A Review on Fish Skin-Derived Gelatin: Elucidating the Gelatin Peptides—Preparation, Bioactivity, Mechanistic Insights, and Strategies for Stability Improvement
Abstract
:1. Introduction
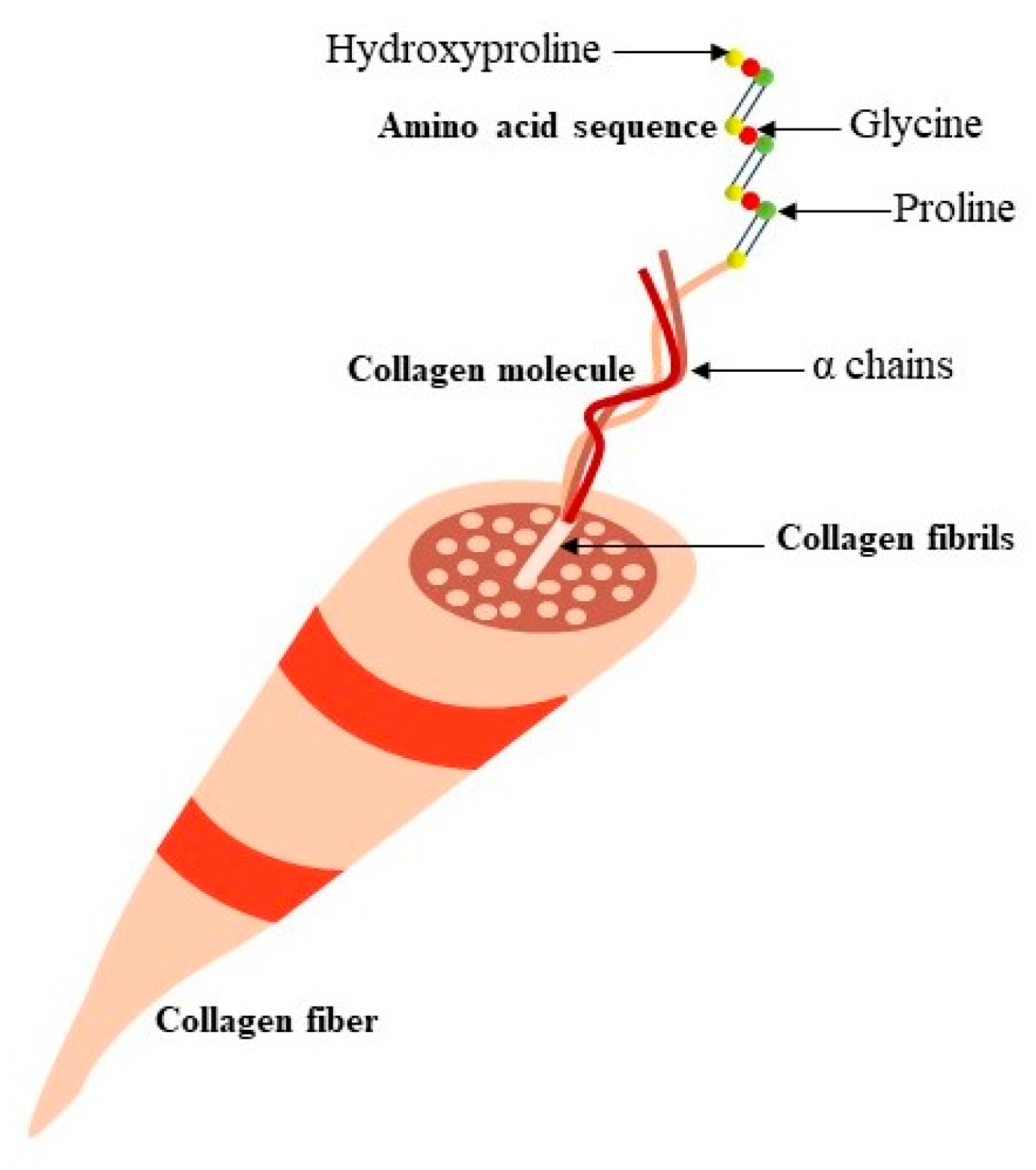
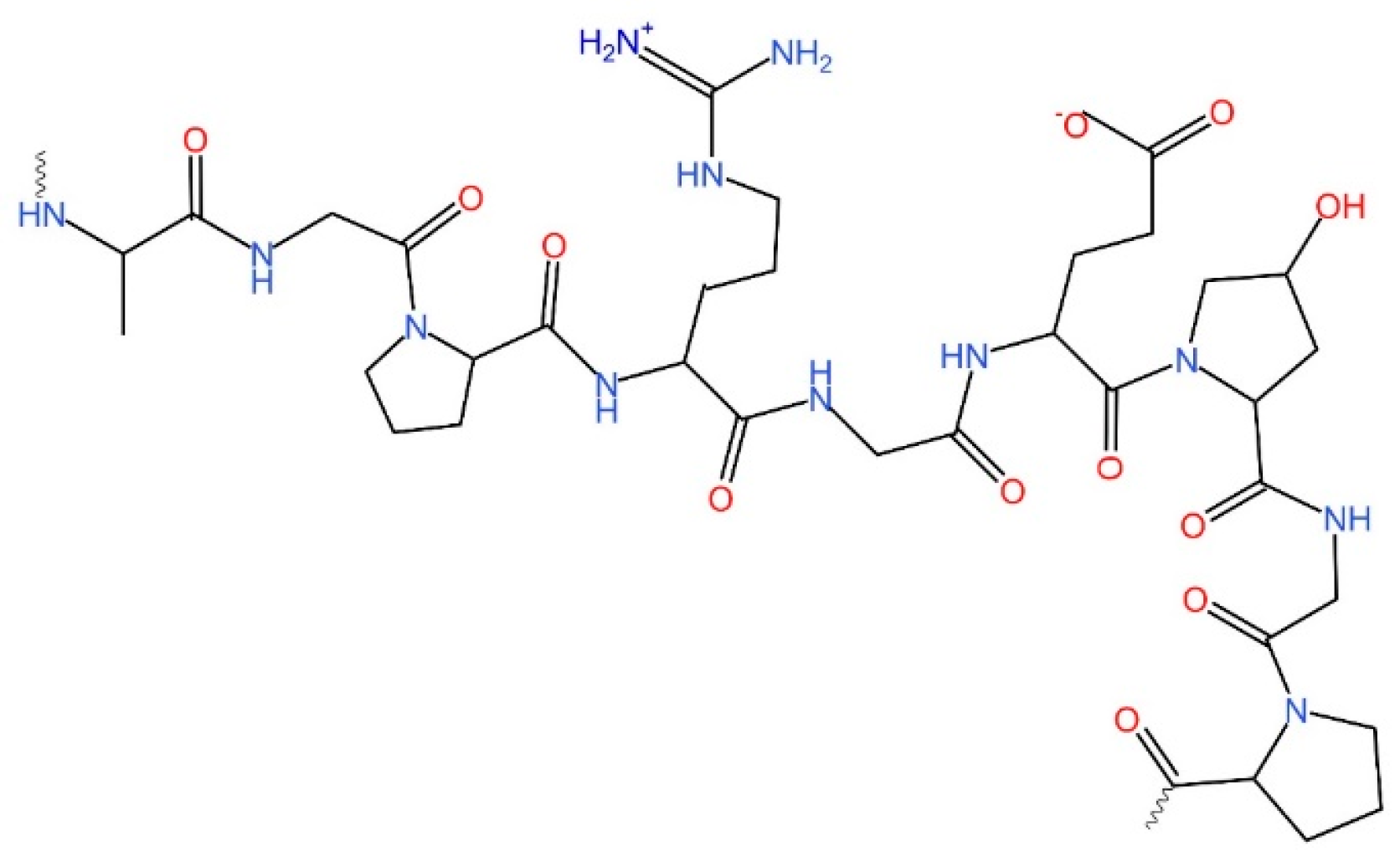
2. Collagen: The Precursor Molecule to Gelatin
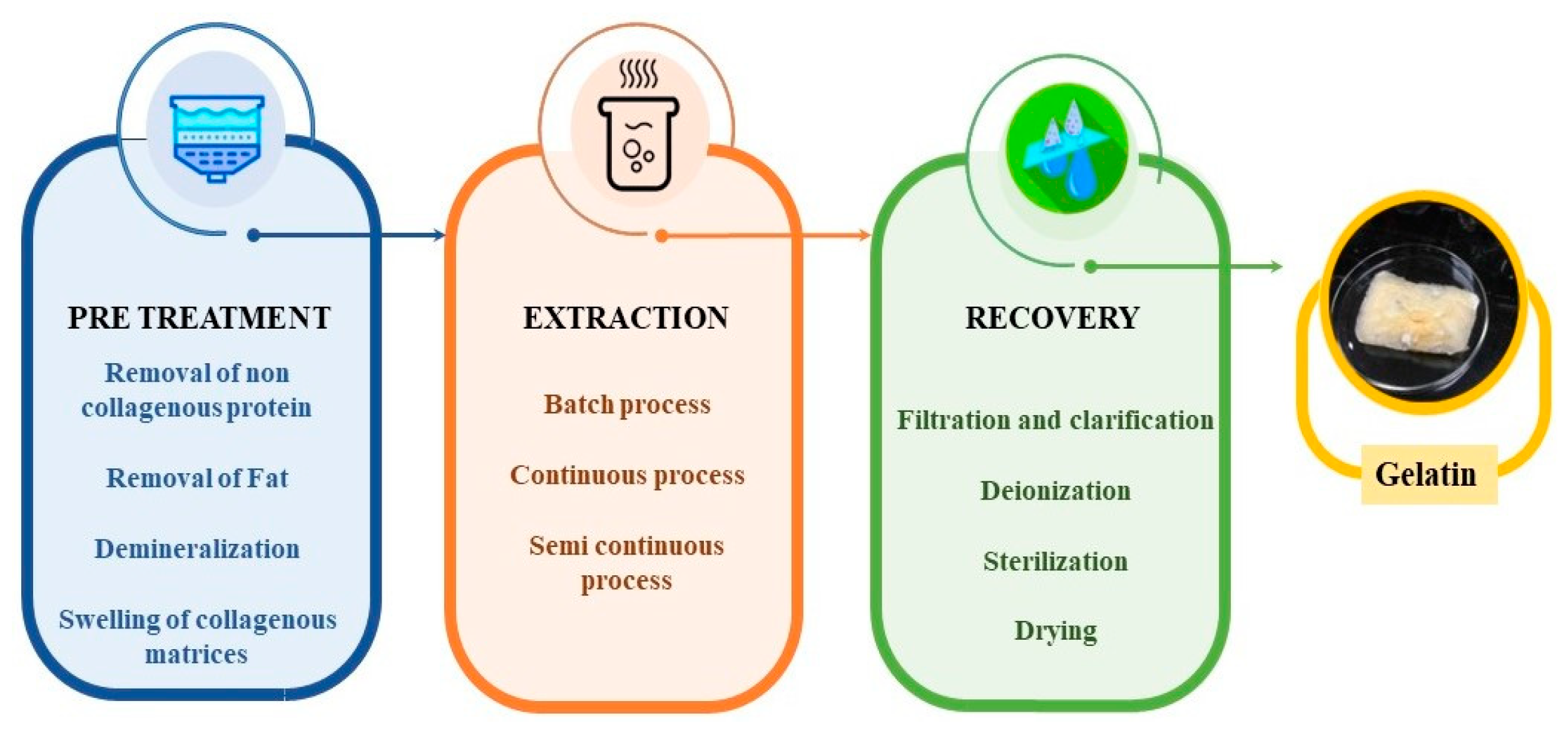
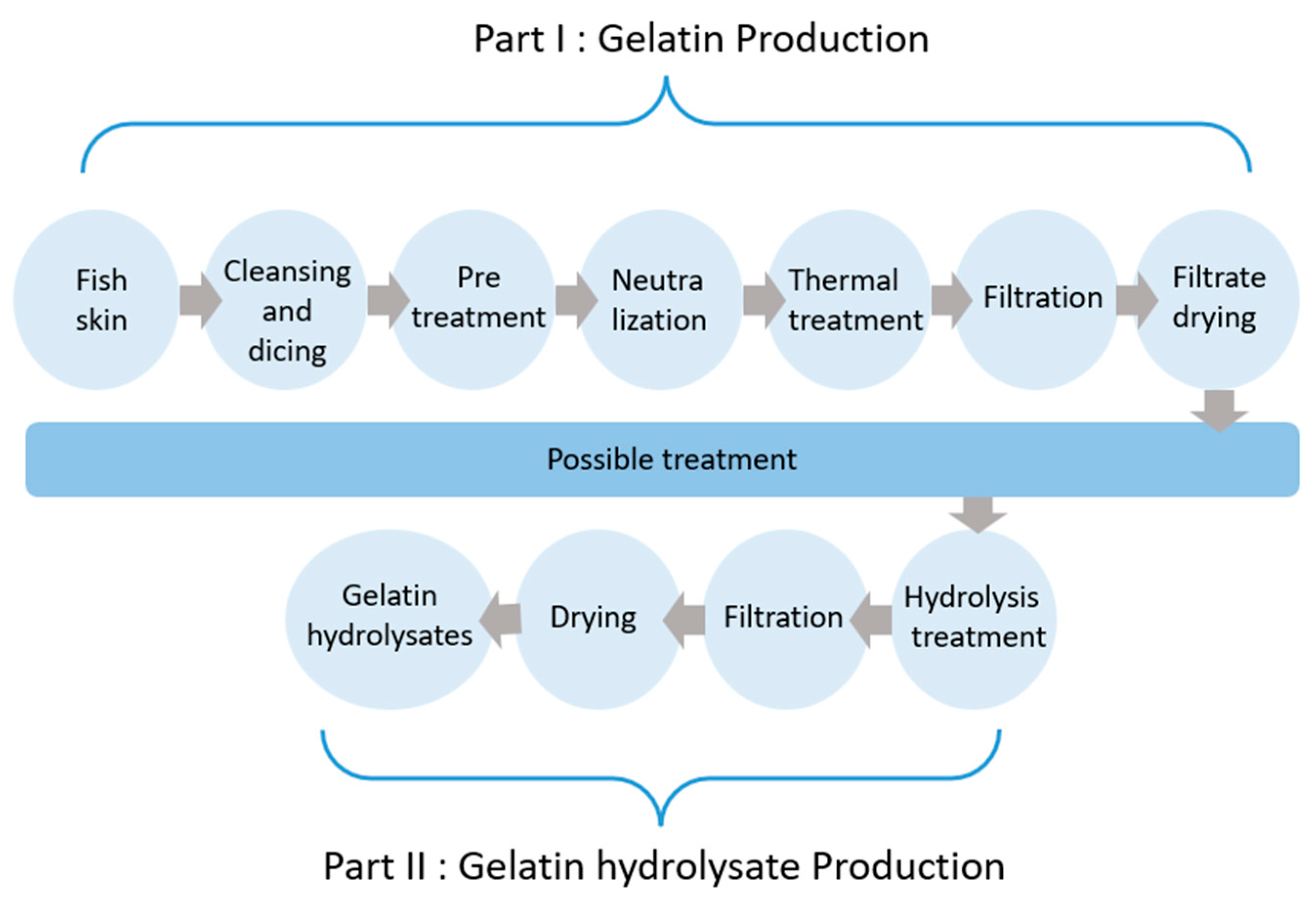
3. Collagen-to-Gelatin Extraction Protocol
4. Rationale behind the Search for Alternatives to Commercial Gelatin
5. Exploring Fish Gelatin as a Feasible Replacement for Mammalian Gelatin
6. Characteristics of Fish Gelatin
6.1. Physical Attributes
- a.
- Melting and gelling temperature: Gelatin creates and maintains stable three-dimensional gels through hydrogen bonds with water [51]. The temperatures at which gelatin melts and gels are directly related to the levels of proline and hydroxyproline present in the initial collagen [11]. These specific amino acids significantly influence the thermal properties of gelatin, determining how it behaves under different temperature conditions. Higher concentrations of proline and hydroxyproline typically result in gelatin with higher melting and gelling points, reflecting the stability and structural integrity derived from the original collagen source. The key difference between mammal and fish gelatins lies in their gelation temperatures. Mammal gelatin has about 30%, warm-water fish gelatin has 22–25%, and cold-water fish gelatin has about 17% proline and hydroxyproline. Cold-water fish gelatin has lower melting and gelling points due to the lower proline and hydroxyproline content, as in the case of cod fish, which reduces the propensity for intermolecular helix formation. Warm-water fish gelatin like that from tilapia and Nile perch behaves like mammalian gelatin due to the comparable proline and hydroxyproline content. For instance, Nile perch (Lates niloticus) skin has the potential to serve as an excellent source of gelatin due to its rich imino acid content, which imparts strong gelling properties. Research by Norziah et al. [52] found cod skin gelatin had lower melting points than bovine and shortfin scad gelatins. The ability of gelatin to melt below body temperature is advantageous for the food industry, which improves the flavor release during consumption and influences the sensory experience in food products. The study by Choi and Regenstein [53] demonstrated that the decreased melting point of cold-water fish gelatin contributes to an improved flavor release, enhanced fruit aroma, and faster melt rate in water-based gel desserts.
- b.
- Gel strength: An important physical property of gelatin is its gel strength, which is affected by several factors, including the molecular weight and complex interactions determined by the amino acid composition and the ratio of α/β-chains within the gelatin matrix [54]. Schrieber and Gareis [55] detailed that gel strength largely depends on the concentration of components with molecular weights around 100,000 g/mol. Moreover, gelatin with a higher content of α-chains generally exhibits greater gel strength. In contrast, an increased presence of peptides with molecular weights