Basic Theory of Ice Crystallization Based on Water Molecular Structure and Ice Structure
Abstract
1. Introduction
2. Structure of Water Molecular Cluster
3. Mechanism of Ice Formation
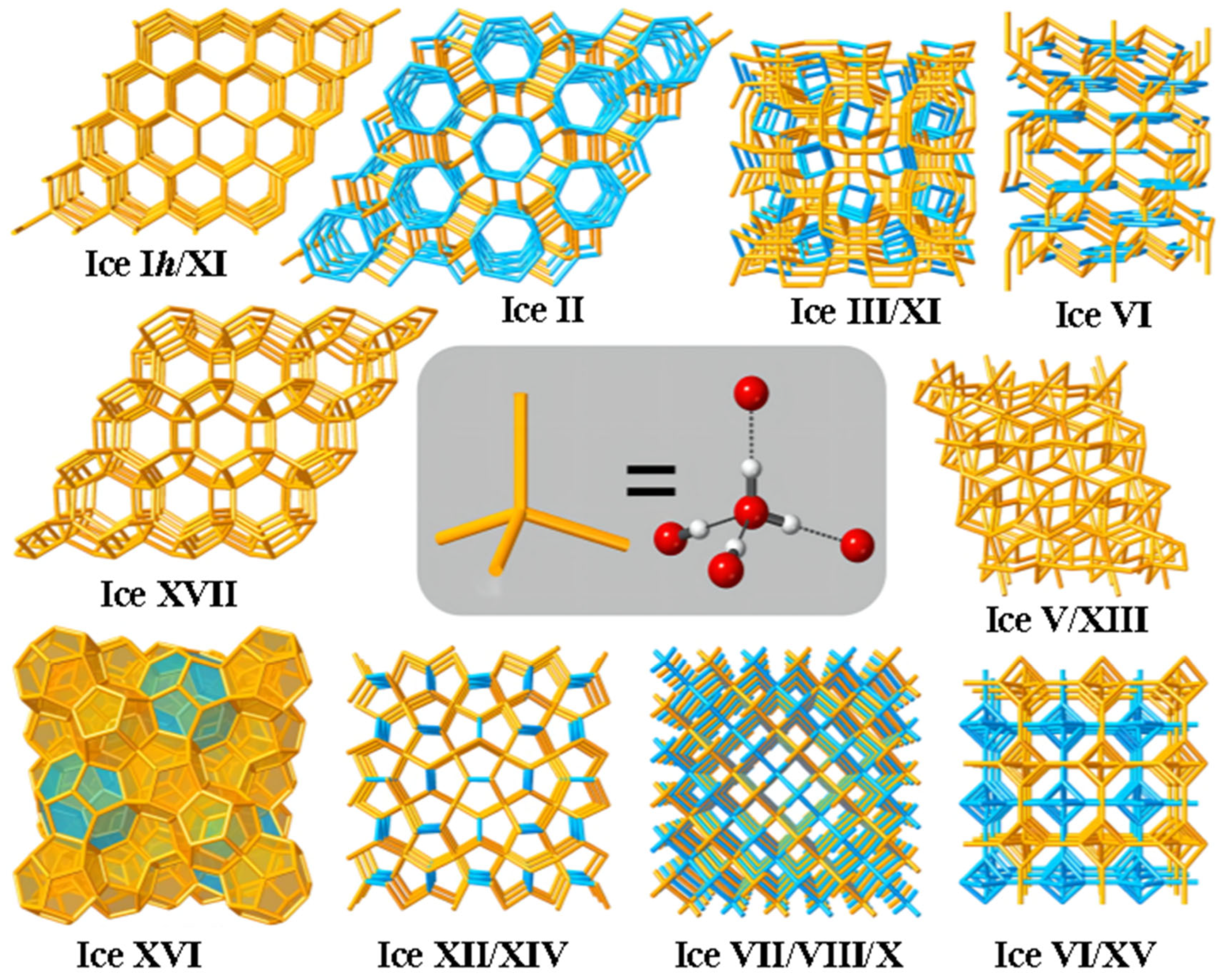
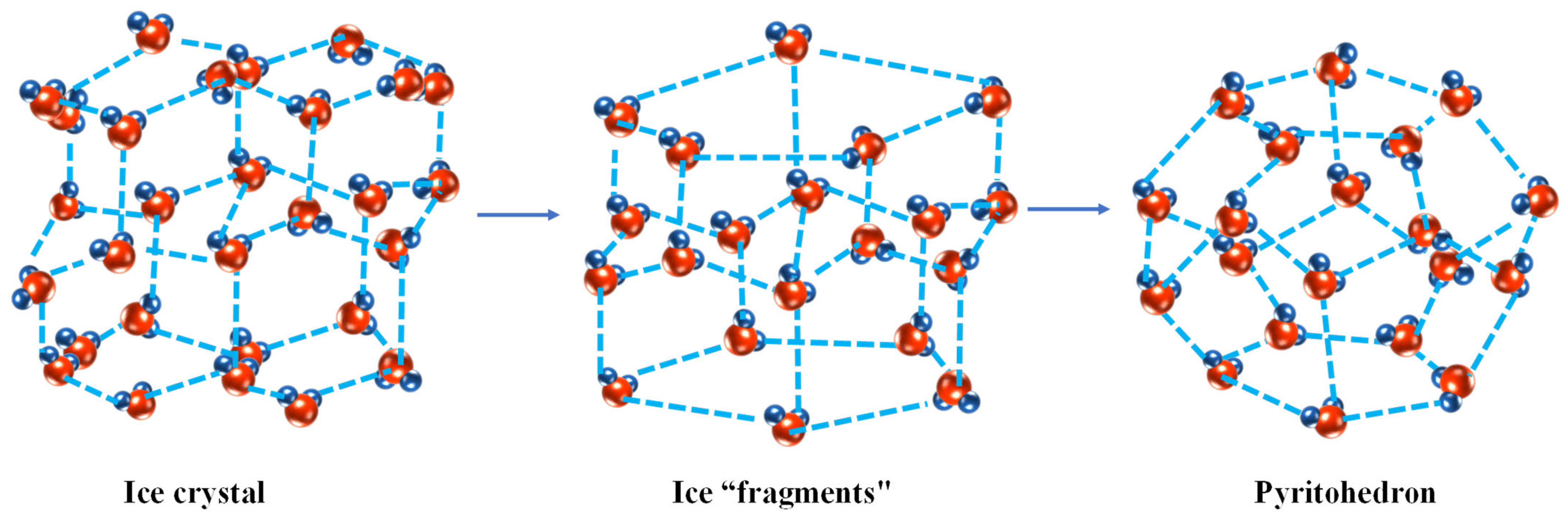
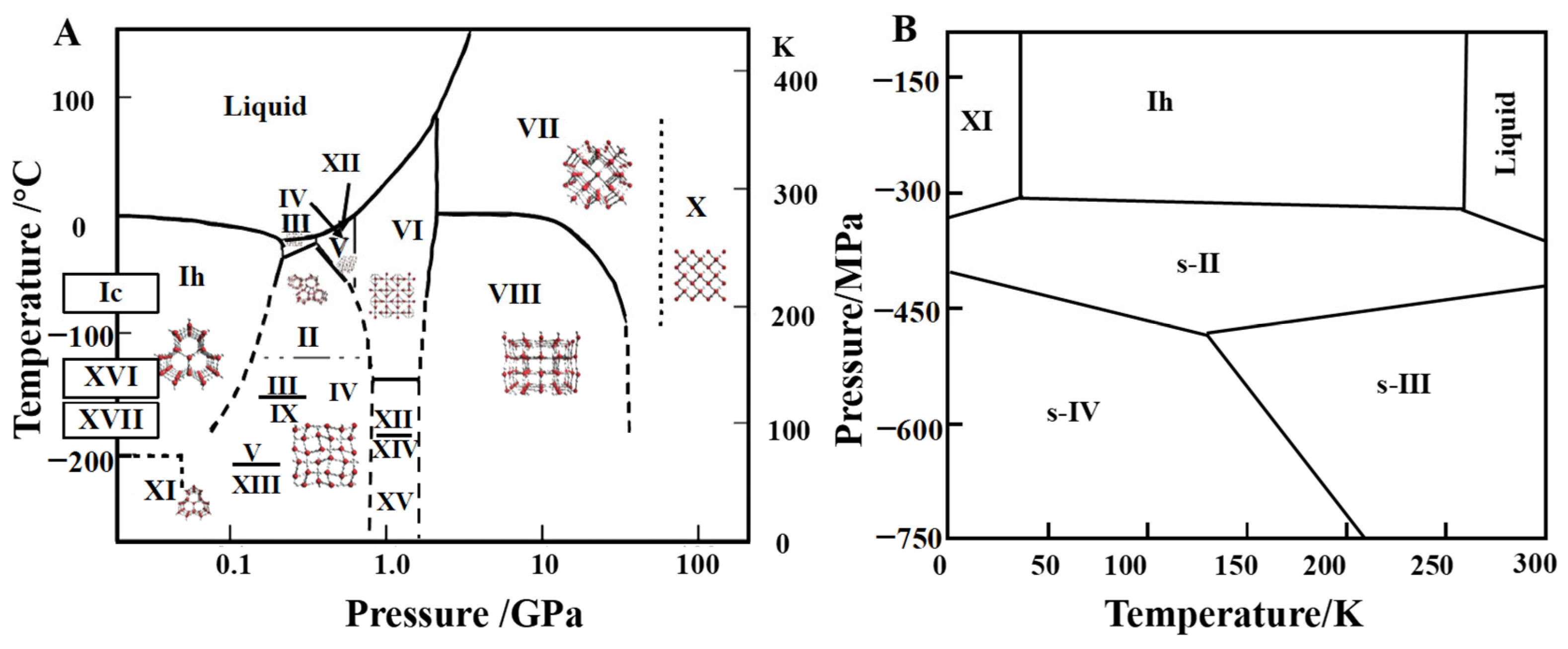
4. Structure of Ice Crystal
5. Characterization Methods for Ice Crystals
5.1. Light Microscopy
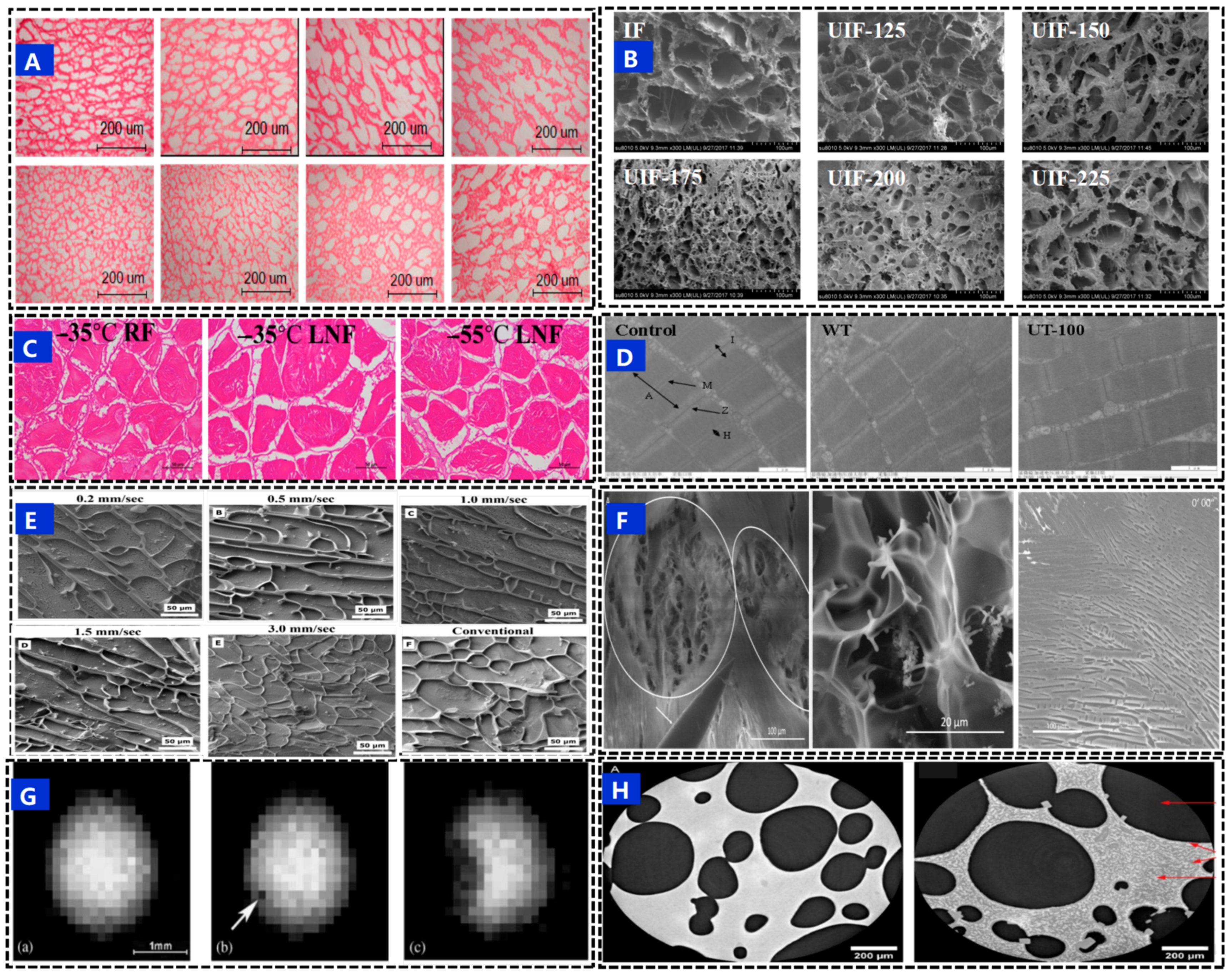
5.2. Electron Microscope
5.3. DSC
5.4. X-ray
5.5. Nuclear Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI)
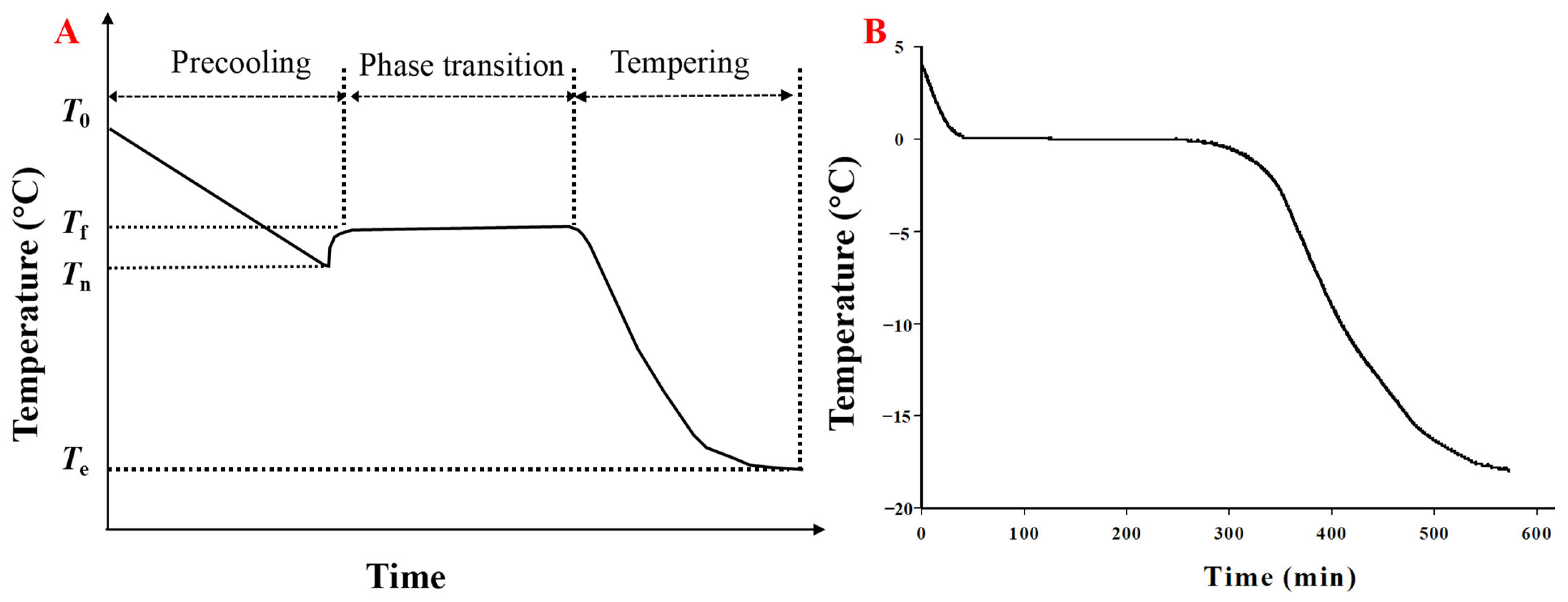
6. Modeling of Ice Crystal Formation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Saki, N.; Ghaffari, M.; Nikoo, M. Effect of active ice nucleation bacteria on freezing and the properties of surimi during frozen storage. LWT-Food Sci. Technol. 2023, 176, 114548. [Google Scholar] [CrossRef]
- Sabikun, N.; Bakhsh, A.; Ismail, I.; Hwang, Y.H.; Joo, S.T. Changes in physicochemical characteristics and oxidative stability of pre- and post-rigor frozen chicken muscles during cold storage. J. Food Sci. Technol. 2019, 56, 4809–4816. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, J.; Chen, X.; Zhang, Q.; Cai, X.; Ding, Y.; Zhou, X.; Wang, S. Dual cryoprotective strategies for ice-binding and stabilizing of frozen seafood: A review. Trends Food Sci. Technol. 2021, 111, 223–232. [Google Scholar] [CrossRef]
- Tan, M.; Mei, J.; Xie, J. The formation and control of ice crystal and its impact on the quality of frozen aquatic products: A review. Crystals 2021, 11, 68. [Google Scholar] [CrossRef]
- Otero, L.; Rodriguez, A.C.; Perez-Mateos, M.; Sanz, P.D. Effects of magnetic fields on freezing: Application to biological products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 646–667. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Jha, P.K.; Tavakoli, J.; Daraei-Garmakhany, A.; Xanthakis, E.; Le-Bail, A. Review on identification, underlying mechanisms and evaluation of freezing damage. J. Food Eng. 2019, 255, 50–60. [Google Scholar] [CrossRef]
- Kiani, H.; Sun, D.W. Water crystallization and its importance to freezing of foods: A review. Trends Food Sci. Technol. 2011, 22, 407–426. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Q.; Sun, D.W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, H.; Yang, X.Z.L. Development of low immunogenic antifreeze peptides for cryopreservation. Ind. Eng. Chem. Res. 2023, 62, 12063–12072. [Google Scholar] [CrossRef]
