Physicochemical Properties, Functionalities, and Antioxidant Activity of Protein Extracts from New Zealand Wild Sea Cucumbers (Australostichopus mollis)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Sea Cucumber Protein Extract
2.3. Physicochemical Properties of Sea Cucumber Protein Extract
2.3.1. Functional Groups Determination
2.3.2. Thermal Properties Determination
2.3.3. Molecular Weight Distribution of Sea Cucumber Protein Extracts
2.3.4. Amino Acid (AA) Composition Analysis
2.4. Functionality of Sea Cucumber Protein Extract
2.4.1. Solubility
2.4.2. Foaming Properties
2.4.3. Emulsifying Properties
2.5. Antioxidant Activity of Sea Cucumber Protein Extract
2.5.1. DPPH Free Radical Scavenging Activity Assay
2.5.2. ABTS Free Radical Scavenging Activity Assay
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Properties of Sea Cucumber Protein Extracts
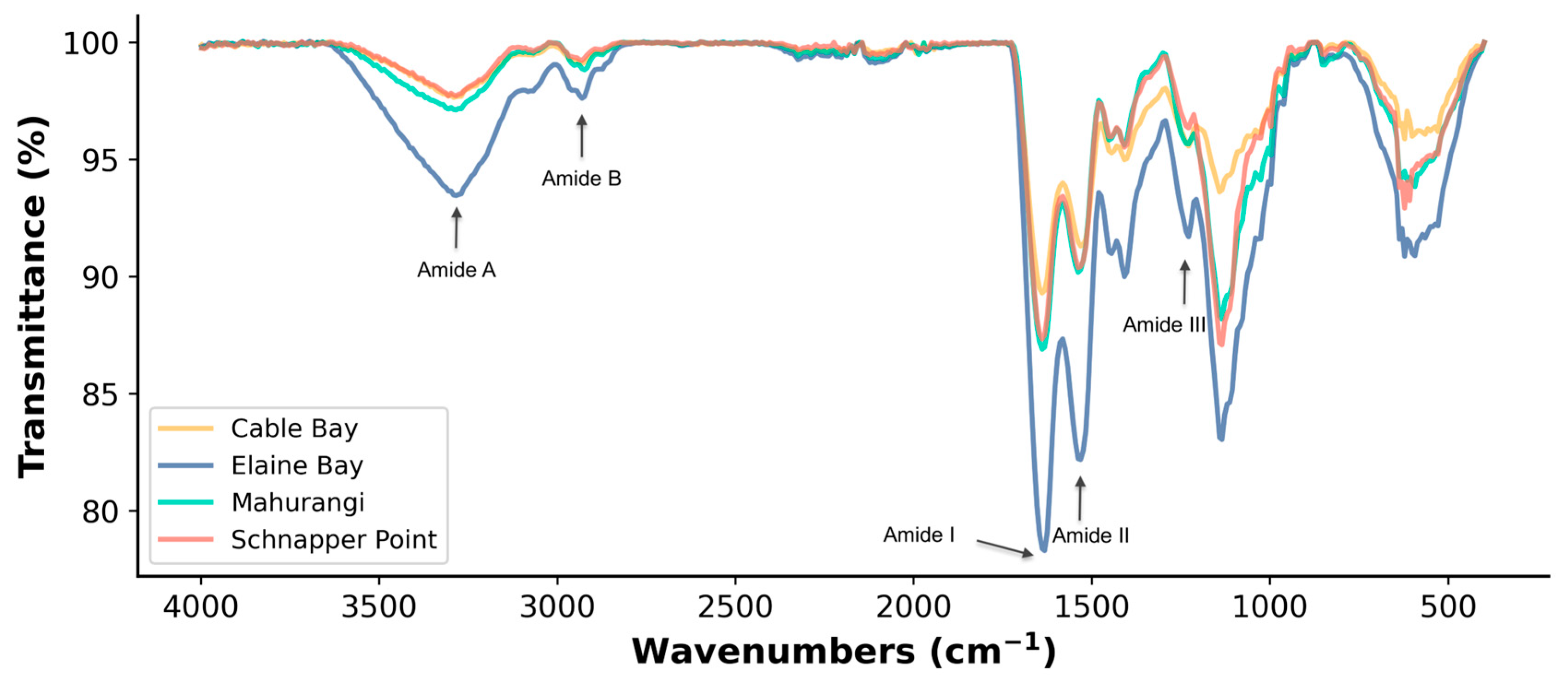
3.1.1. Functional Groups
3.1.2. Thermal Properties
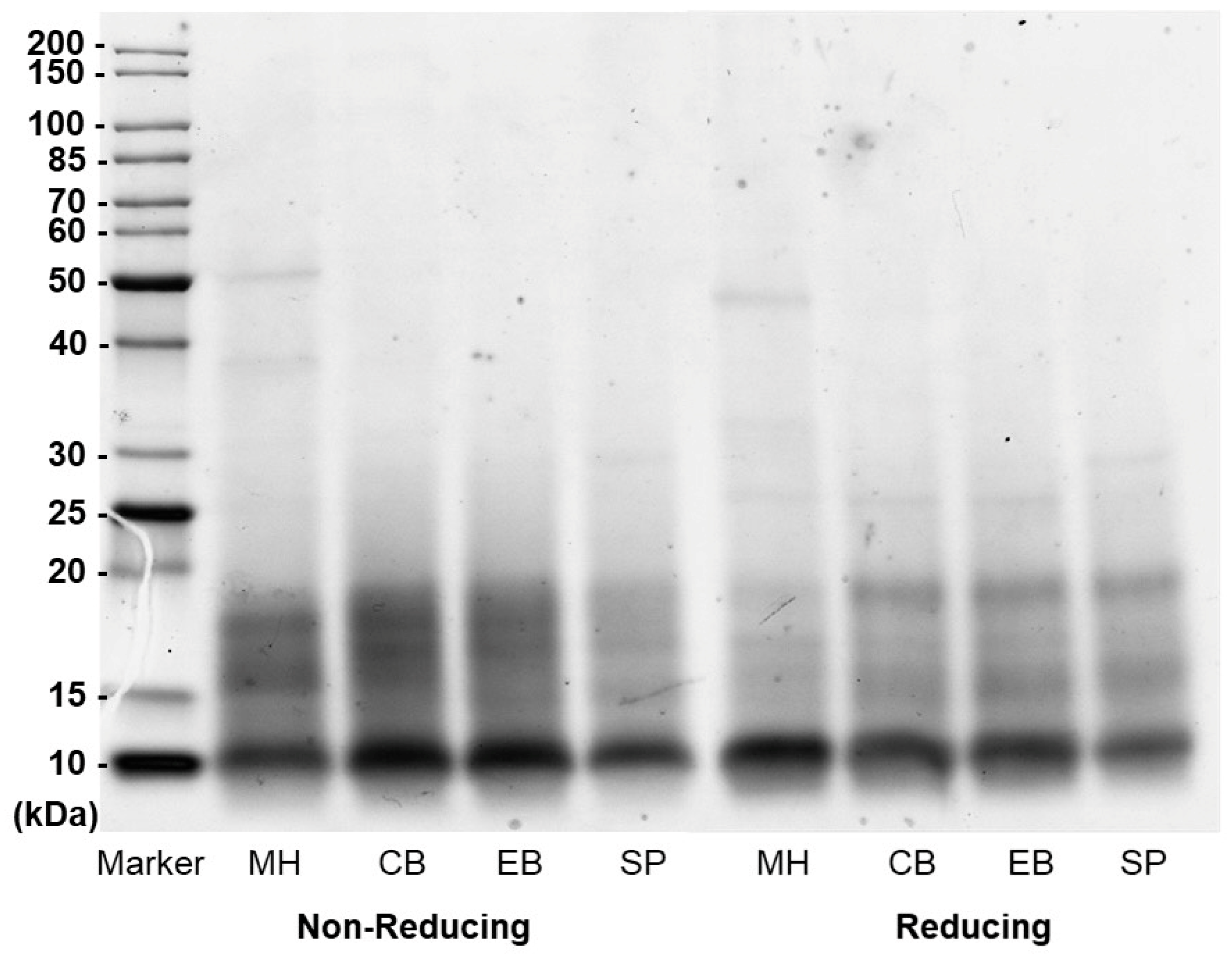
3.1.3. Molecular Weight Distribution
3.1.4. Amino Acid Composition
3.2. Functional Properties of Sea Cucumber Protein Extracts
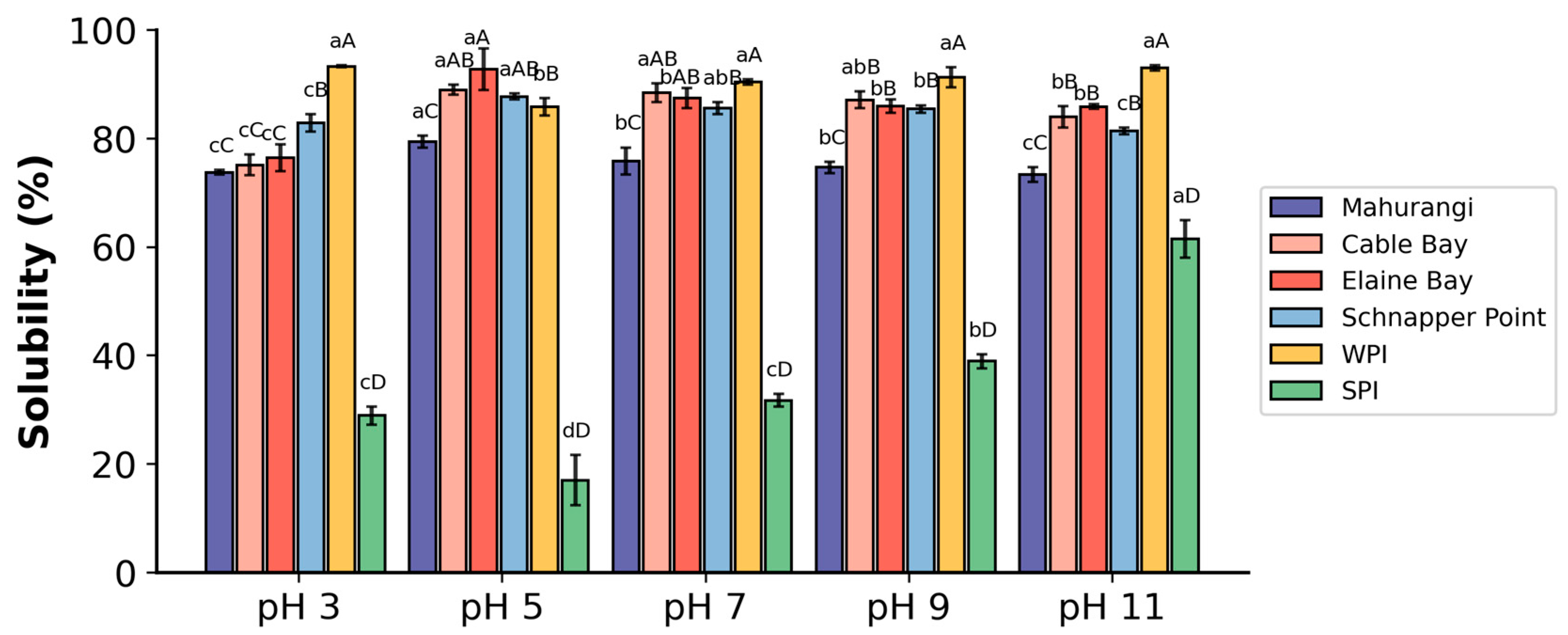
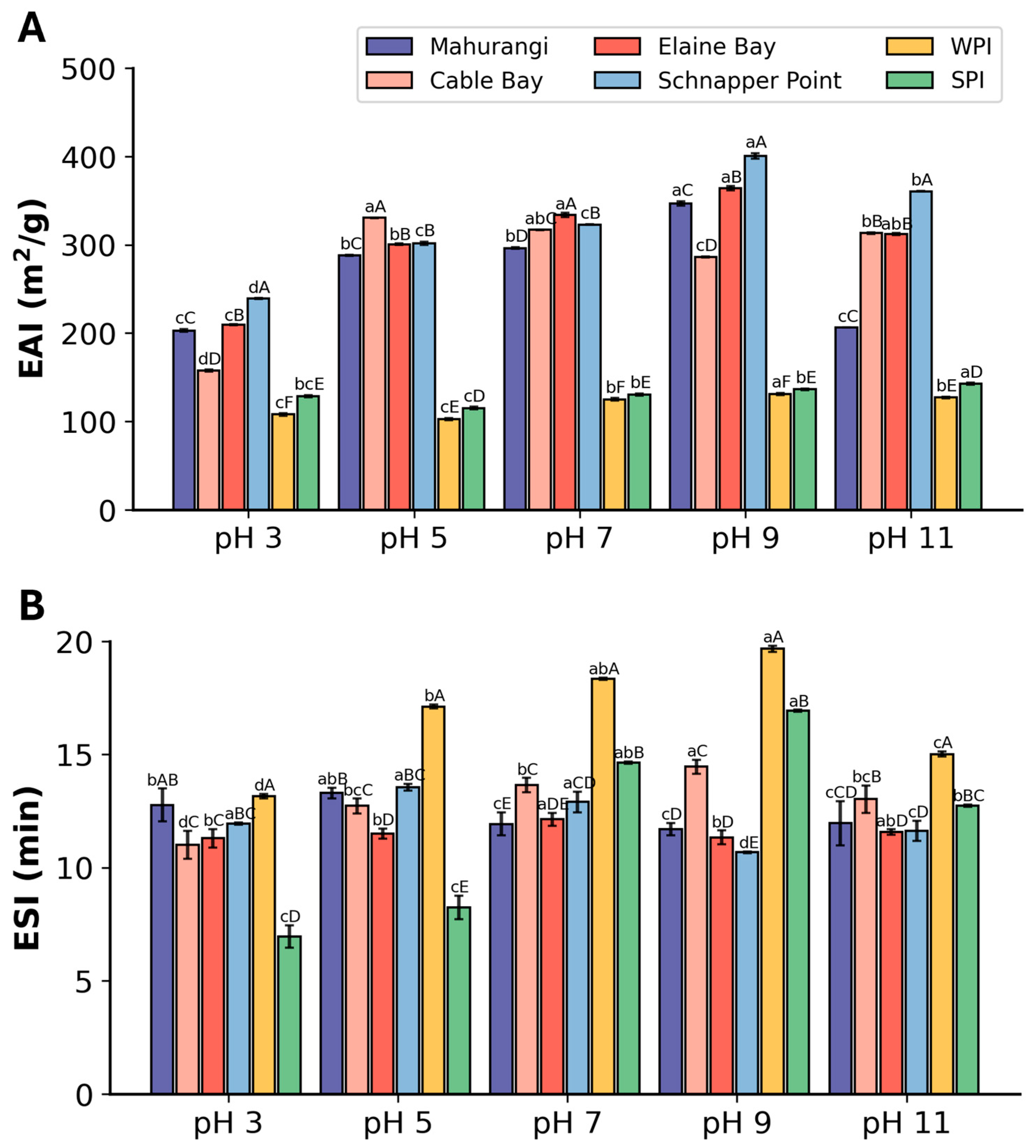
3.2.1. Solubility
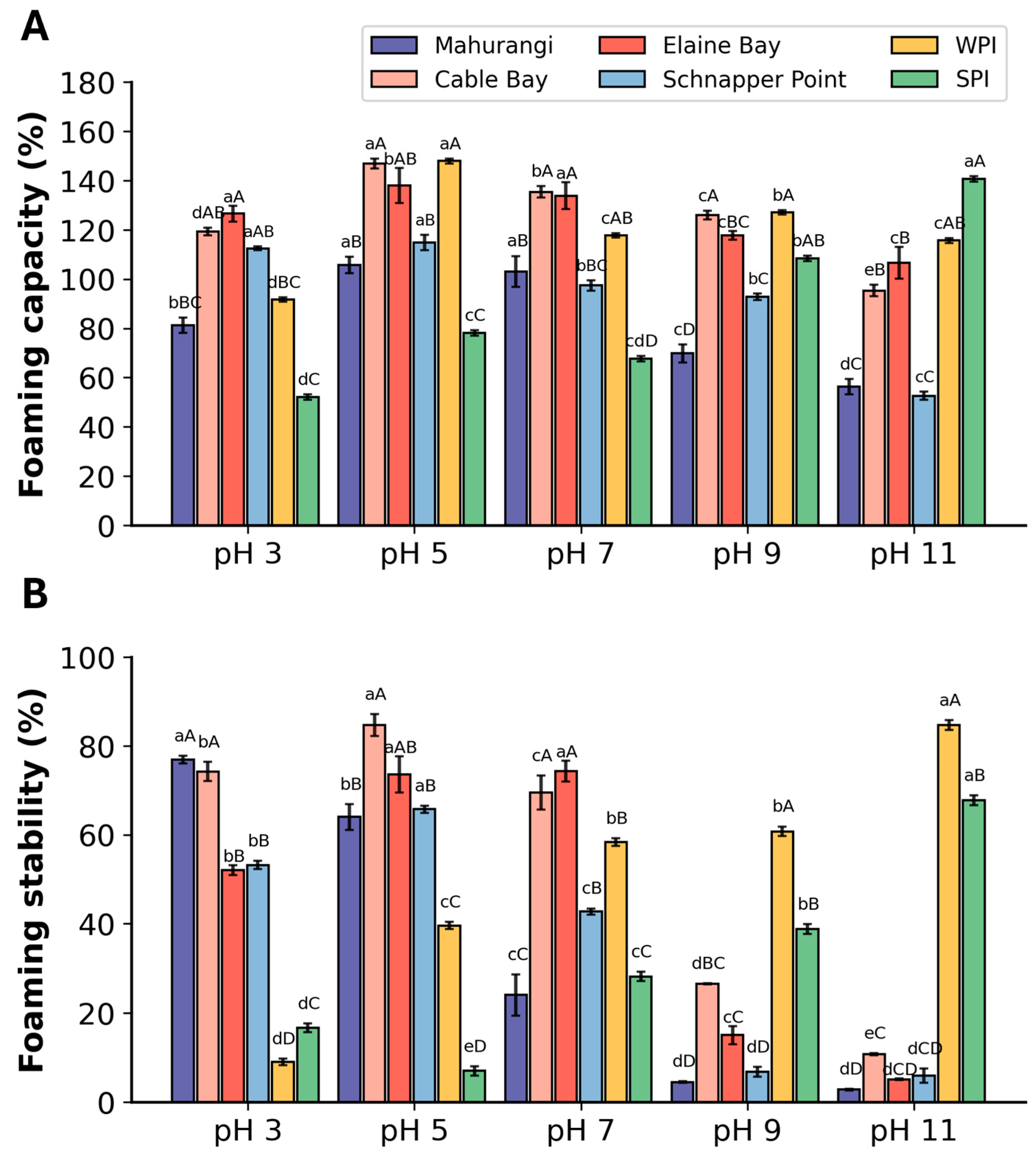
3.2.2. Foaming Properties
3.2.3. Emulsifying Properties
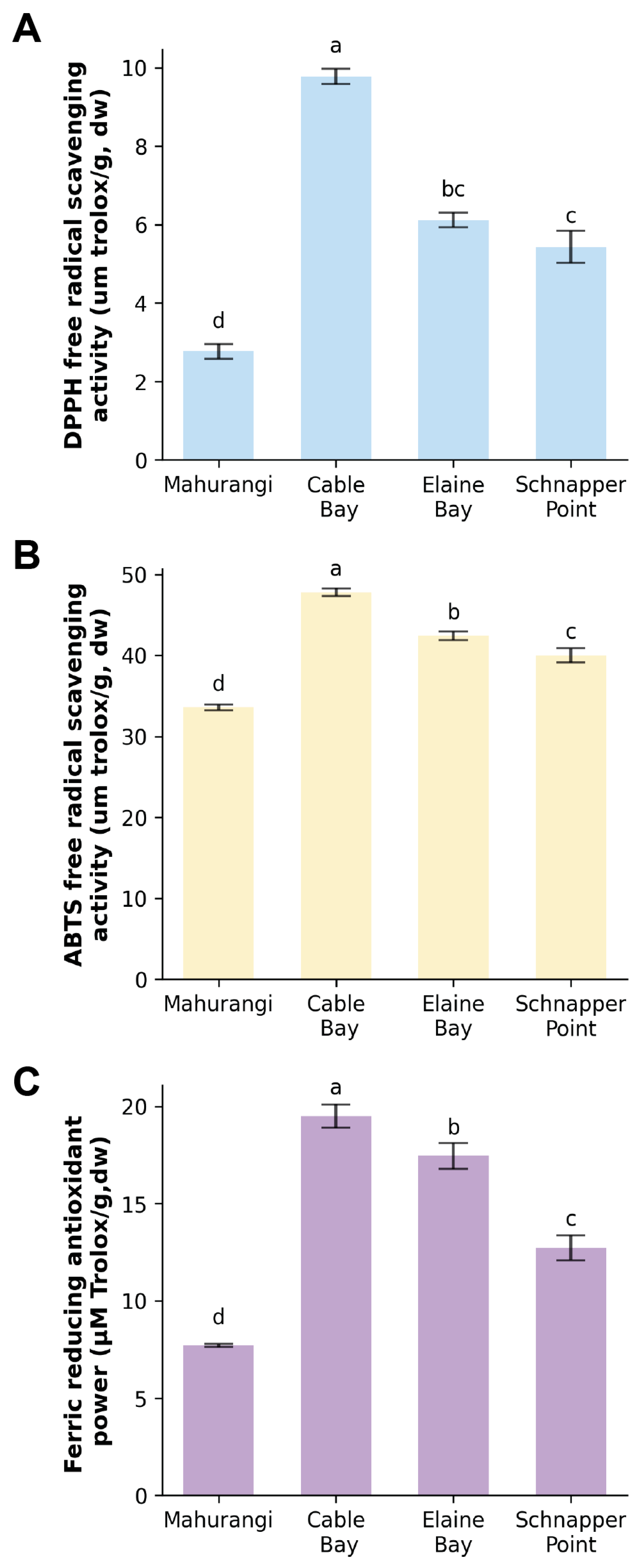
3.3. Antioxidant Activity of Protein Extracts from Sea Cucumber Body Wall
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.W. Value, Market Preferences and Trade of Beche-De-Mer from Pacific Island Sea Cucumbers. PLoS ONE 2014, 9, e95075. [Google Scholar] [CrossRef] [PubMed]
- Aprianto, R.; Amir, N.; Kasmiati; Matusalach; Fahrul; Syahrul; Tresnati, J.; Tuwo, A.; Nakajima, M. Economically important sea cucumber processing techniques in South Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 370, 012082. [Google Scholar] [CrossRef]
- Dereli, H.; Aydın, M. Sea cucumber fishery in Turkey: Management regulations and their efficiency. Reg. Stud. Mar. Sci. 2021, 41, 101551. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, T.; Hu, C.; Ren, C. Isolation and Characterization of Collagen from the Body Wall of Sea Cucumber Stichopus monotuberculatus. J. Food Sci. 2015, 80, C671–C679. [Google Scholar] [CrossRef]
