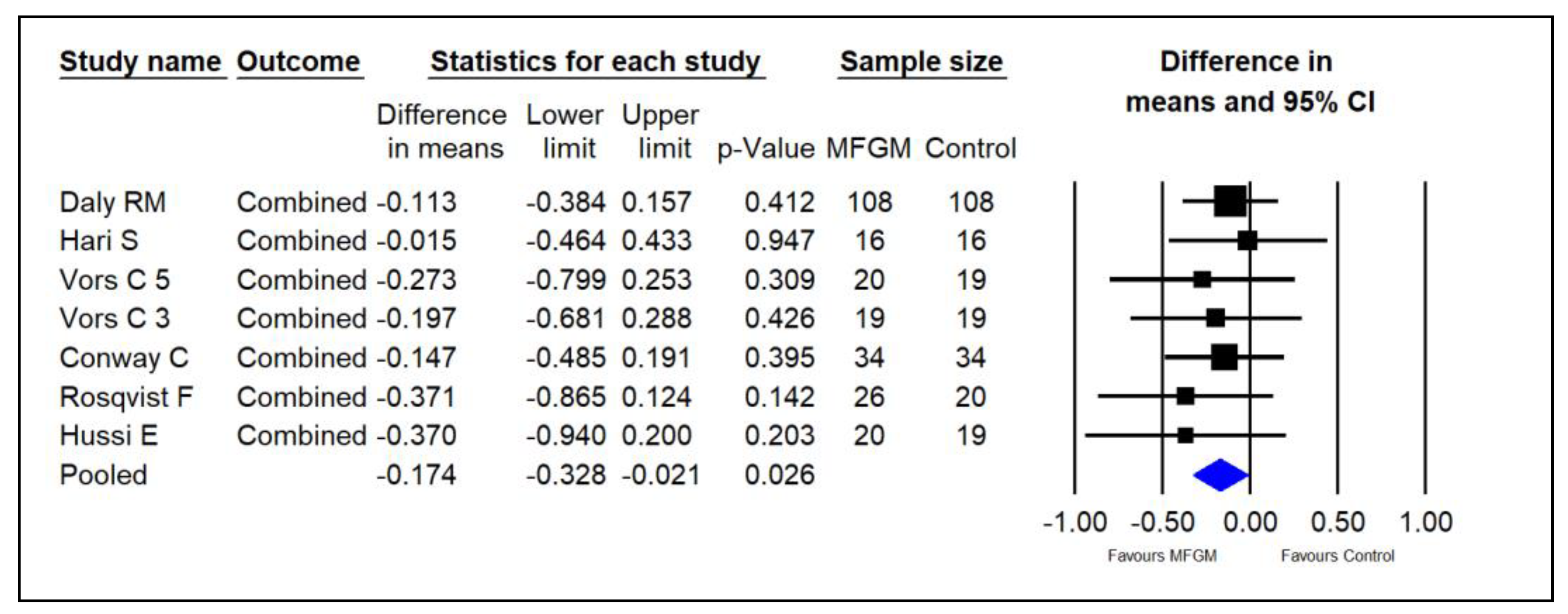
3.7. Overall—Combined Analysis
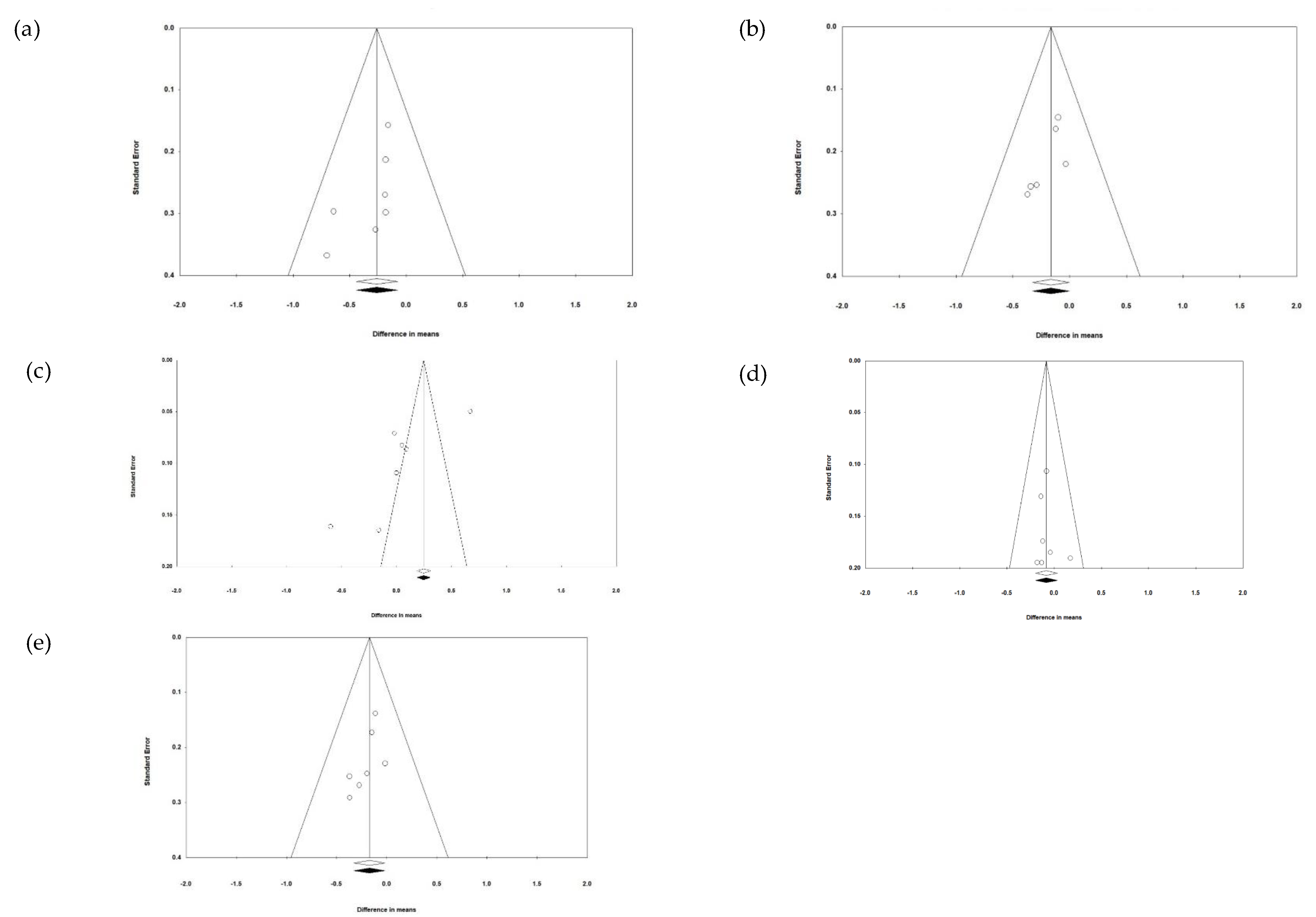
3.8. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Ng, R.; Sutradhar, R.; Yao, Z.; Wodchis, W.P.; Rosella, L.C. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 2020, 49, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Pahan, K. Lipid-lowering drugs. Cell. Mol. Life Sci. 2006, 63, 1165–1178. [Google Scholar] [CrossRef]
- Golomb, B.A.; Evans, M.A. Statin adverse effects. A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovas. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-associated side effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Lifestyle strategies for risk factor reduction, prevention, and treatment of cardiovascular disease. In Lifestyle Medicine, 3rd ed.; Rippe, J.M., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 19–36. [Google Scholar] [CrossRef]
- Basu, A.; Basu, P.; Morris, S.; Lyons, T.J. Lipids and lipoproteins as biomarkers of vascular complications in diabetes and their modulation by dietary phytochemicals. In Biomarkers in Cardiovascular Disease; Patel, V.B., Preedy, V.R., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 653–672. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The cholesterol-lowering effect of oats and oat beta-glucan: Modes of action and potential role of bile acids and the microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Zhou, N.; Shen, Y.; Li, B.; Chen, B.E.; Li, X. Association between omega-3 fatty acid intake and dyslipidemia: A continuous dose–response meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2023, 12, e029512. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. eClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef]
- Anto, L.; Wen Warykas, S.; Torres-Gonzales, M.; Blesso, C.N. Milk polar lipids: Underappreciated lipids with emerging health benefits. Nutrients 2020, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Conway, V.; Couture, P.; Richard, C.; Gauthier, S.F.; Pouliot, Y.; Lamarche, B. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1255–1262. [Google Scholar] [CrossRef]
- Vors, C.; Joumard-Cubizolles, L.; Lecomte, M.; Combe, E.; Ouchchane, L.; Drai, J.; Raynal, K.; Joffre, F.; Meiller, L.; Le Barz, M.; et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: Towards a gut sphingomyelin-cholesterol interplay. Gut 2020, 69, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Pokala, A.; Kraft, J.; Taormina, V.M.; Michalski, M.-C.; Vors, C.; Torres-Gonzales, M.; Bruno, R.S. Whole milk dairy foods and cardiometabolic health: Dairy fat and beyond. Nutr. Res. 2024, 126, 99–122. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jimenez-Flores, R. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef]