- Stebel, M.; Smolka, J.; Palacz, M.; Eikevik, T.M.; Tolstorebrov, I. Numerical modelling of conjugate heat and mass transfer during hydrofluidisation food freezing in different water solutions. Innov. Food Sci. Emerg. 2022, 75, 102898. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, W.; Sun, D.W. Effects of liquid nitrogen quick freezing on polyphenol oxidase and peroxide activities, cell water states and epidermal microstructure of wolfberry. LWT-Food Sci. Technol. 2020, 120, 108923. [Google Scholar] [CrossRef]
- Jia, G.; Chen, Y.; Sun, A.; Orlien, V. Control of ice crystal nucleation and growth during the food freezing process. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2433–2454. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.N.; English, N.J. Vibrational, energetic-dynamical and dissociation properties of water clusters in static electric fields: Non-equilibrium molecular-dynamics insights. Chem. Phys. Lett. 2018, 710, 207–214. [Google Scholar] [CrossRef]
- El-Sisi, A.S. Impact of replacement of gelatin with chitosan on the physicochemical properties of ice-milk. Int. J. Dairy Sci. 2015, 10, 36–43. [Google Scholar] [CrossRef]
- Suck, S.H.; Wetmore, A.E.; Chen, T.S.; Kassner, J.L. Role of various water clusters in IR absorption in the 8–14-m window region. Appl. Opt. 1982, 21, 1610–1614. [Google Scholar] [CrossRef]
- Michalarias, I.; Gao, X.L.; Ford, R.C.; Li, J.C. Recent progress on our understanding of water around biomolecules. J. Mol. Liq. 2005, 117, 107–116. [Google Scholar] [CrossRef]
- Xantheas, S.S.; Dunning, T.H. Abinitio studies of cyclic water clusters (H2O)n, n = 1–6. I. Optimal structures and vibrational spectra. J. Chem. Phys. 1993, 99, 8774–8792. [Google Scholar] [CrossRef]
- Tian, J.; Walayat, N.; Ding, Y.T.; Liu, J.H. The role of trifunctional cryoprotectants in the frozen storage of aquatic foods: Recent developments and future recommendations. Compr. Rev. Food Sci. Food Saf. 2022, 21, 321–339. [Google Scholar] [CrossRef]
- Du, X.; Wang, B.; Li, H.J.; Liu, H.T.; Shi, S.; Feng, J.; Pan, N.; Xia, X.F. Research progress on quality deterioration mechanism and control technology of frozen muscle foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4812–4846. [Google Scholar] [CrossRef]