- Khotimchenko, Y. Pharmacological Potential of Sea Cucumbers. Int. J. Mol. Sci. 2018, 19, 1342. [Google Scholar] [CrossRef]
- Shi, S.; Feng, W.; Hu, S.; Liang, S.; An, N.; Mao, Y. Bioactive compounds of sea cucumbers and their therapeutic effects. Chin. J. Oceanol. Limnol. 2016, 34, 549–558. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, R.; Wen, Z. Bioactive compounds and biological functions of sea cucumbers as potential functional foods. J. Funct. Foods 2018, 49, 73–84. [Google Scholar] [CrossRef]
- Guo, K.; Su, L.; Wang, Y.; Liu, H.; Lin, J.; Cheng, P.; Yin, X.; Liang, M.; Wang, Q.; Huang, Z. Antioxidant and anti-aging effects of a sea cucumber protein hydrolyzate and bioinformatic characterization of its composing peptides. Food Funct. 2020, 11, 5004–5016. [Google Scholar] [CrossRef]
- Li, P.-H.; Lu, W.-C.; Chan, Y.-J.; Ko, W.-C.; Jung, C.-C.; Le Huynh, D.T.; Ji, Y.-X. Extraction and characterization of collagen from sea cucumber (Holothuria cinerascens) and its potential application in moisturizing cosmetics. Aquaculture 2020, 515, 734590. [Google Scholar] [CrossRef]
- González-Wangüemert, M.; Roggatz, C.C.; Rodrigues, M.J.; Barreira, L.; da Silva, M.M.; Custódio, L. A new insight into the influence of habitat on the biochemical properties of three commercial sea cucumber species. Int. Aquat. Res. 2018, 10, 361–373. [Google Scholar] [CrossRef]
- Aydın, M.; Sevgili, H.; Tufan, B.; Emre, Y.; Köse, S. Proximate composition and fatty acid profile of three different fresh and dried commercial sea cucumbers from Turkey. Int. J. Food Sci. Technol. 2011, 46, 500–508. [Google Scholar] [CrossRef]
- Muhammad Husin, M.J.; Mazlan, N.; Shalom, J.; Saud, S.N.; Abdullah Sani, M.S. Evaluation of microplastics ingested by sea cucumber Stichopus horrens in Pulau Pangkor, Perak, Malaysia. Environ. Sci. Pollut. Res. 2021, 28, 61592–61600. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.-X.; Xue, C.-H.; Li, Z.-J.; Zhang, Y.-Q.; Dong, P.; Fu, X.-Y.; Gao, X. Characterization and subunit composition of collagen from the body wall of sea cucumber Stichopus japonicus. Food Chem. 2007, 100, 1120–1125. [Google Scholar] [CrossRef]