either significantly above or below those of α-chains tends to reduce the gel strength [56]. The gelation strength of commercial gelatins is quantified through the bloom values. The higher the bloom value, the stronger the gel. The bloom value can be categorized into three groups: high (220 to 300 bloom), medium (150 to 220 bloom), or low (<150 bloom). Furthermore, gelatin possessing a higher viscosity is deemed more commercially desirable and, as a result, commands a premium price in the market [57]. Yellowfin tuna skin is considered a suitable raw material for producing gelatin with high gel strength [58]. Table 2 illustrates the diverse functional attributes of gelatin-based food products as determined by their gel strength.
- c.
- Viscosity: Viscosity is a crucial functional characteristic for process control. A gelatin solution’s ability to gel depends on the temperature and the viscosity of the gelatin in the water [60]. The species of fish, habitat, origin, and amino acid content are a few of the variables that might affect whether fish gelatin has gelling or non-gelling qualities [3]. The viscosity of gelatin derived from cold-water fish is very high, which may not be appropriate for numerous applications [61]. In contrast to the gel strength, the viscosity demonstrates a weaker correlation with textural characteristics. The molecular weight distribution predominantly influences the viscosity. Gelatin samples characterized by substantial molecular weight fractions yield elevated viscosity; however, this does not inevitably translate to equally high gel strength [61].
- d.
- pH: The pH is an important physical parameter that influences the quality of gelatin. The production process influences the pH of gelatin, and this pH range determines the types of gelatins:
- e.
- Thermoreversibility: Gelatin falls into the group of physical gels involving Van der Waals forces and hydrogen bonds; the interactions or bonds between the constituent chains are primarily of a physical nature, with an energy of approximately 2 Kcal/mol. The bonding energy within gelatin is relatively modest, which enables gelatin to form thermoreversible gels. When compared to other gel-forming substances like proteins and polysaccharides, gelatin has greater thermoreversibility due to the non-random presence of imino acids (such as proline or hydroxyproline) [64,65,66]. This implies that it achieves softness, transforms into a liquid state, and also reverses to a gel-like consistency upon cooling.
- f.
- Texture: Gelatin is vitreous in gel form, with a taste and smell that are nearly imperceptible. Its dried appearance ranges from pale yellow to white. It is offered in a variety of physical configurations, including sheets, fine powders, and coarse granules [1].
- g.
- Stability: Gelatin remains stable for at least five years when stored [67] properly in sealed containers under typical storage conditions to prevent moisture changes. The bloom value can vary slightly due to modest changes in the moisture levels. These bloom variations, nevertheless, may be precisely computed from the moisture content and do not signify a change in the underlying intensity or quality of the bloom [68].