- Sutariya, S.G.; Sunkesula, V. Food freezing: Emerging techniques for improving quality and process efficiency a comprehensive review. Innov. Food Sci. Emerg. 2021, 36–63. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F.; Marx, J.C.; Gerday, C. The climate of snow and ice as boundary condition for microbial life. Psychrophiles: From Biodiversity to Biotechnology; Springer: Berlin, Germany, 2008; Chapter 1; pp. 3–15. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.A.; Xu, Y.; Dong, J. A review on supercooling of phase change materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Kang, T.; Shafel, T.; Lee, D.; Lee, C.J.; Lee, S.H.; Jun, S. Quality retention of fresh tuna stored using supercooling technology. Foods 2020, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Ickes, L.; André, W.; Hoose, C.; Lohmann, U. Classical nucleation theory of homogeneous freezing of water: Thermodynamic and kinetic parameters. Phys. Chem. Chem. Phys. 2015, 17, 5514–5537. [Google Scholar] [CrossRef]
- Kasper, J.C.; Friess, W. The freezing step in lyophilization: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur. J. Pharm. Biopharm. 2011, 78, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, D.; Lee, A.Y.; Myerson, A.S. Nucleation of crystals from solution: Classical and two-step models. Acc. Chem. Res. 2009, 42, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.; Zhu, Z.; Sun, D.W. Glass transitions as affected by food compositions and by conventional and novel freezing technologies: A review. Trends Food Sci Technol. 2019, 94, 1–11. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, X.; Zhang, C.; Xia, X.; Sun, F.; Kong, B. Ultrasound-assisted immersion freezing accelerates the freezing process and improves the quality of common carp (Cyprinus carpio) at different power levels. LWT-Food Sci. Technol. 2019, 108, 106–112. [Google Scholar] [CrossRef]
- Zhao, J.H.; Kumar, P.K.; Sablani, S.S. Glass transitions in frozen systems as influenced by molecular weight of food components. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4683–4715. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Wang, L.; Qian, H.; Qi, X.; Xiao, J. Effect of barley antifreeze protein on thermal properties and water state of dough during freezing and freeze-thaw cycles. Food Hydrocoll. 2015, 47, 32–40. [Google Scholar] [CrossRef]