- Cohn, J.S.; Kamali, A.; Wat, E.; Chung, R.W.S.; Tandy, S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2010, 2, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Vesper, H.; Schmelz, E.-M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H., Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Ogawa, T.; Ono, J.; Miyashita, R.; Aida, K.; Oda, Y.; Ohnishi, M. Analysis of sphingolipid classes and their contents in meals. Biosci. Biotech. Biochem. 2008, 72, 222–225. [Google Scholar] [CrossRef]
- Chen, G.-C.; Szeto, I.M.Y.; Chen, L.-H.; Han, S.-F.; Li, Y.-J.; van Hekezen, R.; Qin, L.-Q. Dairy products consumption and metabolic syndrome in adults: Systematic review and meta-analysis of observational studies. Sci. Rep. 2015, 5, 14606. [Google Scholar] [CrossRef] [PubMed]
- Hussi, E.; Miettinen, T.A.; Ollus, A.; Kostiainen, E.; Ehnholm, C.; Haglund, B.; Huttunen, J.K.; Manninen, V. Lack of serum cholesterol-lowering effect of skimmed milk and butter milk under controlled conditions. Atherosclerosis 1981, 39, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Smedman, A.; Lindmark-Månsson, H.; Paulsson, M.; Petrus, P.; Straniero, S.; Rudling, M.; Dahlman, I.; Risérus, U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: A randomized study. Am. J. Clin. Nutr. 2015, 102, 20–30. [Google Scholar] [CrossRef]
- Hari, S.; Ochiai, R.; Shioya, Y.; Katsuragi, Y. Safety evaluation of the consumption of high dose milk fat globule membrane in healthy adults: A double-blind, randomized controlled trial with parallel group design. Biosci. Biotechnol. Biochem. 2015, 79, 1172–1177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daly, R.M.; Gianoudis, J.; De Ross, B.; O’Connell, S.L.; Kruger, M.; Schollum, L.; Gunn, C. Effects of a multinutrient-fortified milk drink combined with exercise on functional performance, muscle strength, body composition, inflammation, and oxidative stress in middle-aged women: A 4-month, double-blind, placebo-controlled, randomized trial. Am. J. Clin. Nutr. 2020, 112, 427–446. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kiesswetter, E.; Stadelmaier, J.; Petropoulou, M.; Morze, J.; Grummich, K.; Roux, I.; Lay, R.; Himmelsbach, L.; Kussmann, M.; Roeger, C.; et al. Effects of dairy intake on markers of cardiometabolic health in adults: A systematic review with network meta-analysis. Adv. Nutr. 2023, 14, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Raza, G.S.; Herzig, K.-H.; Leppäluoto, J. Milk fat globule membrane—A possible panacea for neurodevelopment, infections, cardiometabolic diseases, and frailty. J. Dairy Sci. 2021, 104, 7345–7363. [Google Scholar] [CrossRef]