6.2. Chemical Attributes
- Amino acid composition: The amino acid content significantly affects the melting and setting temperatures of gelatin derived from various sources. Proline and hydroxyproline exert notable impacts on both the gel strength and the temperature at which gelling occurs [67]. Furthermore, the levels of aspartic acid, glutamic acid, arginine, histidine, lysine, and hydroxylysine are crucial for the formation of cross-links and electrostatic interactions [77].
- Peptide size and quality: Collagen tissues are treated with acid/alkali, heat, and water to disrupt the fibrils, yielding gelatin’s irreversibly. Gelatin comprises collagen fractions above 30 kDa; lower-molecular-weight forms are gelatin hydrolysates, aiding in gel formation. Heat at ~40 °C frees the α-chains via bond breakage in new collagen while the mature bonds of collagen endure. Higher heat breaks the covalent bonds, yielding smaller α-chains and varied weights. Diverse collagen amino acids cause random bond breaks, causing variability. Raw materials add impurities like proteins, lipids, and minerals; the water in commercial gelatin (9–14%) varies based on the production and storage.
7. Versatile Uses and Applications of Gelatin
7.1. Food Industry
7.2. Pharmaceutical and Medical Industry
7.3. Nutritional and Health Benefits
8. Challenges Associated with Fish-Derived Gelatin
9. Enhancing Fish Gelatin: Solutions for Its Limitations
- Developing composite gelation systems involves blending fish gelatin with high-bloom gelatins, as substantiated by the research conducted by P. Gilsenan [106], P. M. Gilsenan and Ross-Murphy [107] and Zhou et al. [108]. Alternatively, these systems can incorporate appropriate plant hydrocolloids, as exemplified by Haug et al. [109] and Pranoto et al. [110]. These methods promise to augment the gel strength, improve the gelling properties, and elevate the melting temperature.
- Modifying the properties of gelatin through the introduction of solutes, such as various types of salts, as deliberated by Elysée-Collen and Lencki [111].
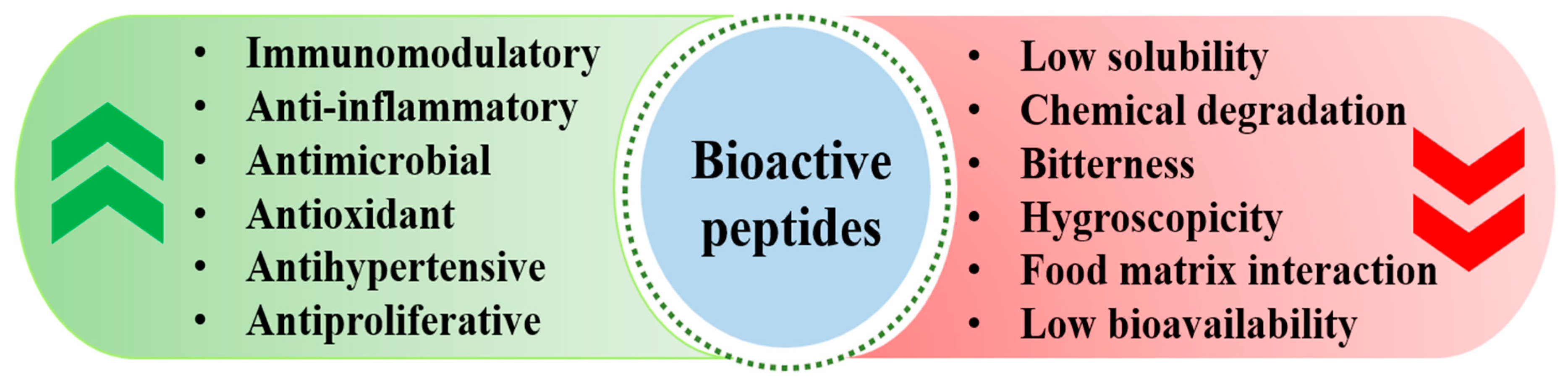
10. Bioactive Peptides Derived from Gelatin: Their Potential Efficacies
11. Production Methods for Fish Gelatin Hydrolysates and Peptides
- a.
- Ultrasonication
- b.
- Microwave pretreatment
- c.
- Hydrothermal pretreatment
- d.
- High pressure (HP)
- e.
- Autolysis
- f.
- Thermal Hydrolysis
- g.
- Bacterial Fermentation
- h.
- Enzymatic hydrolysis
- i.
- Subcritical water hydrolysis
12. Fish Gelatin Hydrolysates and Its Significance for Food and Biomedical Applications
13. Bio-Functionalities of Peptides Derived from Distinct Fish Skin Gelatin Sources
- a.
- Antioxidant
- b.
- Antihypertensive
- c.
- Antimicrobial
- d.
- Antidiabetic
14. Stability Improvement Strategies for Peptides
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Boran, G.; Regenstein, J.M. Fish Gelatin. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 60, pp. 119–143. ISBN 978-0-12-380944-5. [Google Scholar]
- Ozcan, Y.; Kurt, A.; Ozmen, D.; Toker, O.S. Gelatin Production from Turkey (Meleagris gallopavo) Skin as a New Source: From Waste to a Sustainable Food Gelling Agent. J. Sci. Food Agric. 2023, 103, 5511–5520. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Bhat, R. Gelatin Alternatives for the Food Industry: Recent Developments, Challenges and Prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Asiamah, E.; Atter, A.; Ofori, H.; Akonor, P.T.; Nketia, S.; Koivula, H.; Lee, Y.; Agyakwah, S. Effect of Seasonal Variation and Farming Systems on the Properties of Nile Tilapia Gelatin Extracted from Scales. Heliyon 2024, 10, e24504. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Kahtani, H.A.; Jaswir, I.; AbuTarboush, H.; Ismail, E.A. Extraction and Characterization of Gelatin from Camel skin (Potential Halal Gelatin) and Production of Gelatin Nanoparticles. Saudi J. Biol. Sci. 2020, 27, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Ramli, S.S.; Nizar, N.N.A.; Heng, J.Y.Y.; Karde, V.; Abidin, S.A.S.Z.; Taib, M.N. Gelatin Substitute. In Innovation of Food Products in Halal Supply Chain Worldwide; Elsevier: Amsterdam, The Netherlands, 2023; pp. 87–98. ISBN 978-0-323-91662-2. [Google Scholar]
- Rosli, N.S.M.; Ahmed, S.; Sarbon, N.M. Gelatin Alternative: Extractability and Functional and Bioactivity Properties. In Natural Gums; Elsevier: Amsterdam, The Netherlands, 2023; pp. 507–551. ISBN 978-0-323-99468-2. [Google Scholar]
- Alfaro, A.D.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Ahmad Anuar, N.A.; Tukiran, N.A.; Jamaludin, M.A. Gelatin in Halal Pharmaceutical Products. Malays. J. Syariah Law. 2023, 11, 64. [Google Scholar] [CrossRef]
- Joy, J.M.; Dara, P.K.; Amruth, P.; Jacob, M.R.; Dhandapani, N.; Mathew, S.; Anandan, R. Evaluation of the Physicochemical and Techno-Functional Properties of Gelatin Extracted from Fish Processing Waste. IJBSM 2023, 14, 733–742. [Google Scholar] [CrossRef]
- Alam, M.R.; Shahid, M.A.; Alimuzzaman, S.; Khan, A.N. Sources, Extractions and Applications of Bio-Maker Collagen—A Review. Biomed. Eng. Adv. 2022, 4, 100064. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Biochemie; Springer-Lehrbuch; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 978-3-662-08290-4. [Google Scholar]
- Boran, G.; Regenstein, J.M. Optimization of Gelatin Extraction from Silver Carp Skin. J. Food Sci. 2009, 74, E432–E441. [Google Scholar] [CrossRef]
- Ames, W.M. The Conversion of Collagen to Gelatin and Their Molecular Structures. J. Sci. Food Agric. 1952, 3, 454–463. [Google Scholar] [CrossRef]
- Farooq, S.; Ahmad, M.I.; Zheng, S.; Ali, U.; Li, Y.; Shixiu, C.; Zhang, H. A Review on Marine Collagen: Sources, Extraction Methods, Colloids Properties, and Food Applications. Collagen Leather 2024, 6, 11. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Kishimura, H.; Benjakul, S. Physicochemical and Molecular Properties of Gelatin from Skin of Golden carp (Probarbus jullieni) as Influenced by Acid Pretreatment and Prior-Ultrasonication. Food Hydrocoll. 2018, 82, 164–172. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Fu, Y.; Yu, Y.; Zhu, H.; Wang, H.; Sun, Y.; Tan, H.; Zhang, Y. Effect of Microwave Extraction Temperature on the Chemical Structure and Oil-Water Interface Properties of Fish Skin Gelatin. Innov. Food Sci. Emerg. Technol. 2021, 74, 102835. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; He, Q.; Li, X.; Zhang, A. Extraction Optimization and Characterization of Gelatin from Half-Smooth Tongue sole (Cynoglossus semilaevis Gunther) Skin. J. Aquat. Food Prod. Technol. 2019, 28, 637–648. [Google Scholar] [CrossRef]
- Niu, L.; Zhou, X.; Yuan, C.; Bai, Y.; Lai, K.; Yang, F.; Huang, Y. Characterization of Tilapia (Oreochromis niloticus) Skin Gelatin Extracted with Alkaline and Different Acid Pretreatments. Food Hydrocoll. 2013, 33, 336–341. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of Antioxidant Peptides in Enzymatic Hydrolysates of Carp (Cyprinus carpio) Skin Gelatin. Molecules 2018, 24, 97. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, R.; Ding, M.; Tao, L.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Effect of Extraction Methods on the Structural Characteristics, Functional Properties, and Emulsion Stabilization Ability of Tilapia Skin Gelatins. Food Chem. 2020, 328, 127114. [Google Scholar] [CrossRef]
- Zhang, Y.; Dutilleul, P.; Li, C.; Simpson, B.K. Alcalase-Assisted Production of Fish Skin Gelatin Rich in High Molecular Weight (HMW) Polypeptide Chains and Their Characterization for Film Forming Capacity. LWT 2019, 110, 117–125. [Google Scholar] [CrossRef]
- Ideia, P.; Pinto, J.; Ferreira, R.; Figueiredo, L.; Spínola, V.; Castilho, P.C. Fish Processing Industry Residues: A Review of Valuable Products Extraction and Characterization Methods. Waste Biomass Valor. 2020, 11, 3223–3246. [Google Scholar] [CrossRef]
- Binsi, P.K. Overview of Waste Generation in Fish and Shellfish Processing Industry; ICAR-Central Institute of Fisheries Technology: Kochi, India, 2018; pp. 18–27. [Google Scholar]
- Karayannakidis, P.D.; Zotos, A. Fish Processing By-Products as a Potential Source of Gelatin: A Review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- Regenstein, J.M.; Zhou, P. Collagen and Gelatin from Marine By-Products. In Maximising the Value of Marine By-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 279–303. ISBN 978-1-84569-013-7. [Google Scholar]