- Libbrecht, K.G. Physical dynamics of ice crystal growth. Annu. Rev. Mater. Res. 2017, 47, 271–295. [Google Scholar] [CrossRef]
- Bartels-Rausch, T.; Bergeron, V.; Cartwright, J.H.E.; Escribano, R.; Finney, J.L.; Grothe, H.; Gutierrez, P.J.; Haapala, J.; Kuhs, W.F.; Pettersson, J.B.C.; et al. Ice structures, patterns, and processes: A view across the ice-fields. Rev. Mod. Phys. 2012, 84, 885–944. [Google Scholar] [CrossRef]
- Komatsu, K.; Machida, S.; Noritake, F.; Hattori, T.; Kagi, H. Ice Ic without stacking disorder by evacuating hydrogen from hydrogen hydrate. Nat. Commun. 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.J.; Bertram, A.K. Formation and stability of cubic ice in water droplets. Phys. Chem. Chem. Phys. 2006, 8, 186–192. [Google Scholar] [CrossRef]
- Malkin, T.L.; Murray, B.J.; Brukhno, A.V.; Anwar, J.; Salzmann, C.G. Structure of ice crystallized from supercooled water. Proc. Natl. Acad. Sci. USA 2012, 109, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, C. Advances in the experimental exploration of water’s phase diagram. J. Chem. Phys. 2019, 150, 060901. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Rosu-Finsen, A.; Sharif, Z.; Radaelli, P.G.; Finney, J.L. Detailed crystallographic analysis of the ice v to ice xiii hydrogen-ordering phase transition. J. Chem. Phys. 2021, 154, 134504. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Kishi, K.; Otosu, T.; Yagasaki, T.; Yamaguchi, S. Experimental and theoretical Raman spectroscopy of isotopically pure and diluted ice VI. J. Phys. Chem. C 2022, 126, 17359–17365. [Google Scholar] [CrossRef]
- Trybel, F.; Cosacchi, M.; Meier, T.; Axt, V.M.; Steinle-Neumann, G. Proton dynamics in high-pressure ice-VII from density functional theory. Phys. Rev. B 2021, 102, 184310. [Google Scholar] [CrossRef]
- Kosyakov, V.I.; Shestakov, V.A. On the possibility of the existence of a new ice phase under negative pressures. Dokl. Phys. Chem. 2001, 376, 49–51. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, X.; Kong, B.; Sun, Q.; Ji, H.; Liu, S. Effects of magnetic field-assisted immersion freezing at different magnetic field intensities on the muscle quality of golden pompano (Trachinotus ovatus). Food Chem. 2023, 407, 135092. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, F.; Xia, X.; Xu, H.; Kong, B. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason. Sonochem. 2019, 51, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 85, 10–18. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Sun, Q.; Zheng, O.; Wei, S.; Xia, Q.; Ji, H.; Deng, C.; Hao, J.; Xu, J. Insight into muscle quality of golden pompano (Trachinotus ovatus) frozen with liquid nitrogen at different temperatures. Food Chem. 2022, 374, 131737. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Hu, Y.Q.; Takaki, K.; Yu, H.X.; Yuan, C.H. Effect of water ice-glazing on the quality of frozen swimming crab (Portunus trituberculatus) by liquid nitrogen spray freezing during frozen storage. Int. J. Refrig. 2021, 131, 1010–1015. [Google Scholar] [CrossRef]
- Sun, Q.; Kong, B.; Liu, S.; Zheng, O.; Zhang, C. Ultrasound-assisted thawing accelerates the thawing of common carp (Cyprinus carpio) and improves its muscle quality. LWT-Food Sci. Technol. 2021, 141, 111080. [Google Scholar] [CrossRef]
- Gillis, J.D.; Holt, W.V.; Penfold, L.M.; Woad, K.J.; Graham, J.K.; Watts, J.A.; Gardner, D.S.; Yon, L. Cryo-scanning electron microscopy demonstrates that ice morphology is not associated with the post-thaw survival of domestic boar (Sus domesticus) spermatozoa: A comparison of directional and conventional freezing methods. Cryobiology 2022, 108, 10–18. [Google Scholar] [CrossRef]
- Vetráková, U.; Neděla, V.; Runtuk, J.; Tihlaíková., E.; Shalaev, E. Dynamical in-situ observation of the lyophilization and vacuum-drying processes of a model biopharmaceutical system by an environmental scanning electron microscope. Int. J. Pharmaceut. 2020, 585, 119448. [Google Scholar] [CrossRef]
- Hindmarsh, J.P.; Buckley, C.; Russell, A.B.; Chen, X.D.; Gladden, L.F.; Wilson, D.I.; Johns, M.L. Imaging droplet freezing using MRI. Chem. Eng. Sci. 2004, 59, 2113–2122. [Google Scholar] [CrossRef]
- Zennoune, A.; Latil, P.; Flin, F.; Perrin, J.; Weitkamp, T.; Scheel, M.; Geindreau, C.; Benkhelifa, H.; Ndoye, F.T. Investigating the influence of freezing rate and frozen storage conditions on a model sponge cake using synchrotron X-rays micro-computed tomography. Food Res Int. 2022, 162 Pt B, 112116. [Google Scholar] [CrossRef]