- Sun, N.; Cui, P.; Jin, Z.; Wu, H.; Wang, Y.; Lin, S. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chem. 2017, 230, 627–636. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Li, Y.; Zhang, X.; Qi, H. Authentication of the sea cucumber (Apostichopus japonicus) using amino acids carbon stable isotope fingerprinting. Food Control 2018, 91, 128–137. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Minakova, A.S.; Anumol, T.; Keller, A.A. Quantitative analysis of changes in amino acids levels for cucumber (Cucumis sativus) exposed to nano copper. NanoImpact 2018, 12, 9–17. [Google Scholar] [CrossRef]
- Hefter, G.T.; Tomkins, R.P.T. The Experimental Determination of Solubilities; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Du, Y.-N.; Guo, X.-K.; Han, Y.-T.; Han, J.-R.; Yan, J.-N.; Shang, W.-H.; Wu, H.-T. Physicochemical and functional properties of protein isolate from sea cucumber (Stichopus japonicus) guts. J. Food Process. Preserv. 2019, 43, e13957. [Google Scholar] [CrossRef]
- Rawiwan, P.; Quek, S.Y. Physicochemical and functional attributes of RuBisCo-enriched Brassicaceae leaf protein concentrates. Food Hydrocoll. 2024, 151, 109887. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Dong, X.; Woo, M.W.; Quek, S.Y. The physicochemical properties, functionality, and digestibility of hempseed protein isolate as impacted by spray drying and freeze drying. Food Chem. 2024, 433, 137310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chhun, S.; Xiang, J.; Tangjaidee, P.; Peng, Y.; Quek, S.Y. Microencapsulation of Cyclocarya paliurus (Batal.) Iljinskaja Extracts: A Promising Technique to Protect Phenolic Compounds and Antioxidant Capacities. Foods 2021, 10, 2910. [Google Scholar] [CrossRef]
- Han, Y.-T.; Zhao, C.-C.; Han, J.-R.; Yan, J.-N.; Du, Y.-N.; Shang, W.-H.; Wu, H.-T. Antioxidant activity of sea cucumber (Stichopus japonicus) gut hydrolysates-ribose Maillard reaction products derived from organic reagent extraction. J. Food Meas. Charact. 2019, 13, 2790–2797. [Google Scholar] [CrossRef]
- Binsan, W.; Benjakul, S.; Visessanguan, W.; Roytrakul, S.; Tanaka, M.; Kishimura, H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 2008, 106, 185–193. [Google Scholar] [CrossRef]
- Si, L.; Fan, Y.; Wang, Y.; Sun, L.; Li, B.; Xue, C.; Hou, H. Thermal degradation behavior of collagen from sea cucumber (Stichopus japonicus) using TG-FTIR analysis. Thermochim. Acta 2018, 659, 166–171. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Weymuth, T.; Jacob, C.R.; Reiher, M. A Local-Mode Model for Understanding the Dependence of the Extended Amide III Vibrations on Protein Secondary Structure. J. Phys. Chem. B 2010, 114, 10649–10660. [Google Scholar] [CrossRef]