- Nurilmala, M.; Suryamarevita, H.; Husein Hizbullah, H.; Jacoeb, A.M.; Ochiai, Y. Fish Skin as a Biomaterial for Halal Collagen and Gelatin. Saudi J. Biol. Sci. 2022, 29, 1100–1110. [Google Scholar] [CrossRef]
- Alves, A.L.; Fraguas, F.J.; Carvalho, A.C.; Valcárcel, J.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A.; Silva, T.H. Characterization of Codfish Gelatin: A Comparative Study of Fresh and Salted Skins and Different Extraction Methods. Food Hydrocoll. 2022, 124, 107238. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile tilapia skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef]
- Giacomelli da Silva, C.; Souza Rodrigues, A.; Carolina Lima, A.; de Oliveira Mello, R.; dal Pont Morisso, F.; Cristina Prestes Dornelles, R.; Hashime Kubota, E. Gelatin Extracted from Jundiá Skin (Rhamdia Quelen): An Alternative to the Discarded by-Product. Food Res. Int. 2022, 161, 111829. [Google Scholar] [CrossRef] [PubMed]
- Renuka, V.; Rao Ravishankar, C.N.; Zynudheen, A.A.; Bindu, J.; Joseph, T.C. Characterization of Gelatin Obtained from Unicorn leatherjacket (Aluterus monoceros) and Reef cod (Epinephelus diacanthus) Skins. LWT 2019, 116, 108586. [Google Scholar] [CrossRef]
- Jamilah, B.; Harvinder, K.G. Properties of Gelatins from Skins of Fish—Black tilapia (Oreochromis mossambicus) and Red tilapia (Oreochromis nilotica). Food Chem. 2002, 77, 81–84. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and Physico-Chemical Characterisation of Nile Perch (Lates Niloticus) Skin and Bone Gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Cheow, C.S.; Norizah, M.S.; Kyaw, Z.Y.; Howell, N.K. Preparation and Characterisation of Gelatins from the Skins of Sin croaker (Johnius dussumieri) and Shortfin scad (Decapterus macrosoma). Food Chem. 2007, 101, 386–391. [Google Scholar] [CrossRef]
- Kasankala, L.M.; Xue, Y.; Weilong, Y.; Hong, S.D.; He, Q. Optimization of Gelatine Extraction from Grass carp (Catenopharyngodon idella) Fish Skin by Response Surface Methodology. Bioresour. Technol. 2007, 98, 3338–3343. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Rawdkuen, S.; Chaijan, M.; Benjakul, S.; Osako, K.; Tanaka, M. Chemical Compositions and Characterisation of Skin Gelatin from Farmed Giant Catfish (Pangasianodon gigas). LWT—Food Sci. Technol. 2010, 43, 161–165. [Google Scholar] [CrossRef]
- Mohtar, N.F.; Perera, C.; Quek, S.-Y. Optimisation of Gelatine Extraction from Hoki (Macruronus novaezelandiae) Skins and Measurement of Gel Strength and SDS–PAGE. Food Chem. 2010, 122, 307–313. [Google Scholar] [CrossRef]
- Zeng, S.; Yan, X.; Cao, W.; Hong, P.; Zhang, C.; Li, L. Original Article: Optimisation of Extraction Conditions and Characteristics of Skin Gelatin from Nile tilapia (Oreochromis niloticus). Int. J. Food Sci. Technol. 2010, 45, 1807–1813. [Google Scholar] [CrossRef]
- Tabarestani, H.S.; Maghsoudlou, Y.; Motamedzadegan, A.; Sadeghi Mahoonak, A.R. Optimization of Physico-Chemical Properties of Gelatin Extracted from Fish Skin of Rainbow trout (Onchorhynchus mykiss). Bioresour. Technol. 2010, 101, 6207–6214. [Google Scholar] [CrossRef] [PubMed]
- Koli, J.M.; Basu, S.; Nayak, B.B.; Patange, S.B.; Pagarkar, A.U.; Gudipati, V. Functional Characteristics of Gelatin Extracted from Skin and Bone of Tiger-Toothed Croaker (Otolithes Ruber) and Pink perch (Nemipterus japonicus). Food Bioprod. Process. 2012, 90, 555–562. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Kishimura, H. Characteristics and Functional Properties of Gelatin from Splendid squid (Loligo formosana) Skin as Affected by Extraction Temperatures. Food Hydrocoll. 2012, 29, 389–397. [Google Scholar] [CrossRef]
- Jridi, M.; Nasri, R.; Lassoued, I.; Souissi, N.; Mbarek, A.; Barkia, A.; Nasri, M. Chemical and Biophysical Properties of Gelatins Extracted from Alkali-Pretreated Skin of Cuttlefish (Sepia officinalis) Using Pepsin. Food Res. Int. 2013, 54, 1680–1687. [Google Scholar] [CrossRef]
- Shyni, K.; Hema, G.S.; Ninan, G.; Mathew, S.; Joshy, C.G.; Lakshmanan, P.T. Isolation and Characterization of Gelatin from the Skins of Skipjack tuna (Katsuwonus pelamis), Dog shark (Scoliodon sorrakowah), and Rohu (Labeo rohita). Food Hydrocoll. 2014, 39, 68–76. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Bashari, M.; Alekhorshied, M.; Idrissa Cissouma, A.; Yang, N.; Xu, X. Physicochemical Properties of Skin Gelatin from Farmed Amur Sturgeon (Acipenser schrenckii) as Influenced by Acid Pretreatment. Food Biosci. 2014, 5, 19–26. [Google Scholar] [CrossRef]
- Jridi, M.; Lassoued, I.; Kammoun, A.; Nasri, R.; Chaâbouni, M.; Nasri, M.; Souissi, N. Screening of Factors Influencing the Extraction of Gelatin from the Skin of Cuttlefish Using Supersaturated Design. Food Bioprod. Process. 2015, 94, 525–535. [Google Scholar] [CrossRef]
- Silva, E.V.C.D.; Lourenço, L.D.F.H.; Pena, R.S. Optimization and Characterization of Gelatin from Kumakuma (Brachyplatystoma filamentosum) Skin. CyTA—J. Food 2017, 15, 361–368. [Google Scholar] [CrossRef]
- Djabourov, M. Architecture of Gelatin Gels. Contemp. Phys. 1988, 29, 273–297. [Google Scholar] [CrossRef]
- Norziah, M.H.; Al-Hassan, A.; Khairulnizam, A.B.; Mordi, M.N.; Norita, M. Characterization of Fish Gelatin from Surimi Processing Wastes: Thermal Analysis and Effect of Transglutaminase on Gel Properties. Food Hydrocoll. 2009, 23, 1610–1616. [Google Scholar] [CrossRef]
- Choi, S.-S.; Regenstein, J.M. Physicochemical and Sensory Characteristics of Fish Gelatin. J. Food Sci. 2000, 65, 194–199. [Google Scholar] [CrossRef]
- Cho, S.M.; Kwak, K.S.; Park, D.C.; Gu, Y.S.; Ji, C.I.; Jang, D.H.; Lee, Y.B.; Kim, S.B. Processing Optimization and Functional Properties of Gelatin from Shark (Isurus oxyrinchus) Cartilage. Food Hydrocoll. 2004, 18, 573–579. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Liu, H.; Li, D.; Guo, S. Rheological Properties of Channel catfish (Ictalurus punctaus) Gelatine from Fish Skins Preserved by Different Methods. LWT—Food Sci. Technol. 2008, 41, 1425–1430. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Cho, S.M.; Gu, Y.S.; Kim, S.B. Extracting Optimization and Physical Properties of Yellowfin tuna (Thunnus albacares) Skin Gelatin Compared to Mammalian Gelatins. Food Hydrocoll. 2005, 19, 221–229. [Google Scholar] [CrossRef]
- Abedinia, A.; Mohammadi Nafchi, A.; Sharifi, M.; Ghalambor, P.; Oladzadabbasabadi, N.; Ariffin, F.; Huda, N. Poultry Gelatin: Characteristics, Developments, Challenges, and Future Outlooks as a Sustainable Alternative for Mammalian Gelatin. Trends Food Sci. Technol. 2020, 104, 14–26. [Google Scholar] [CrossRef]
- Masuelli, M.A.; Sansone, M.G. Hydrodynamic Properties of Gelatin—Studies from Intrinsic Viscosity Measurements. In Products and Applications of Biopolymers; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Chiou, B.-S.; Avena-Bustillos, R.J.; Shey, J.; Yee, E.; Bechtel, P.J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Rheological and Mechanical Properties of Cross-Linked Fish Gelatins. Polymer 2006, 47, 6379–6386. [Google Scholar] [CrossRef]
- Kumar, D.P.; Chandra, M.V.; Elavarasan, K.; Shamasundar, B.A. Structural Properties of Gelatin Extracted from Croaker fish (Johnius sp. ) Skin Waste. Int. J. Food Prop. 2017, 20, S2612–S2625. [Google Scholar] [CrossRef]
- Eastoe, J.E.; Leach, A.A. Chemical Constitution of Gelatin. In the Science and Technology of Gelatin; Academic Press: New York, NY, USA, 1977; pp. 73–105. [Google Scholar]
- Limpisophon, K.; Tanaka, M.; Weng, W.; Abe, S.; Osako, K. Characterization of Gelatin Films Prepared from Under-Utilized Blue Shark (Prionace glauca) Skin. Food Hydrocoll. 2009, 23, 1993–2000. [Google Scholar] [CrossRef]
- Yang, M.; Yang, L.; Xu, J.; Nie, Y.; Wu, W.; Zhang, T.; Wang, X.; Zhong, J. Comparison of Silver Carp Fin Gelatins Extracted by Three Types of Methods: Molecular Characteristics, Structure, Function, and Pickering Emulsion Stabilization. Food Chem. 2022, 368, 130818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, R.; Ding, M.; Li, L.; Tao, N.; Wang, X.; Zhong, J. Commercial Cold-Water Fish Skin Gelatin and Bovine Bone Gelatin: Structural, Functional, and Emulsion Stability Differences. LWT 2020, 125, 109207. [Google Scholar] [CrossRef]
- Ledward, D.A. Gelation of Gelatin. In Functional Properties of Food Macromolecules; Elsevier Applied Science Publishers: London, UK, 1986; pp. 171–201. [Google Scholar]