- Watanabe, S.; Takahashi, T.; Tanaka, L.; Haruta, Y.; Shiota, M.; Hosokawa, M.; Miyashita, K. The effect of milk polar lipids separated from butter serum on the lipid levels in the liver and the plasma of obese-model mouse (KK-Ay). J. Funct. Foods 2011, 3, 313–320. [Google Scholar] [CrossRef]
- Norris, G.H.; Jiang, C.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef]
- Wat, E.; Tandy, S.; Kapera, E.; Kamili, A.; Chung, R.W.; Brown, A.; Rowney, M.; Cohn, J.S. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis 2009, 205, 144–150. [Google Scholar] [CrossRef]
- Milard, M.; Laugerette, F.; Durand, A.; Buisson, C.; Meugnier, E.; Loizon, E.; Louche-Pelissier, C.; Sauvinet, V.; Garnier, L.; Viel, S.; et al. Milk polar lipids in a high-fat diet can prevent body weight gain: Modulated abundance of gut bacteria in relation with fecal loss of Specific fatty acids. Mol. Nut. Food Res. 2019, 63, 1801078. [Google Scholar] [CrossRef]
- Lecomte, M.; Couëdelo, L.; Meugnier, E.; Plaisancié, P.; Létisse, M.; Benoit, B.; Gabert, L.; Penhoat, A.; Durand, A.; Pineau, G.; et al. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet cells in high-fat fed mice. Mol. Nutr. Food Res. 2016, 60, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Ward, R.E. Milk polar lipids modulate lipid metabolism, gut permeability, and systemic inflammation in high-fat-fed C57BL/6J ob/ob mice, a model of severe obesity. J. Dairy Sci. 2019, 102, 4816–4831. [Google Scholar] [CrossRef] [PubMed]
- Kamili, A.; Wat, E.; Chung, R.S.W.; Tandy, S.; Weir, J.M.; Meikle, P.J.; Cohn, J.S. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr. Metab. 2010, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Hintze, K.J.; Jimenez-Flores, R.; Ward, R.E. Dietary fat composition influences tissue lipid profile and gene expression in Fischer-344 rats. Lipids 2012, 47, 1119–1130. [Google Scholar] [CrossRef]
- Conway, V.; Couture, P.; Gauthier, S.; Pouliot, Y.; Lamarche, B. Effect of buttermilk consumption on blood pressure in moderately hypercholesterolemic men and women. Nutrition 2014, 30, 116–119. [Google Scholar] [CrossRef]
- Conway, V.; Gauthier, S.F.; Pouliot, Y. Buttermilk: Much more than a source of milk phospholipids. Animal Front. 2014, 4, 44–51. [Google Scholar] [CrossRef]
- Homan, R.; Hamelehle, K.L. Phospholipase A2 relieves phosphatidylcholine inhibition of micellar cholesterol absorption and transport by human intestinal cell line Caco-2. J. Lipid Res. 1998, 39, 1197–1209. [Google Scholar] [CrossRef]
- Beil, F.U.; Grundy, S.M. Studies on plasma lipoproteins during absorption of exogenous lecithin in man. J. Lipid Res. 1980, 21, 525–536. [Google Scholar] [CrossRef]