- De Meutter, J.; Goormaghtigh, E. FTIR Imaging of Protein Microarrays for High Throughput Secondary Structure Determination. Anal. Chem. 2021, 93, 3733–3741. [Google Scholar] [CrossRef]
- Farrell, H.M.; Wickham, E.D.; Unruh, J.J.; Qi, P.X.; Hoagland, P.D. Secondary structural studies of bovine caseins: Temperature dependence of β-casein structure as analyzed by circular dichroism and FTIR spectroscopy and correlation with micellization. Food Hydrocoll. 2001, 15, 341–354. [Google Scholar] [CrossRef]
- Baronio, C.M.; Barth, A. The Amide I Spectrum of Proteins—Optimization of Transition Dipole Coupling Parameters Using Density Functional Theory Calculations. J. Phys. Chem. B 2020, 124, 1703–1714. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- De Meutter, J.; Goormaghtigh, E. Amino acid side chain contribution to protein FTIR spectra: Impact on secondary structure evaluation. Eur. Biophys. J. 2021, 50, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Zhang, J.; Song, L.; Li, D.; Wu, Z.; Zhu, B.; Nakamura, Y.; Shahidi, F.; Yu, C.; et al. Isolation and identification of zinc-chelating peptides from sea cucumber (Stichopus japonicus) protein hydrolysate. J. Sci. Food Agric. 2019, 99, 6400–6407. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-M.; Li, D.-Y.; Liu, Z.-Q.; Liu, Y.-X.; Zhou, J.-Z.; Zhang, M.; Zhou, D.-Y.; Zhu, B.-W. Effects of heat treatments on texture of abalone muscles and its mechanism. Food Biosci. 2021, 44, 101402. [Google Scholar] [CrossRef]
- Liu, F.; Zamora, L.; Jeffs, A.; Quek, S.Y. Biochemical composition of the Australasian sea cucumber, Australostichopus mollis, from a nutritional point of view. Nutrire 2017, 42, 12. [Google Scholar] [CrossRef]
- Dong, X.; Yang, X.; Li, H.; Che, H.; Song, L.; Chen, X.; Xie, W.; Qi, H. Effects of oxidation on the structure of collagen fibers of sea cucumber (Apostichopus japonicus) body wall during thermal processing. LWT 2021, 138, 110528. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.; Bu, Y.; Li, J.; Li, X. Effects of nanowarming on water holding capacity, oxidation and protein conformation changes in jumbo squid (Dosidicus gigas) mantles. LWT 2020, 129, 109511. [Google Scholar] [CrossRef]