- Efthymiou, C.; Williams, M.A.K.; McGrath, K.M. Revealing the structure of high-water content biopolymer networks: Diminishing freezing artefacts in cryo-SEM images. Food Hydrocolloid. 2017, 73, 203–212. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Wang, X.; Zheng, S. Thermal analysis of melting and freezing processes of phase change materials (PCMs) based on dynamic DSC test. Energ. Build. 2016, 130, 388–396. [Google Scholar] [CrossRef]
- Xu, B.G.; Zhang, M.; Bhandari, B.; Cheng, X.F.; Islam, M.N. Effect of ultrasound-assisted freezing on the physico-chemical properties and volatile compounds of red radish. Ultrason. Sonochem. 2015, 27, 316–324. [Google Scholar] [CrossRef]
- Bainy, E.M.; Corazza, M.L.; Lenzi, M.K. Measurement of freezing point of tilapia fish burger using differential scanning calorimetry (DSC) and cooling curve method. J. Food Eng. 2015, 161, 82–86. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, C.; Zhao, J.; Ouyang, Q. Recent advances in emerging imaging techniques for non-destructive detection of food quality and safety. TrAC-Trend Anal. Chem. 2013, 52, 261–274. [Google Scholar] [CrossRef]
- Quirine, K.; Henning, L. Upscaling ice crystal growth dynamics in snow: Rigorous modeling and comparison to 4D X-ray tomography data. Acta Mater. 2018, 151, 478–487. [Google Scholar]
- Dai, C.; Zhou, X.; Zhang, S.; Zhou, N. Influence of ultrasound-assisted nucleation on freeze-drying of carrots. Dry. Technol. 2016, 34, 1196–1203. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, Q.; Chen, Q.; Liu, Q.; Kong, B. Effectiveness of ultrasound-assisted immersion thawing on the thawing rate and physicochemical properties of chicken breast muscle. J. Food Sci. 2021, 86, 1692–1703. [Google Scholar] [CrossRef]
- Mahdjoub, R.; Chouvenc, P.; Seurin, M.J.; Andrieu, J.; Briguet, A. Sucrose solution freezing studied by magnetic resonance imaging. Carbohydr. Res. 2006, 341, 492–498. [Google Scholar] [CrossRef]
- Fan, A.D. A Monte Carlo simulation of solidification structure formation under melt shearing. Mat. Sci. Eng. A 2004, 365, 330–335. [Google Scholar]