- GME. Standardised Methods for the Testing of Edible Gelatin. In Gelatin Monograph; Gelatin Manufacturers of Europe (GME): Brussels, Belgium, 2000. [Google Scholar]
- Jellouli, K.; Balti, R.; Bougatef, A.; Hmidet, N.; Barkia, A.; Nasri, M. Chemical Composition and Characteristics of Skin Gelatin from Grey triggerfish (Balistes capriscus). LWT—Food Sci. Technol. 2011, 44, 1965–1970. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, L.; Jia, H.; Li, Q.; Jin, W.; Dong, X.; Pan, J. Physiochemical and Functional Properties of Chum salmon (Oncorhynchus keta) Skin Gelatin Extracted at Different Temperatures. J. Sci. Food Agric. 2017, 97, 5406–5413. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.V.; Shamasundar, B.A. Texture Profile Analysis and Functional Properties of Gelatin from the Skin of Three Species of Fresh Water Fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Characteristics of Gelatin from the Skin of Unicorn leatherjacket (Aluterus monoceros) as Influenced by Acid Pretreatment and Extraction Time. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- See, S.F.; Hong, P.K.; Ng, K.L.; Wan-Aida, W.M.; Babki, A.S. Physicochemical Properties of Gelatins Extracted from Skins of Different Freshwater Fish Species. Int. Food Res. J. 2010, 17, 809–816. [Google Scholar]
- Kim, H.-W.; Song, D.-H.; Choi, Y.-S.; Kim, H.-Y.; Hwang, K.-E.; Park, J.-H.; Kim, Y.-J.; Choi, J.-H.; Kim, C.-J. Effects of Soaking pH and Extracting Temperature on the Physicochemical Properties of Chicken Skin Gelatin. Korean J. Food Sci. Anim. Resour. 2012, 32, 316–322. [Google Scholar] [CrossRef]
- Kim, T.-K.; Ham, Y.-K.; Shin, D.-M.; Kim, H.-W.; Jang, H.W.; Kim, Y.-B.; Choi, Y.-S. Extraction of Crude Gelatin from Duck Skin: Effects of Heating Methods on Gelatin Yield. Poult. Sci. 2020, 99, 590–596. [Google Scholar] [CrossRef]
- Sompie, M.; Siswosubroto, S.E.; Rembet, G.D.; Ponto, J.W. Effect of Different Types of Acid Solvent on Functional and Microbiological Properties of Chicken Leg Skin Gelatin. IOP Conf. Ser. Earth Environ. Sci. 2019, 387, 012128. [Google Scholar] [CrossRef]
- Engel, J.; Bächinger, H.P. Structure, Stability and Folding of the Collagen Triple Helix. In Collagen; Brinckmann, J., Notbohm, H., Müller, P.K., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; Volume 247, pp. 7–33. ISBN 978-3-540-23272-8. [Google Scholar]
- Altan Kamer, D.D.; Palabiyik, I.; Işık, N.O.; Akyuz, F.; Demirci, A.S.; Gumus, T. Effect of Confectionery Solutes on the Rheological Properties of Fish (Oncorhynchus mykiss) Gelatin. LWT 2019, 101, 499–505. [Google Scholar] [CrossRef]
- Eddy, S.; Editya, F. The Effect of Concentrations of Ephinephelus Sp. Skin Gelatin on the Quality of Halal Marshmallows. Russ. J. Agric. Socio-Econ. Sci. 2020, 97, 120–125. [Google Scholar]
- Tinratat, S.; Sila-asna, M. Optimization of Gelatin Extraction and Physico-Chemical Properties of Fish Skin and Bone Gelatin: Its Application to Panna Cotta Formulas. Curr. Res. Nutr. Food Sci. 2017, 5, 263–273. [Google Scholar] [CrossRef]
- Uddin, S.M.K.; Hossain, M.A.M.; Sagadevan, S.; Al Amin, M.; Johan, M.R. Halal and Kosher Gelatin: Applications as Well as Detection Approaches with Challenges and Prospects. Food Biosci. 2021, 44, 101422. [Google Scholar] [CrossRef]
- Hartel, R.W.; von Elbe, J.H.; Hofberger, R. Caramel, Fudge and Toffee. In Confectionery Science and Technology; Springer International Publishing: Cham, Switzerland, 2018; pp. 273–299. ISBN 978-3-319-61740-4. [Google Scholar]
- Choobkar, N.; Branch, K.; Iran, K.; Aghajani, A.; Iran, Q.; Jokar, A.; Iran, M. The Effect of Replacement of Cow’s Gelatin by Cyprinus Carpio Skin Gelatin on the Some Mineral Contents and Color Parameters of Functional Pastill. Iran. Assoc. Aqua. Anim. Health 2018, 4, 40–54. [Google Scholar] [CrossRef]
- Yousefi, M.; Jafari, S.M. Recent Advances in Application of Different Hydrocolloids in Dairy Products to Improve Their Techno-Functional Properties. Trends Food Sci. Technol. 2019, 88, 468–483. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 92–115. ISBN 978-1-84569-758-7. [Google Scholar]
- Pang, Z.; Deeth, H.; Yang, H.; Prakash, S.; Bansal, N. Evaluation of Tilapia Skin Gelatin as a Mammalian Gelatin Replacer in Acid Milk Gels and Low-Fat Stirred Yogurt. J. Dairy Sci. 2017, 100, 3436–3447. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.H.; Lim, B.L.; Chow, K.H.; Chong, S.M.; Chang, Y.C. Using Fish Gelatin and Pectin to Make a Low-Fat Spread. Food Hydrocoll. 2008, 22, 1637–1640. [Google Scholar] [CrossRef]
- Reineccius, G.; Meng, Y. Gelatin and Other Proteins for Microencapsulation. In Microencapsulation in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 293–308. ISBN 978-0-12-821683-5. [Google Scholar]
- Soper, J.C. Method of Encapsulating Food or Flavor Particles Using Warm Water Fish Gelatin, and Capsules Produced Therefrom. U.S. Patent 08/367,072, 1999. [Google Scholar]
- Choi, D.J.; Kho, Y.; Park, S.J.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Effect of Cross-Linking on the Dimensional Stability and Biocompatibility of a Tailored 3D-Bioprinted Gelatin Scaffold. Int. J. Biol. Macromol. 2019, 135, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, V. Fish Gelatin: A Versatile Ingredient for the Food and Pharmaceutical Industries. In Marine Proteins and Peptides; Kim, S., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 271–295. ISBN 978-1-118-37506-8. [Google Scholar]
- Jiang, Y.; Yuan, I.H.; Dutille, E.K.; Bailey, R.; Shaker, M.S. Preventing Iatrogenic Gelatin Anaphylaxis. Ann. Allergy Asthma Immunol. 2019, 123, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Al-Tabakha, M.M.; Arida, A.I.; Fahelelbom, K.M.S.; Sadek, B.; Saeed, D.A.; Abu Jarad, R.A.; Jawadi, J. Influence of Capsule Shell Composition on the Performance Indicators of Hypromellose Capsule in Comparison to Hard Gelatin Capsules. Drug Dev. Ind. Pharm. 2015, 41, 1726–1737. [Google Scholar] [CrossRef]
- Tabibi, S.E.; Gupta, S.L. Soft Gelatin Capsules Development. In Water-Insoluble Drug Formulation; CRC Press: Boca Raton, FL, USA, 2008; p. 20. ISBN 978-0-429-12236-1. [Google Scholar]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Nomura, Y.; Oohashi, K.; Watanabe, M.; Kasugai, S. Increase in Bone Mineral Density through Oral Administration of Shark Gelatin to Ovariectomized Rats. Nutrition 2005, 21, 1120–1126. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global Burden of Hypertension: Analysis of Worldwide Data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, P. The Collagen Diet: Rejuvenate Skin, Strengthen Joints and Feel Younger by Boosting Collagen Intake and Production; Ulysses Press: Berkeley, CA, USA, 2018; ISBN 978-1-61243-832-0. [Google Scholar]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and Rheological Properties of Fish Gelatin Compared to Mammalian Gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Leuenberger, B.H. Investigation of Viscosity and Gelation Properties of Different Mammalian and Fish Gelatins. Food Hydrocoll. 1991, 5, 353–361. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and Physical Properties of Gelatin Extracted from Different Marine Species: A Comparative Study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Asghar, A.; Henrickson, R.L. Chemical, Biochemical, Functional, and Nutritional Characteristics of Collagen in Food Systems. In Advances in Food Research; Elsevier: Amsterdam, The Netherlands, 1982; Volume 28, pp. 231–372. ISBN 978-0-12-016428-8. [Google Scholar]
- Norland, R.E. Fish Gelatin. In Advances in Fisheries Technology and Biotechnology for Increased Profitability; Voigt, M.J., Botta, J.R., Eds.; Technomic Publisher Co., Inc.: Lancaster, UK, 1990; Volume X, pp. 325–333. ISBN 0-87762-785-1. [Google Scholar]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea Cucumber Derived Type I Collagen: A Comprehensive Review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef]
- Yi, J.B.; Kim, Y.T.; Bae, H.J.; Whiteside, W.S.; Park, H.J. Influence of Transglutaminase-Induced Cross-Linking on Properties of Fish Gelatin Films. J. Food Sci. 2006, 71, E376–E383. [Google Scholar] [CrossRef]
- Gilsenan, P. Rheological Characterisation of Gelatins from Mammalian and Marine Sources. Food Hydrocoll. 2000, 14, 191–195. [Google Scholar] [CrossRef]