- Rao, F.; Caflisch, A. The Protein Folding Network. J. Mol. Biol. 2004, 342, 299–306. [Google Scholar] [CrossRef]
- Kaya, H.; Chan, H.S. Polymer principles of protein calorimetric two-state cooperativity. Proteins 2000, 40, 637–661. [Google Scholar] [CrossRef]
- Boye, J.I.; Roufik, S.; Pesta, N.; Barbana, C. Angiotensin I-converting enzyme inhibitory properties and SDS-PAGE of red lentil protein hydrolysates. LWT-Food Sci. Technol. 2010, 43, 987–991. [Google Scholar] [CrossRef]
- Sun, N.; Cui, P.; Lin, S.; Yu, C.; Tang, Y.; Wei, Y.; Xiong, Y.; Wu, H. Characterization of sea cucumber (Stichopus japonicus) ovum hydrolysates: Calcium chelation, solubility and absorption into intestinal epithelial cells. J. Sci. Food Agric. 2017, 97, 4604–4611. [Google Scholar] [CrossRef]
- Sroyraya, M.; Hanna, P.; Siangcham, T.; Tinikul, R.; Jattujan, P.; Poomtong, T.; Sobhon, P. Nutritional components of the sea cucumber Holothuria scabra. Funct. Foods Health Dis. 2017, 7, 168–181. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Q.; Yang, H. Seasonal biochemical changes in composition of body wall tissues of sea cucumber Apostichopus japonicus. Chin. J. Oceanol. Limnol. 2011, 29, 252–260. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Food and Agriculture Organization (FAO); United Nations University (UNU). Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Yu, M.-M.; Fan, Y.-C.; Xu, S.-J.; Liu, Y.-X.; Wu, Z.-X.; Zhou, D.-Y.; Zhu, B.-W. Effects of antioxidants on the texture and protein quality of ready-to-eat abalone muscles during storage. J. Food Compos. Anal. 2022, 108, 104456. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Tuo, F.-Y.; Song, L.; Liu, Y.-X.; Dong, X.-P.; Li, D.-M.; Zhou, D.-Y.; Shahidi, F. Action of trypsin on structural changes of collagen fibres from sea cucumber (Stichopus japonicus). Food Chem. 2018, 256, 113–118. [Google Scholar] [CrossRef]
- Akbari, N.; Mohammadzadeh Milani, J.; Biparva, P. Functional and conformational properties of proteolytic enzyme-modified potato protein isolate. J. Sci. Food Agric. 2020, 100, 1320–1327. [Google Scholar] [CrossRef]