- He, Z.Z.; Liu, J. Modeling ice crystal formation of water in biological system. In Advanced Topics on Crystal Growth; Ferreira, S.O., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Handel, R.; Davidchack, R.L.; Anwar, J.; Brukhno, A. Direct calculation of solid-liquid interfacial free energy for molecular systems: TIP4P ice-water interface. Phys. Rev. Lett. 2008, 100, 036104–036107. [Google Scholar] [CrossRef]
- Singla, S.; Anim-Danso, E.; Islam, A.E.; Ngo, Y.; Dhinojwala, A. Insight on structure of water and ice next to graphene using surface-sensitive spectroscopy. ACS Nano 2017, 11, 4899–4906. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Ohto, T.; Sun, S.; Rouxel, J.R.; Imoto, S.; Backus, E.H.G.; Mukamel, S.; Bonn, M.; Nagata, Y. Molecular structure and modeling of water-air and ice-air interfaces monitored by sum-frequency generation. Chem. Rev. 2020, 120, 3633–3667. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H. Hydrogen bonds between water molecules: Thermal expansivity of ice and water. J. Mol. Liq. 2001, 90, 323–332. [Google Scholar] [CrossRef]

| Method | Sample Processing | Observation Content | Summary of Characteristics | |
|---|---|---|---|---|
| Light microscopy | Frozen section | Freeze substitution or freeze drying | Fractal dimension, ice crystal diameter, ice crystal roundness, elongation, ice crystal area. | Indirectly observing the sizes, shapes, and distribution of ice crystals by observing the surface of the sample |
| Paraffin section | Samples often need to be fixed, dehydrated, waxed, embedded, sliced, and stained | Tissue structure of samples | Indirectly characterizing the effect of ice crystals on food | |
| Electron microscope | Scanning electron microscopy (SEM) | Sample always needs to be fixed, dehydrated, freeze-dried, and sprayed with gold. Freeze drying can be used instead of fixing, dehydration, and freeze drying | Surface microstructure of samples, or the size of ice crystals in frozen food | SEM can provide fine information that light microscope cannot display. Unlike TEM, SEM does not have tight requirements for sample thickness, and sample preparation is simpler. |
| Cryo-electron microscopy | No need for sample preparation | Interior three-dimensional structure of wet samples at low temperature or the nucleation temperature, sizes, shapes, and positions of ice crystals in food and the mechanical effects of ice crystals. | ||
| Environmental SEM (ESEM) | No need for sample preparation and conductive pre-treatment | Ice crystals in freeze-dried samples | ||