- Gilsenan, P.M.; Ross-Murphy, S.B. Viscoelasticity of Thermoreversible Gelatin Gels from Mammalian and Piscine Collagens. J. Rheol. 2000, 44, 871–883. [Google Scholar] [CrossRef]
- Zhou, P.; Mulvaney, S.J.; Regenstein, J.M. Properties of Alaska Pollock Skin Gelatin: A Comparison with Tilapia and Pork Skin Gelatins. J. Food Sci. 2006, 71, C313–C321. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical Behaviour of Fish Gelatin-κ-Carrageenan Mixtures. Carbohydr. Polym. 2004, 56, 11–19. [Google Scholar] [CrossRef]
- Pranoto, Y.; Lee, C.M.; Park, H.J. Characterizations of Fish Gelatin Films Added with Gellan and κ-Carrageenan. LWT—Food Sci. Technol. 2007, 40, 766–774. [Google Scholar] [CrossRef]
- Elysée-Collen, B.; Lencki, R.W. Protein Ternary Phase Diagrams. 1. Effect of Ethanol, Ammonium Sulfate, and Temperature on the Phase Behavior of Type B Gelatin. J. Agric. Food Chem. 1996, 44, 1651–1657. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, M.; Tao, L.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Octenyl Succinic Anhydride Modification of Bovine Bone and Fish Skin Gelatins and Their Application for Fish Oil-Loaded Emulsions. Food Hydrocoll. 2020, 108, 106041. [Google Scholar] [CrossRef]
- Razzak, M.A.; Kim, M.; Chung, D. Elucidation of Aqueous Interactions between Fish Gelatin and Sodium Alginate. Carbohydr. Polym. 2016, 148, 181–188. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S. Physico-Chemical Properties and Fishy Odour of Gelatin from Seabass (Lates calcarifer) Skin Stored in Ice. Food Biosci. 2015, 10, 59–68. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Zotos, A. Physicochemical Properties of Yellowfin tuna (T Hunnus albacare) Skin Gelatin and Its Modification by the Addition of Various Coenhancers: Modification of Yellowfin Tuna Skin Gelatin. J. Food Process. Preserv. 2015, 39, 530–538. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, Morphological and Thermal Behaviour Characterisations of Fish Gelatin Film Incorporated with Basil and Citronella Essential Oils as Affected by Surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of Fish Gelatin and Chitosan in Uncrosslinked and Crosslinked with EDC Films: FT-IR Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Jeevithan, E.; Jeya Shakila, R.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Physico-Functional and Mechanical Properties of Chitosan and Calcium Salts Incorporated Fish Gelatin Scaffolds. Int. J. Biol. Macromol. 2013, 60, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Koli, J.M.; Basu, S.; Nayak, B.B.; Kannuchamy, N.; Gudipati, V. Improvement of Gel Strength and Melting Point of Fish Gelatin by Addition of Coenhancers Using Response Surface Methodology. J. Food Sci. 2011, 76, E503–E509. [Google Scholar] [CrossRef] [PubMed]
- Boran, G.; Mulvaney, S.J.; Regenstein, J.M. Rheological Properties of Gelatin from Silver Carp Skin Compared to Commercially Available Gelatins from Different Sources. J. Food Sci. 2010, 75, E565–E571. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Giménez, B.; Montero, P.; Gómez-Guillén, M.C. Incorporation of Antioxidant Borage Extract into Edible Films Based on Sole Skin Gelatin or a Commercial Fish Gelatin. J. Food Eng. 2009, 92, 78–85. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An Active Peptide Purified from Gastrointestinal Enzyme Hydrolysate of Pacific Cod Skin Gelatin Attenuates Angiotensin-1 Converting Enzyme (ACE) Activity and Cellular Oxidative Stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Akbari-adergani, B. Bioactive Food Derived Peptides: A Review on Correlation between Structure of Bioactive Peptides and Their Functional Properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Yousefi, R.; Eun, J.-B. Bio/Multi-Functional Peptides Derived from Fish Gelatin Hydrolysates: Technological and Functional Properties. Biocatal. Agric. Biotechnol. 2021, 36, 102152. [Google Scholar] [CrossRef]
- Noor, N.Q.I.M.; Razali, R.S.; Ismail, N.K.; Ramli, R.A.; Razali, U.H.M.; Bahauddin, A.R.; Zaharudin, N.; Rozzamri, A.; Bakar, J.; Shaarani, S.M. Application of Green Technology in Gelatin Extraction: A Review. Processes 2021, 9, 2227. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S. Antioxidant Activities of Hydrolysed Collagen from Salmon Scale Ossein Prepared with the Aid of Ultrasound. Int. J. Food Sci. Technol. 2018, 53, 2786–2795. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Kumar, S.; Bhat, H.F.; Aadil, R.M.; Bekhit, A.E.-D.A. Ultrasonication as an Emerging Technology for Processing of Animal Derived Foods: A Focus on in Vitro Protein Digestibility. Trends Food Sci. Technol. 2022, 124, 309–322. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Zeng, W.-C.; Zhang, W.-H.; Liao, X.-P.; Shi, B. Effect of Ultrasonic Pretreatment on Kinetics of Gelatin Hydrolysis by Collagenase and Its Mechanism. Ultrason. Sonochemistry 2016, 29, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Fang, Y.; Chen, Y.; Yang, W.; Ma, N.; Pei, F.; Kimatu, B.M.; Hu, Q.; Qiu, W. Protective Effects of Se-Containing Protein Hydrolysates from Se-Enriched Rice against Pb2+-Induced Cytotoxicity in PC12 and RAW264.7 Cells. Food Chem. 2016, 202, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Xu, R.; Zhang, J.; Wang, M.; Xu, K. Hydrothermal Carbonization of Waste Furniture for Clean Blast Furnace Fuel Production: Physicochemical, Gasification Characteristics and Conversion Mechanism Investigation. Chem. Eng. J. 2023, 469, 143980. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.-W.; Kim, H.-J. Hydrothermal Carbonization of Lignocellulosic Biomass for Carbon Rich Material Preparation: A Review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Xiao, Y.; Lasaga, A.C. Ab Initio Quantum Mechanical Studies of the Kinetics and Mechanisms of Quartz Dissolution: OH− Catalysis. Geochim. Cosmochim. Acta 1996, 60, 2283–2295. [Google Scholar] [CrossRef]
- Ahmed, R.; Chun, B.-S. Subcritical Water Hydrolysis for the Production of Bioactive Peptides from Tuna Skin Collagen. J. Supercrit. Fluids 2018, 141, 88–96. [Google Scholar] [CrossRef]
- Potekhin, S.A.; Senin, A.A.; Abdurakhmanov, N.N.; Tiktopulo, E.I. High Pressure Stabilization of Collagen Structure. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2009, 1794, 1151–1158. [Google Scholar] [CrossRef]
- Bonomi, F.; Fiocchi, A.; Frøkiær, H.; Gaiaschi, A.; Iametti, S.; Poiesi, C.; Rasmussen, P.; Restani, P.; Rovere, P. Reduction of Immunoreactivity of Bovine β-Lactoglobulin upon Combined Physical and Proteolytic Treatment. J. Dairy Res. 2003, 70, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Préstamo, G.; Luisa Baeza, M.; Martínez-Molero, M.I.; Gomez, R. Effects of Combined High Pressure and Enzymatic Treatments on the Hydrolysis and Immunoreactivity of Dairy Whey Proteins. Int. Dairy J. 2006, 16, 831–839. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Gómez-Guillén, M.C.; Montero, P. Enzymatic Hydrolysis of Fish Gelatin under High Pressure Treatment. Int. J. Food Sci. Technol. 2011, 46, 1129–1136. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Benjakul, S. Cryoprotective and Antioxidative Effects of Gelatin Hydrolysate from Unicorn Leatherjacket Skin. Int. J. Refrig. 2015, 49, 69–78. [Google Scholar] [CrossRef]
- Anal, A.K.; Noomhorm, A.; Vongsawasdi, P. Protein Hydrolysates and Bioactive Peptides from Seafood and Crustacean Waste: Their Extraction, Bioactive Properties and Industrial Perspectives. In Marine Proteins and Peptides; Kim, S., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 709–735. ISBN 978-1-118-37506-8. [Google Scholar]
- Vázquez, J.; Meduíña, A.; Durán, A.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.; Rodríguez-Amado, I. Production of Valuable Compounds and Bioactive Metabolites from By-Products of Fish Discards Using Chemical Processing, Enzymatic Hydrolysis, and Bacterial Fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.; Sica, P.; Costa, C.; Márquez, M.C. Enzymatic Hydrolysis of Fish Waste as an Alternative to Produce High Value-Added Products. Waste Biomass Valor. 2021, 12, 847–855. [Google Scholar] [CrossRef]
- Pires, C.; Leitão, M.; Sapatinha, M.; Gonçalves, A.; Oliveira, H.; Nunes, M.L.; Teixeira, B.; Mendes, R.; Camacho, C.; Machado, M.; et al. Protein Hydrolysates from Salmon Heads and Cape Hake By-Products: Comparing Enzymatic Method with Subcritical Water Extraction on Bioactivity Properties. Foods 2024, 13, 2418. [Google Scholar] [CrossRef]