- Cano-Medina, A.; Jiménez-Islas, H.; Dendooven, L.; Herrera, R.P.; González-Alatorre, G.; Escamilla-Silva, E.M. Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Res. Int. 2011, 44, 684–692. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Bamdad, F.; Wang, Y.; Tian, Z.; Temelli, F.; Han, J.; Chen, L. Optimization of lentil protein extraction and the influence of process pH on protein structure and functionality. LWT Food Sci. Technol. 2014, 57, 461–469. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Hossain, A.; Dave, D.; Shahidi, F. Functional and physiochemical properties of protein isolates from different body parts of North Atlantic sea cucumber (Cucumaria frondosa). Food Biosci. 2023, 52, 102511. [Google Scholar] [CrossRef]
- Rao, M.A. Flow and Functional Models for Rheological Properties of Fluid Foods. In Rheology of Fluid, Semisolid, and Solid Foods: Principles and Applications; Springer: Boston, MA, USA, 2014; pp. 27–61. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Guadix, A.; Guadix, E.M.; Jacobsen, C. Physical and oxidative stability of fish oil-in-water emulsions stabilized with fish protein hydrolysates. Food Chem. 2016, 203, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Enser, M.; Bloomberg, G.B.; Brock, C.; Clark, D.C. De novo design and structure-activity relationships of peptide emulsifiers and foaming agents. Int. J. Biol. Macromol. 1990, 12, 118–124. [Google Scholar] [CrossRef]
- Dexter, A.F.; Middelberg, A.P.J. Peptides as Functional Surfactants. Ind. Eng. Chem. Res. 2008, 47, 6391–6398. [Google Scholar] [CrossRef]
- Niu, R.-H.; Chen, F.-S.; Zhao, Z.-T.; Xin, Y.; Duan, X.-J.; Wang, B.-Y. Effect of Papain on the Demulsification of Peanut Oil Body Emulsion and the Corresponding Mechanism. J. Oleo Sci. 2020, 69, 617–625. [Google Scholar] [CrossRef]
- Lin, D.; Sun, L.-C.; Chen, Y.-L.; Liu, G.-M.; Miao, S.; Cao, M.-J. Peptide/protein hydrolysate and their derivatives: Their role as emulsifying agents for enhancement of physical and oxidative stability of emulsions. Trends Food Sci. Technol. 2022, 129, 11–24. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of Hydrolysis Degree on the Functional Properties of Salmon Byproducts Hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Ricardo, F.; Pradilla, D.; Cruz, J.C.; Alvarez, O. Emerging Emulsifiers: Conceptual Basis for the Identification and Rational Design of Peptides with Surface Activity. Int. J. Mol. Sci. 2021, 22, 4615. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Emulsifying and Emulsion-Stabilizing Properties of Gluten Hydrolysates. J. Agric. Food Chem. 2014, 62, 2623–2630. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Antioxidant potential and physicochemical properties of protein hydrolysates from body parts of North Atlantic sea cucumber (Cucumaria frondosa). Food Prod. Process. Nutr. 2021, 3, 3. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Jiang, A. Antioxidant peptides isolated from sea cucumber Stichopus japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, S.; Shi, W.; Qi, X.; Song, W.; Mou, J.; Yang, J. Structure characterization, antioxidant and immunoregulatory properties of a novel fucoidan from the sea cucumber Stichopus chloronotus. Carbohydr. Polym. 2020, 231, 115767. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, C.; Zheng, Q.; Wu, J.; Zhu, K.; Shen, X.; Cao, J. Effect of simulated gastrointestinal digestion in vitro on the antioxidant activity, molecular weight and microstructure of polysaccharides from a tropical sea cucumber (Holothuria leucospilota). Food Hydrocoll. 2019, 89, 735–741. [Google Scholar] [CrossRef]
| Sample | T0 (°C) | Td (°C) | ΔH (J/g Sample) |
|---|---|---|---|
| Mahurangi Harbour | 39.33 ± 0.20 B | 44.36 ± 1.03 B | 0.32 ± 0.05 C |
| Cable Bay | 40.09 ± 0.17 A | 47.45 ± 0.56 A | 0.43 ± 0.03 B |
| Schnapper Point | 37.79 ± 0.42 B | 44.37 ± 1.79 B | 0.48 ± 0.01 B |
| Elaine Bay | 40.33 ± 0.32 A | 45.41 ± 0.21 A | 0.71 ± 0.04 A |
| Amino Acid | Mahurangi Harbour | Cable Bay | Elaine Bay | Schnapper Point | |
|---|---|---|---|---|---|
| EAA | Threonine | 43.89 ± 2.00 eB | 50.75 ± 3.16 deA | 32.25 ± 3.31 fC | 41.2 ± 3.59 eB |
| Lysine | 38.75 ± 2.91 eB | 47.2 ± 4.22 eA | 48.03 ± 4.94 eA | 33.68 ± 1.10 efB | |
| Valine | 21.08 ± 2.25 fgA | 8.83 ± 0.98 hijkC | 15.56 ± 0.66 ghiB | 22.51 ± 0.78 ghA | |
| Leucine | 25.66 ± 1.72 fB | 29.67 ± 1.53 fA | 19.88 ± 2.79 gC | 27.58 ± 1.05 fgAB | |
| Isoleucine | 15.14 ± 1.35 fghA | 16.92 ± 0.40 ghiA | 11.97 ± 1.49 hijkB | 16.24 ± 0.66 hiA | |
| Phenylalanine | 16.71 ± 0.55 fghAB | 18.38 ± 1.63 ghA | 18.25 ± 0.24 ghA | 15.46 ± 0.68 hijB | |
| Methionine | 6.72 ± 1.15 Bij | 7.79 ± 0.21 ijkAB | 8.39 ± 0.14 ijklA | 7.18 ± 0.78 ijklAB | |