- Grzybek, M.; Kubiak, J.; Łach, A.; Przuybyło, M.; Sikorski, A.F. A raft-associated species of phosphatidylethanolamine interacts with cholesterol comparably to sphingomyelin. A Langmuir-Blodgett monolayer study. PLoS ONE 2009, 4, e5053. [Google Scholar] [CrossRef]
- Hollander, D.; Morgan, D. Effect of plant sterols, fatty acids and lecithin on cholesterol absorption in vivo in the rat. Lipids 1980, 15, 395–400. [Google Scholar] [CrossRef]
- Imaizumi, K.; Mawatari, K.; Murata, M.; Ikeda, I.; Sugano, M. The contrasting effect of dietary phosphatidylethanolamine and phosphatidylcholine on serum lipoproteins and liver lipids in rats. J. Nutr. 1983, 113, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, K.; Sekihara, K.; Sugano, M. Hypocholesterolemic action of dietary phosphatidylethanolamine in rats sensitive to exogenous cholesterol. J. Nutr. Biochem. 1991, 2, 251–254. [Google Scholar] [CrossRef]
- Young, S.C.; Hui, D.Y. Pancreatic lipase/colipase-mediated triacylglycerol hydrolysis is required for cholesterol transport from lipid emulsions to intestinal cells. Biochem. J. 1999, 339, 615–620. [Google Scholar] [CrossRef]
- Xu, D.; Sun, G.S. A meta-analysis of β-glucan effects on lipids in mildly hypercholesterolemic individuals. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), nzaa052_062. [Google Scholar] [CrossRef]
- Yu, J.; Xia, J.; Yang, C.; Pan, D.; Xu, D.; Sun, G.; Xia, H. Effects of oat β-glucan intake on lipid profiles in hypercholesterolemic adults: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2022, 14, 2043. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Yu, J.-H.; Sun, J.-H.; Ma, W.-Q.; Wang, J.-J.; Sun, G.-J. Effects of omega-3 fatty acids supplementation on serum lipid profile and blood pressure in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Foods 2023, 12, 725. [Google Scholar] [CrossRef]
- Zhang, H.J.; Gao, X.; Guo, X.-F.; Li, K.-L.; Li, S.; Sinclair, A.J.; Li, D. Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: A systematic review and meta-analysis of data from 33 randomized controlled trials. Clin. Nutr. 2021, 40, 4538–4550. [Google Scholar] [CrossRef] [PubMed]
- Rueda, R. The role of complex lipids in attaining metabolic health. Curr. Cardiovasc. Risk Rep. 2014, 8, 371. [Google Scholar] [CrossRef]



| Author | Country | Age, Range (Mean or Median) y | Participants | Study Design | Intervention | Control Group | Intervention Duration | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Conway et al. [14] | Canada | 18–65 (49.4 ± 12.8 y) | 34 men and women | Crossover | 45 g skim milk + buttermilk powder mixed with water (187.5 mg phospholipids) | 45 g skim milk powder mixed with water (34.6 mg phospholipids) | 4 weeks | TC, TG, HDL, LDL |