- Caruso, G.; Floris, R.; Serangeli, C.; di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- John, E.P.; Mishra, U. Integrated Multitrophic Aquaculture Supply Chain Fish Traceability with Blockchain Technology, Valorisation of Fish Waste and Plastic Pollution Reduction by Seaweed Bioplastic: A Study in Tuna Fish Aquaculture Industry. J. Clean. Prod. 2024, 434, 140056. [Google Scholar] [CrossRef]
- Atma, Y. Fish Gelatin Hydrolysates as Antioxidant, Antihypertensive, and Antidiabetic: The Plausible Mechanisms. MROC 2021, 18, 719–724. [Google Scholar] [CrossRef]
- Ishak, N.H.; Sarbon, N.M. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioprocess. Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. A Review of Fish-Derived Antioxidant and Antimicrobial Peptides: Their Production, Assessment, and Applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Javed, M.; Cheng, M.; Xiong, S.; Liu, Y. Gelatin Hydrolysates from Sliver carp (Hypophthalmichthys molitrix) Improve the Antioxidant and Cryoprotective Properties of Unwashed Frozen Fish Mince. Int. J. Food Sci. Technol. 2022, 57, 2619–2627. [Google Scholar] [CrossRef]
- Mokhtarnezhad, V.; Taheri, A.; Motamedzadegan, A. Bioactive Properties of Marine Fish Skin Gelatin Hydrolysate: Optimisation Using Response Surface Methodology. Indian. J. Fish. 2021, 68, 118. [Google Scholar] [CrossRef]
- Limpisophon, K.; Shibata, J.; Yasuda, Y.; Tanaka, M.; Osako, K. Optimization of Hydrolysis Conditions for Production of Gelatin Hydrolysates from Shark Skin Byproduct and Evaluation of Their Antioxidant Activities. J. Aquat. Food Prod. Technol. 2020, 29, 736–749. [Google Scholar] [CrossRef]
- Viji, P.; Phannendra, T.S.; Jesmi, D.; Madhusudana Rao, B.; Dhiju Das, P.H.; George, N. Functional and Antioxidant Properties of Gelatin Hydrolysates Prepared from Skin and Scale of Sole Fish. J. Aquat. Food Prod. Technol. 2019, 28, 976–986. [Google Scholar] [CrossRef]
- Alolod, G.A.L.; Nuñal, S.N.; Nillos, M.G.G.; Peralta, J.P. Bioactivity and Functionality of Gelatin Hydrolysates from the Skin of Oneknife unicornfish (Naso thynnoides). J. Aquat. Food Prod. Technol. 2019, 28, 1013–1026. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, H.; Wei, H.; Xing, Q.; Zou, Y.; Zhou, Y.; Peng, J. Antioxidative Peptides of Hydrolysate Prepared from Fish Skin Gelatin Using Ginger Protease Activate Antioxidant Response Element-Mediated Gene Transcription in IPEC-J2 Cells. J. Funct. Foods 2018, 51, 104–112. [Google Scholar] [CrossRef]
- Ketnawa, S.; Benjakul, S.; Martínez-Alvarez, O.; Rawdkuen, S. Fish Skin Gelatin Hydrolysates Produced by Visceral Peptidase and Bovine Trypsin: Bioactivity and Stability. Food Chem. 2017, 215, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Martínez-Alvarez, O.; Benjakul, S.; Rawdkuen, S. Gelatin Hydrolysates from Farmed Giant Catfish Skin Using Alkaline Proteases and Its Antioxidative Function of Simulated Gastro-Intestinal Digestion. Food Chem. 2016, 192, 34–42. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Xu, X. Antioxidant and Cryoprotective Effects of Amur Sturgeon Skin Gelatin Hydrolysate in Unwashed Fish Mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sai-Ut, S.; Benjakul, S.; Sumpavapol, P.; Kishimura, H. Antioxidant Activity of Gelatin Hydrolysate Produced from Fish Skin Gelatin Using Extracellular Protease from B Acillus amyloliquefaciens H11: Fish Gelatin Hydrolysate with Antioxidant Activity. J. Food Process. Preserv. 2015, 39, 394–403. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Benjakul, S. Glycyl Endopeptidase from Papaya Latex: Partial Purification and Use for Production of Fish Gelatin Hydrolysate. Food Chem. 2014, 165, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Khiari, Z.; Rico, D.; Martin-Diana, A.B.; Barry-Ryan, C. Structure Elucidation of ACE -inhibitory and Antithrombotic Peptides Isolated from Mackerel Skin Gelatine Hydrolysates. J. Sci. Food Agric. 2014, 94, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.; Yang, N.; Xu, B.; Jin, Z.; Xu, X. Antioxidant and Cryoprotective Effects of a Tetrapeptide Isolated from Amur Sturgeon Skin Gelatin. J. Funct. Foods 2014, 7, 609–620. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Nasri, R.; Aydi, M.; Toldrá, F.; Aristoy, M.-C.; Barkia, A.; Nasri, M. Characterization, Antioxidative and ACE Inhibitory Properties of Hydrolysates Obtained from Thornback ray (Raja clavata) Muscle. J. Proteom. 2015, 128, 458–468. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and Identification of Antioxidant Peptides from the Skin Protein Hydrolysate of Two Marine Fishes, Horse mackerel (Magalaspis cordyla) and Croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Lee, C.W.; Yun, H.; Eun, J.-B. Structure–Function Relationship of Fermented Skate Skin Gelatin-Derived Bioactive Peptides: A Peptidomics Approach. Food Sci. Biotechnol. 2021, 30, 1685–1693. [Google Scholar] [CrossRef]
- Thuanthong, M.; de Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and Characterization of Angiotensin-Converting Enzyme-Inhibitory Peptides from Nile tilapia (Oreochromis niloticus) Skin Gelatine Produced by an Enzymatic Membrane Reactor. J. Funct. Foods 2017, 36, 243–254. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ryu, B.; Kim, S.-K. Angiotensin- I- Converting Enzyme (ACE) Inhibitory Peptides from Pacific Cod Skin Gelatin Using Ultrafiltration Membranes. Process Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kang, K.-H.; Ryu, B.; Vo, T.-S.; Jung, W.-K.; Byun, H.-G.; Kim, S.-K. Angiotensin-I Converting Enzyme Inhibitory Peptides from Antihypertensive skate (Okamejei kenojei) Skin Gelatin Hydrolysate in Spontaneously Hypertensive Rats. Food Chem. 2015, 174, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Antihypertensive Effect of Novel Angiotensin I Converting Enzyme Inhibitory Peptide from Chum salmon (Oncorhynchus keta) Skin in Spontaneously Hypertensive Rats. J. Funct. Foods 2014, 7, 381–389. [Google Scholar] [CrossRef]
- Byun, H.-G.; Kim, S.-K. Structure and Activity of Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Alaskan Pollack Skin. BMB Rep. 2002, 35, 239–243. [Google Scholar] [CrossRef]
- Sila, A.; Martinez-Alvarez, O.; Haddar, A.; Gómez-Guillén, M.C.; Nasri, M.; Montero, M.P.; Bougatef, A. Recovery, Viscoelastic and Functional Properties of Barbel Skin Gelatine: Investigation of Anti-DPP-IV and Anti-Prolyl Endopeptidase Activities of Generated Gelatine Polypeptides. Food Chem. 2015, 168, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant Properties of a Radical-Scavenging Peptide Purified from Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Comp. Rev. Food Sci. Food Safe 2004, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.M.; Amruth, P.; Jacob, M.R.; Dara, P.K.; Renuka, V.; Anandan, R. Liposome Mediated Encapsulation and Role of Chitosan on Modulating Liposomal Stability to Deliver Potential Bioactives-A Review. Food Hydrocoll. Health 2023, 4, 100142. [Google Scholar] [CrossRef]
| Fish | Extraction Method | Yield (%) | Reference |
|---|---|---|---|
| Black tilapia (Oreochromis mossambicus) Red tilapia (Oreochromis nilotica) | Pretreatment with NaOH (2%, w/v), H2SO4 (2%, w/v) and citric acid (1%, w/v); hot water 45 °C for 12 h | 5.39 7.81 | [36] |
| Nile perch (Lates niloticus) | Pretreated with H2SO4 (0.01 M); hot water 50, 60 and 70 °C for 5 h | 1.2–4.9 | [37] |
| Shortfin scad (Decapterus macrosomia Sin croaker (Johnius dussumieri) | Pretreatment with NaOH (0.2%, w/v), H2SO4 (0.2%, w/v) and citric acid (1%, w/v); hot water 40–50 °C for 12 h | 7.25 14.3 | [38] |
| Grass carp (Catenopharyngodon Idella) | HCl (1.19%, 24 h) pretreatment followed by hot water extraction at 52.61 °C for 5.12 h | 19.83 | [39] |
| Farmed giant catfish (Pangasianodon gigas) | NaOH (0.2 M)-treated skin soaked in acetic acid (0.05 M) and stirred for 3 h at 25 °C | 20.1 | [40] |
| Hoki (Macruronus novaezelandiae) | 0.75 M NaCl pretreatment for 9 min and hot water extraction at 49.3 °C for 60 min | 17.6 | [41] |
| Nile tilapia (Oreochromis niloticus) | Alkali-treated (NaOH 3.2% w/v, 2.3 h) skin soaked in HCl (0.7%, 84 min) followed by hot water extraction 60 °C for 3 h | 19.3 | [42] |
| Rainbow trout (Oncorhynchus mykiss) | Pretreated with NaOH (0.19 N) and acetic acid (0.121 N) for 3 h, followed by hot water extraction of 50 °C for 16 h | 9.36 | [43] |