| Histidine | 5.01 ± 0.16 Bij | 5.42 ± 0.32 jkA | 4.52 ± 0.17 klmC | 4.24 ± 0.17 jklC | |
| NEAA | Glycine | 172.5 ± 26.6 aAB | 202.8 ± 20.0 aAB | 168.8 ± 11.3 aB | 212.6 ± 24.6 aA |
| Glutamic acid | 100.4 ± 3.92 bC | 121.1 ± 10.8 bB | 150.7 ± 10.5 bA | 105.7 ± 4.51 bC | |
| Aspartic acid | 87.32 ± 4.04 cBC | 92.11 ± 4.96 cB | 108.4 ± 5.71 cA | 79.85 ± 5.20 cC | |
| Proline | 58.09 ± 3.37 dB | 59.86 ± 2.93 dB | 65.65 ± 0.44 dA | 60.8 ± 0.49 dB | |
| Arginine | 20.69 ± 2.26 fgA | 23.55 ± 1.5 fgA | 21.18 ± 0.31 gA | 20.56 ± 2.08 ghA | |
| Tyrosine | 17.39 ± 3.00 fghB | 23.65 ± 1.49 fgA | 7.35 ± 0.03 jklmD | 12.94 ± 0.76 hijkC | |
| Alanine | 12.39 ± 0.68 hijB | 13.56 ± 0.24 ghijA | 13.61 ± 0.33 ghijA | 13.25 ± 0.43 hijA | |
| Cysteine | 6.26 ± 0.78 ijB | 6.71 ± 0.32 ijkB | 8.40 ± 0.50 ijklA | 4.57 ± 0.24 jklC | |
| Serine | 1.85 ± 0.30 jA | 2.14 ± 0.26 kA | 2.15 ± 0.24 lmA | 1.82 ± 0.26 klA | |
| Total AA | 650.0 ± 38.0 C | 730.6 ± 12.0 A | 705.2 ± 20.2 AB | 679.9 ± 20.7 BC | |
| Total EAA | 173.0 ± 7.38 AB | 185.0 ± 10.5 A | 158.9 ± 5.25 B | 168.1 ± 3.63 BC | |
| Total NEAA | 476.9 ± 31.9 B | 545.5 ± 14.6 A | 546.2 ± 18.2 A | 511.7 ± 22.0 AB | |
| EAA/NEAA | 0.36 ± 0.02 A | 0.34 ± 0.03 A | 0.29 ± 0.01 B | 0.33 ± 0.02 A | |
| EAAI | 65.50 ± 1.82 B | 66.65 ± 3.23 AB | 57.66 ± 1.51 C | 62.04 ± 0.62 B | |
| Limiting AA | Histidine | Valine | Histidine | Histidine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Dong, X.; Zamora, L.N.; Jeffs, A.G.; Quek, S.Y. Physicochemical Properties, Functionalities, and Antioxidant Activity of Protein Extracts from New Zealand Wild Sea Cucumbers (Australostichopus mollis). Foods 2024, 13, 2735. https://doi.org/10.3390/foods13172735
Wen Y, Dong X, Zamora LN, Jeffs AG, Quek SY. Physicochemical Properties, Functionalities, and Antioxidant Activity of Protein Extracts from New Zealand Wild Sea Cucumbers (Australostichopus mollis). Foods. 2024; 13(17):2735. https://doi.org/10.3390/foods13172735
Chicago/Turabian StyleWen, Yuan, Xuan Dong, Leonardo N. Zamora, Andrew G. Jeffs, and Siew Young Quek. 2024. "Physicochemical Properties, Functionalities, and Antioxidant Activity of Protein Extracts from New Zealand Wild Sea Cucumbers (Australostichopus mollis)" Foods 13, no. 17: 2735. https://doi.org/10.3390/foods13172735