| Vors et al. [15] | France | 56–62 (49.4 ± 12.8 y) | 58 overweight postmenopausal women (20 in 5 g experimental group, 19 in 3 g experimental group, 19 in control group) | Parallel | Full-fat cream cheese with 3000 mg or 5000 mg of phospholipids | Full-fat cream cheese with 0 mg phospholipids | 4 weeks | TC, TG, HDL, LDL |
| Hussi et al. [22] | Finland | Not provided in text | 39 healthy male prisoners (20 in experimental group, 19 in control group) | Parallel | 2.0 L buttermilk | 3 dL milk | 3 weeks | TC, TG, HDL |
| Rosqvist et al. [23] | Sweden | 20–70 y (median 58.5 y in control and 60.5 y in MFGM) | 57 overweight men and women (26 in experimental group, 20 in control group) | Parallel | 1 dL whipping cream (40% fat)/d, 1 dL fat-free milk (0.1% fat)/d, and 1 scone/d (19.8 mg phospholipids) | 1 dL fat-free milk (0.1% fat)/d and 1 scone/d (1.3 mg phospholipids) | 8 weeks | TC, TG, HDL, LDL |
| Hari et al. [24] | Japan | 20–64 (42.0 ± 11.9 y) | 32 healthy men and women (16 in experimental group, 16 in control group) | Parallel | 6.5 g MFGM tablet (231 mg sphingomyelin) | 6.5 g whole milk powder tablet (4 mg sphingomyelin) | 4 weeks | TC, TG, HDL, LDL |
| Daly et al. [25] | Australia | 45–65 (55 ± 5 y in MFGM, 56 ± 5 y in placebo) | 244 healthy women (123 in experimental group, 121 in control group) | Parallel | 60 g milk powder + MFGM mixed with water (400 mg phospholipids) | 60 g rice powder mixed with water (0 mg phospholipids) | 16 weeks | TC, TG, HDL, LDL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanon, A.P.; Spies, S.J.; MacGibbon, A.K.H.; Fuad, M. Milk Fat Globule Membrane Is Associated with Lower Blood Lipid Levels in Adults: A Meta-Analysis of Randomized Controlled Trials. Foods 2024, 13, 2725. https://doi.org/10.3390/foods13172725
Kanon AP, Spies SJ, MacGibbon AKH, Fuad M. Milk Fat Globule Membrane Is Associated with Lower Blood Lipid Levels in Adults: A Meta-Analysis of Randomized Controlled Trials. Foods. 2024; 13(17):2725. https://doi.org/10.3390/foods13172725
Chicago/Turabian StyleKanon, Alexander P., Sarah J. Spies, Alastair K. H. MacGibbon, and Maher Fuad. 2024. "Milk Fat Globule Membrane Is Associated with Lower Blood Lipid Levels in Adults: A Meta-Analysis of Randomized Controlled Trials" Foods 13, no. 17: 2725. https://doi.org/10.3390/foods13172725
APA StyleKanon, A. P., Spies, S. J., MacGibbon, A. K. H., & Fuad, M. (2024). Milk Fat Globule Membrane Is Associated with Lower Blood Lipid Levels in Adults: A Meta-Analysis of Randomized Controlled Trials. Foods, 13(17), 2725. https://doi.org/10.3390/foods13172725