| Pink perch (Nemipterus japonicas) Tiger-toothed croaker (Otoliths ruber) | Pretreated with NaOH (0.2%, w/v), H2SO4 (0.2%, w/v), and citric acid (1%, w/v) for 40 min, followed by hot water extraction at 45 °C for 12 h | 5.57 7.56 | [44] |
| Splendid squid (Loligo formosana) | Skin soaked in NaOH (0.05 M, 6 h) and phosphoric acid (0.05 M, 24 h) followed by hot water extraction at different temperatures (50, 60, 70, and 80 °C) for 12 h. | 8.8–45.3 | [45] |
| Cuttlefish (Sepia officinalis) | Alkali-pretreated skin (NaOH, 0.05 M) subjected to 5, 10 and 15 U pepsin/g for 18 h at 4 °C, pH 2 | 6.59–9.22 | [46] |
| Dog shark (Scoliodon sorrakowah) Rohu (Labeo rohita) Skipjack tuna (Katsuwonus pelamis) | Pretreated with NaOH (0.1 M) and acetic acid (0.2 M) followed by hot water at 45 °C for 12 h | 19.7 11.3 17.2 | [47] |
| Farmed amur sturgeon (Acipenser schrenckii) | Alkali (NaOH, 0.1 M for 6 h, 4 °C) and acid (acetic acid, 0.05–0.2 M, 3–6 h)-treated skin followed by extraction at 50 °C for 1 h | 5.04–24.11 | [48] |
| Octopus (Octopus vulgaris) | Alkali (0.05 M NaOH, 1 h at 4 °C)-treated skin soaked in glycine-HCl buffer (10 h at 4 °C) added pepsin (0–15 U) incubated at 40 °C for 4 h. | 3.21–7.78 | [49] |
| Kumakuma (Brachyplatystoma flamentosum) | Pretreatment with NaCl (0.6 M) followed by NaOH (0.3 M), followed by acetic acid treatment (0.02 M) for min | 19.7 | [50] |
| Application | Bloom Strength (g) | Concentration and Function |
|---|---|---|
| Gelatin desserts and gummy bears | 175–275 | 7–9% as gel formation [59]. |
| Sausages, broths, meat products, and canned meats | 175–275 | 1–5% as an emulsion stabilizer and binding agent |
| Dairy products | 150–250 | 0.2–1.0% as syneresis stabilizer [59]. |
| Frozen foods | 200–250 | 0.1–0.5% reducing water loss agent [59]. |
| Beverage industry | 100–20 | 0.002–0.015% as clarifying agent [59]. |
| Skin Source | Gel Strength (Bloom Grams) | Gelling Temp (°C) | Melting Temp (°C) | Viscosity (cP) | Water Holding (%) | Fat Binding (%) | Emulsion | Foaming | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EAI (m2/g) | ESI (min) | FE (%) | FS (%) | ||||||||
| Cold-water fish gelatin | |||||||||||
| Rohu | 124 | 13.8 | 18.2 | 2.50 | 163 | 360 | NA | NA | 17.4 | 10.5 | [47] |
| Gray triggerfish | 168.3 | NA | NA | NA | ~190 | ~340% | 21.44 | 42.77 | 123.04 | 117.22 | [69] |
| Chum salmon | 87.7 | NA | NA | NA | NA | NA | ~24 | ~100 | ~119 | ~103 | [70] |
| Indian carp | 367.66 | NA | NA | NA | NA | NA | 0.87 * | 90 | 61.8 | [71] | |
| Rohu | 258.0 | NA | NA | NA | NA | NA | 0.552 * | ND | 92 | 72.9 | |
| White carp | 343.0 | NA | NA | NA | NA | NA | 0.669 | NA | 80 | 77.1 | |
| Warm-water fish gelatin | |||||||||||
| Yellowfin tuna | 426 | 18.7 | 24.3 | NA | NA | NA | NA | NA | NA | NA | [58] |
| Skipjack tuna | 177 | 18.7 | 24.2 | 4.37 | 452 | 452 | NA | NA | 19.2 | 14.4 | [47] |
| Dog shark | 206 | 20.8 | 25.8 | 5.6 | 256 | 347 | NA | NA | 21.5 | 17.6 | |
| Unicorn leatherjacket | 149.77 | NA | NA | NA | NA | NA | 39.17 | 8.58 | 116.35 | 111.11 | [72] |
| Snakehead | 311.18 | NA | NA | 3.40 | NA | NA | NA | NA | NA | NA | [73] |
| Catfish | 278.72 | NA | NA | 5.24 | NA | NA | NA | NA | NA | NA | |
| Pangasius catfish | 324.53 | NA | NA | 3.82 | NA | NA | NA | NA | NA | NA | |
| Red tilapia | 487.61 | NA | NA | 1.73 | NA | NA | NA | NA | NA | NA | |
| Porcine gelatin | |||||||||||
| Porcine | 295 | 25.6 | 36.5 | NA | NA | NA | NA | NA | NA | NA | [58] |
| Bovine gelatin | |||||||||||
| Hoki | 197 | NA | 16.6 | 10.8 | NA | NA | NA | NA | NA | NA | [41] |
| Shortfin scad | 177 | 9.9 | 23.8 | NA | NA | NA | NA | NA | NA | NA | [38] |
| Bovine | 216 | 23.8 | 33.8 | NA | NA | NA | NA | NA | NA | NA | [58] |
| Poultry gelatin | |||||||||||
| Chicken | 270.5 | NA | 39.83 | NA | NA | NA | NA | NA | NA | NA | [74] |
| Duck | 210–260 | NA | 31.2–33.8 | 56.9–77.8 | NA | NA | NA | NA | NA | NA | [75] |
| Chicken leg | 75–85 | NA | NA | 7 to 8.9 | NA | NA | NA | NA | NA | NA | [76] |
| Modification | Fish Skin Source | Tailored Properties | Reference |
|---|---|---|---|
| Sucrose, glucose and fructose addition | Tilapia | Characteristics of the structure, the functions, and the capability of stabilizing the emulsion | [24] |
| Octenyl succinic anhydride modification | Cold-water fish | Structural, functional, and emulsion stability | [112] |
| Sodium alginate addition | Cod, pollock, and haddock | Foam and emulsion stabilization | [113] |
| Spray drying with citric acid, adding pretreatment | Seabass (L. calcarifer) | Physico-chemical properties and fishy odor | [114] |
| NaH2PO4, MgCl2, CaCl2, and glycerol addition | Yellowfin tuna | Physicochemical properties | [115] |
| Basil and citronella essential oils addition | Alaska pollock | Structural, morphological, and thermal properties | [116] |
| Proteins (chitosan) addition | Baltic cod | Physico-chemical properties | [117] |
| Chitosan and calcium acetate addition | Catfish (Pungaseous fungaseous) | Physico-functional and mechanical properties | [118] |
| Coenhancers (MgSO4, sucrose, and transglutaminase) addition | Tiger-toothed croaker | Gel strength and melting point | [119] |
| Adjust extraction conditions (alkali and acid treatment, water extraction) | Silver carp | Sensory and instrumental characteristics | [120] |
| Addition of glycerol and sorbitol | Sole (Solea spp.) | Sensory characteristics | [121] |
| Source of Skin Gelatin | Enzyme | Mechanism | Peptide Sequence | Reference |
|---|---|---|---|---|
| (a) Antioxidant activity | ||||
| Silver carp | Alcalase, papain and flavoenzyme | Flavorsome hydrolysate has the highest DPPH-scavenging activity | NA | [151] |
| Catfish | Protamex |
| NA | [152] |
| Shark | Alcalase |
| NA | [153] |
| Carp | Protamex |
| Ala-Tyr dipeptide | [23] |
| Sole fish | Alcalase |
| NA | [154] |
| Oneknife unicornfish | Crude protease from Bacillus sp. |
| NA | [155] |
| Nile tilapia | Ginger protease |
| Gly-Pro-Y-type X-Hyp-Gly-type | [156] |
| Giant catfish | Viscera peptidase of gait catfish |
| NA | [157] |
| Giant catfish | Visceral alkaline proteases from giant catfish, Izyme AL and trypsin |
| NA | [158] |
| Amur sturgeon | Alcalase or flavoenzyme |
| NA | [159] |
| Unicorn leatherjacket | Protease from Bacillus amyloliquefaciens and Alcalase |
| NA | [160] |
| Tilapia | Papaya crude extract |
| NA | [161] |
| Atlantic mackerel | Pepsin |
| NA | [162] |
| Amur sturgeon | Alcalase and flavoenzyme |
| Pro–Ala–Gly–Tyr (PAGT) | [163] |
| Thornback ray | - |
| AVGAT | [164] |
| Horse mackerel | - |
| NHRYDR | [165] |
| (b) Antihypertensive | ||||
| Skate | Subtilisin and actinidin |
| 1. GRPGNRGE (IC50 0.74 mg/mL) 2. AKDYEVDAT (IC50 0.52 mg/mL) | [166] |
| Oneknife unicornfish | Crude protease from Bacillus sp. |
| NA | [155] |
| Nile tilapia | Alcalase |
| GIV, GAP*GF, GFA*GPA, SGNIGFP*GPK, GIPGPIGPP*GRP | [167] |
| Giant catfish | Viscera peptidase of gait catfish |
| NA | [157] |
| Atlantic mackerel | Pepsin |
| NA | [162] |
| Pacific cod | Pepsin |
| GASSGMPG (6.9 µM) LAYA (1.45 µM) | [168] |
| Skate | Alcalase/protease |
| MVGSAPGVL LGPLGHQ | [169] |
| Salmon | Trypsin |
| GLPLNLP | [170] |
| Alaska pollock | - |
| GPL | [171] |
| (c) Antimicrobial activity | ||||
| Skate | Subtilisin and actinidin |
| 1. GRPGNRGE 2. AKDYEVDAT | [166] |
| (d) Antidiabetic activity | ||||
| Barbel | Commercial proteases |
| ND | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joy, J.M.; Padmaprakashan, A.; Pradeep, A.; Paul, P.T.; Mannuthy, R.J.; Mathew, S. A Review on Fish Skin-Derived Gelatin: Elucidating the Gelatin Peptides—Preparation, Bioactivity, Mechanistic Insights, and Strategies for Stability Improvement. Foods 2024, 13, 2793. https://doi.org/10.3390/foods13172793
Joy JM, Padmaprakashan A, Pradeep A, Paul PT, Mannuthy RJ, Mathew S. A Review on Fish Skin-Derived Gelatin: Elucidating the Gelatin Peptides—Preparation, Bioactivity, Mechanistic Insights, and Strategies for Stability Improvement. Foods. 2024; 13(17):2793. https://doi.org/10.3390/foods13172793
Chicago/Turabian StyleJoy, Jean Mary, Amruth Padmaprakashan, Akshay Pradeep, Preethy Treesa Paul, Rosemol Jacob Mannuthy, and Suseela Mathew. 2024. "A Review on Fish Skin-Derived Gelatin: Elucidating the Gelatin Peptides—Preparation, Bioactivity, Mechanistic Insights, and Strategies for Stability Improvement" Foods 13, no. 17: 2793. https://doi.org/10.3390/foods13172793