| Transmission electron microscopy (TEM) | Pre-treatment of TEM samples is tedious, including sample fixation, polymerization, and staining pre-treatment | Internal structure of frozen food | The sample preparation process is complex, indirectly reflecting the role of ice crystals by collecting refined information within the sample | |
| DSC | No need for sample preparation | Amount of freezable and non-freezable water, freezing point, and associated enthalpy | Indirectly reflecting the impact of ice crystals on food through changes in freezing parameters | |
| X-ray | No need for sample preparation. It can be imaged directly in a frozen state or freeze-dried before imaging | Used for monitoring ice crystals in frozen foods or studying the pores formed by ice crystals after freeze drying | Although X-ray imaging is an accurate method to determine the morphology, size distribution, and volume fraction of ice crystals, it does not show the structural details, and freeze drying can lead to the destruction of cell structure through shrinkage. | |
| Nuclear magnetic resonance | No need for sample preparation | The state of water, including bound water, free water, and immobilized water | Indirectly characterizing the effect of ice crystals on food structure through changes in moisture state | |
| Magnetic resonance imaging | No need for sample preparation | The spatial distribution status of water | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, O.; Zhang, L.; Sun, Q.; Liu, S. Basic Theory of Ice Crystallization Based on Water Molecular Structure and Ice Structure. Foods 2024, 13, 2773. https://doi.org/10.3390/foods13172773
Zheng O, Zhang L, Sun Q, Liu S. Basic Theory of Ice Crystallization Based on Water Molecular Structure and Ice Structure. Foods. 2024; 13(17):2773. https://doi.org/10.3390/foods13172773
Chicago/Turabian StyleZheng, Ouyang, Li Zhang, Qinxiu Sun, and Shucheng Liu. 2024. "Basic Theory of Ice Crystallization Based on Water Molecular Structure and Ice Structure" Foods 13, no. 17: 2773. https://doi.org/10.3390/foods13172773
APA StyleZheng, O., Zhang, L., Sun, Q., & Liu, S. (2024). Basic Theory of Ice Crystallization Based on Water Molecular Structure and Ice Structure. Foods, 13(17), 2773. https://doi.org/10.3390/foods13172773







