Enzymatic Preparation, Identification by Transmembrane Channel-like 4 (TMC4) Protein, and Bioinformatics Analysis of New Salty Peptides from Soybean Protein Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of SPI Enzymatic Hydrolysate
2.3. Molecular Weight Distribution of SPIEH
2.4. Determination of Free Amino Acid Content in SPIEH
2.5. Evaluation of Salt Taste
2.5.1. Sensory Evaluation
2.5.2. Electronic Tongue Analysis
2.6. Separation and Purification Salty Peptides from SPIEH
2.6.1. Separation by UF
2.6.2. Purification by GFC
2.7. Identification of the Peptide Sequence
2.8. Construction of Saltiness Receptor Model TMC4
2.9. Molecular Docking of Potential Peptides with TMC4
2.10. In Silico Screening of Premium Salty Peptides
2.11. Statistical Analysis
3. Results and Discussion
3.1. Molecular Weight Distribution of the Hydrolysate
3.2. Free Amino Acid Content Analysis
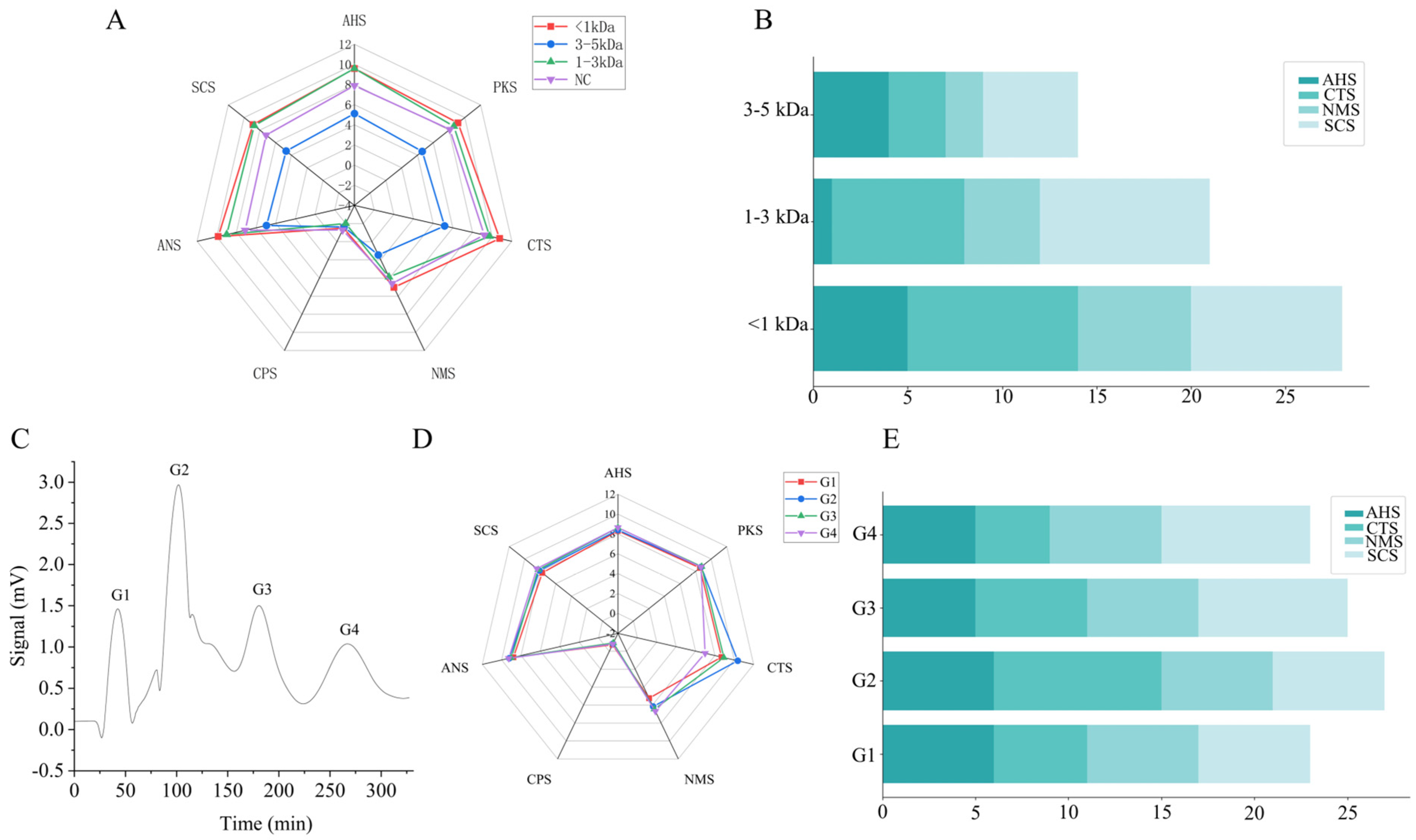
3.3. Separation and Purification of SPIEH
3.4. Peptide Sequence Identification
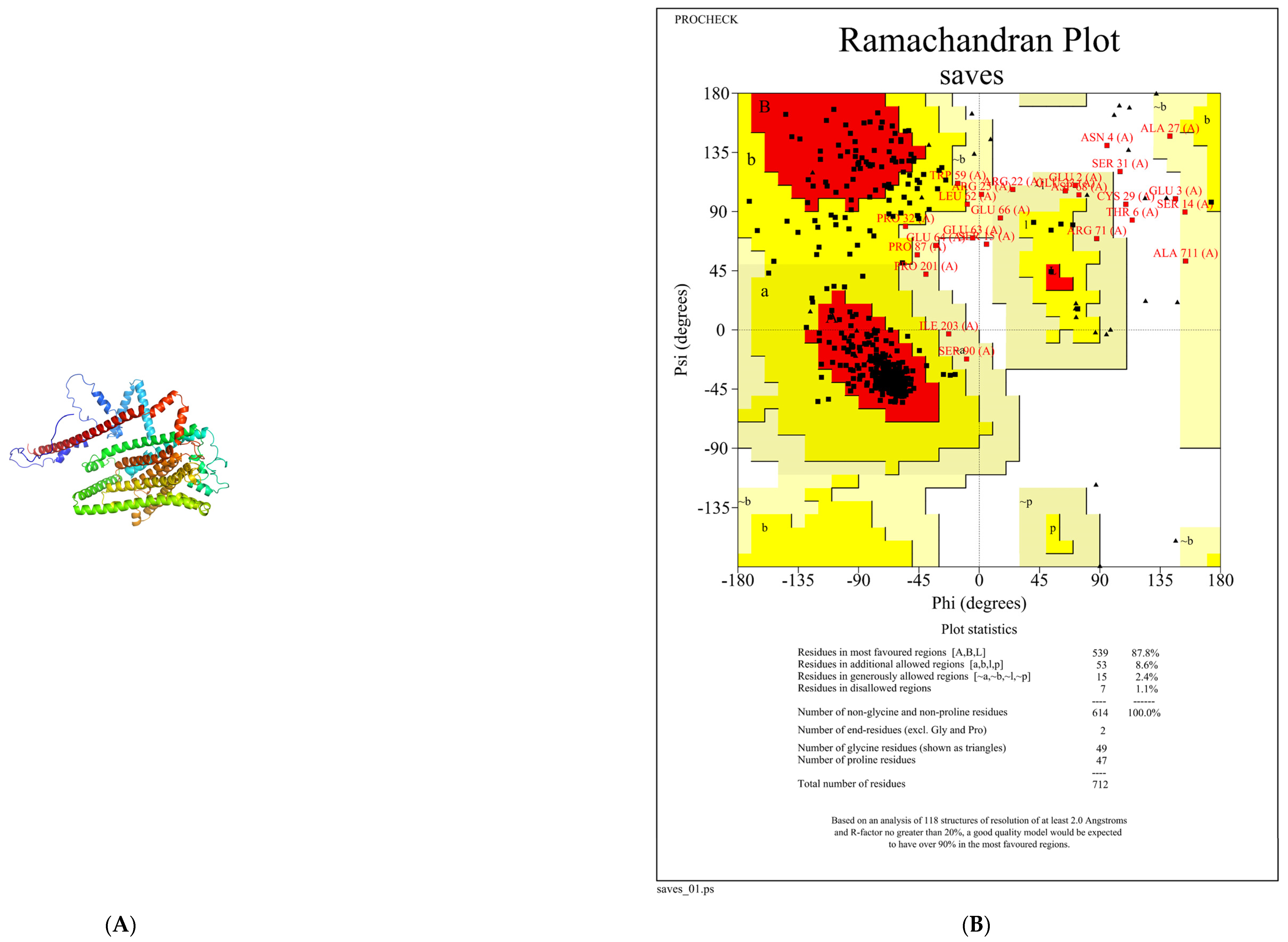
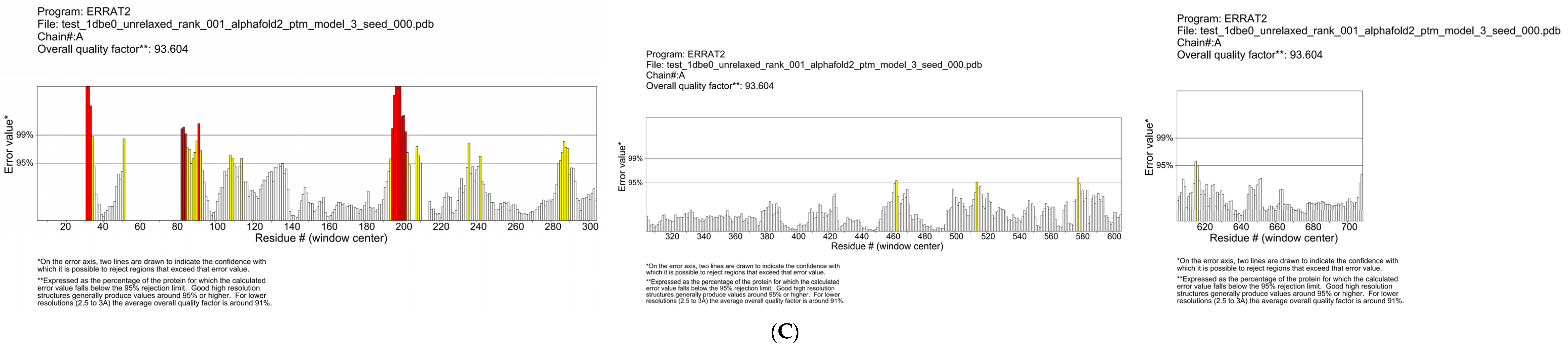
3.5. Construction and Evaluation of Salty Receptor TMC4
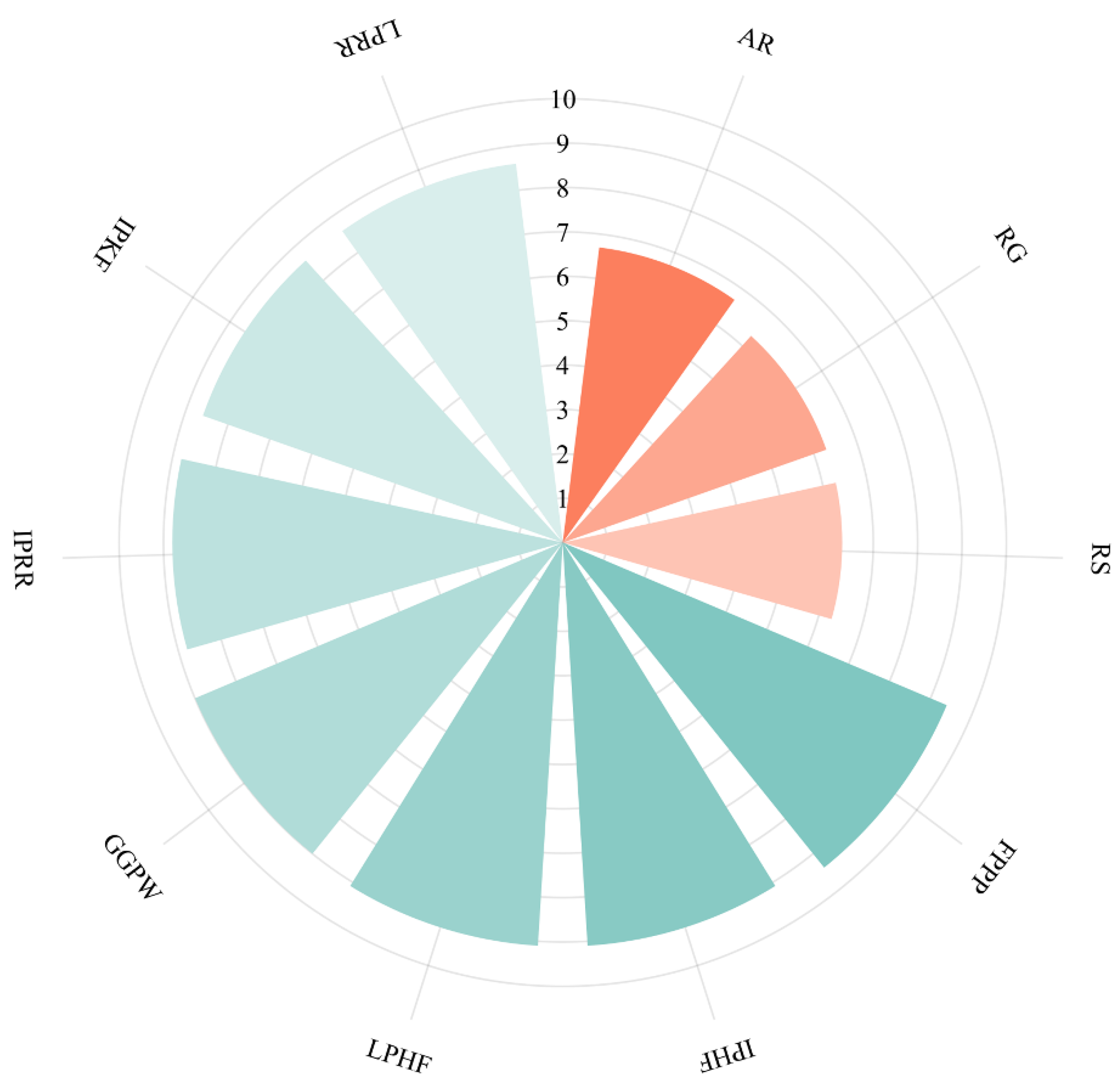
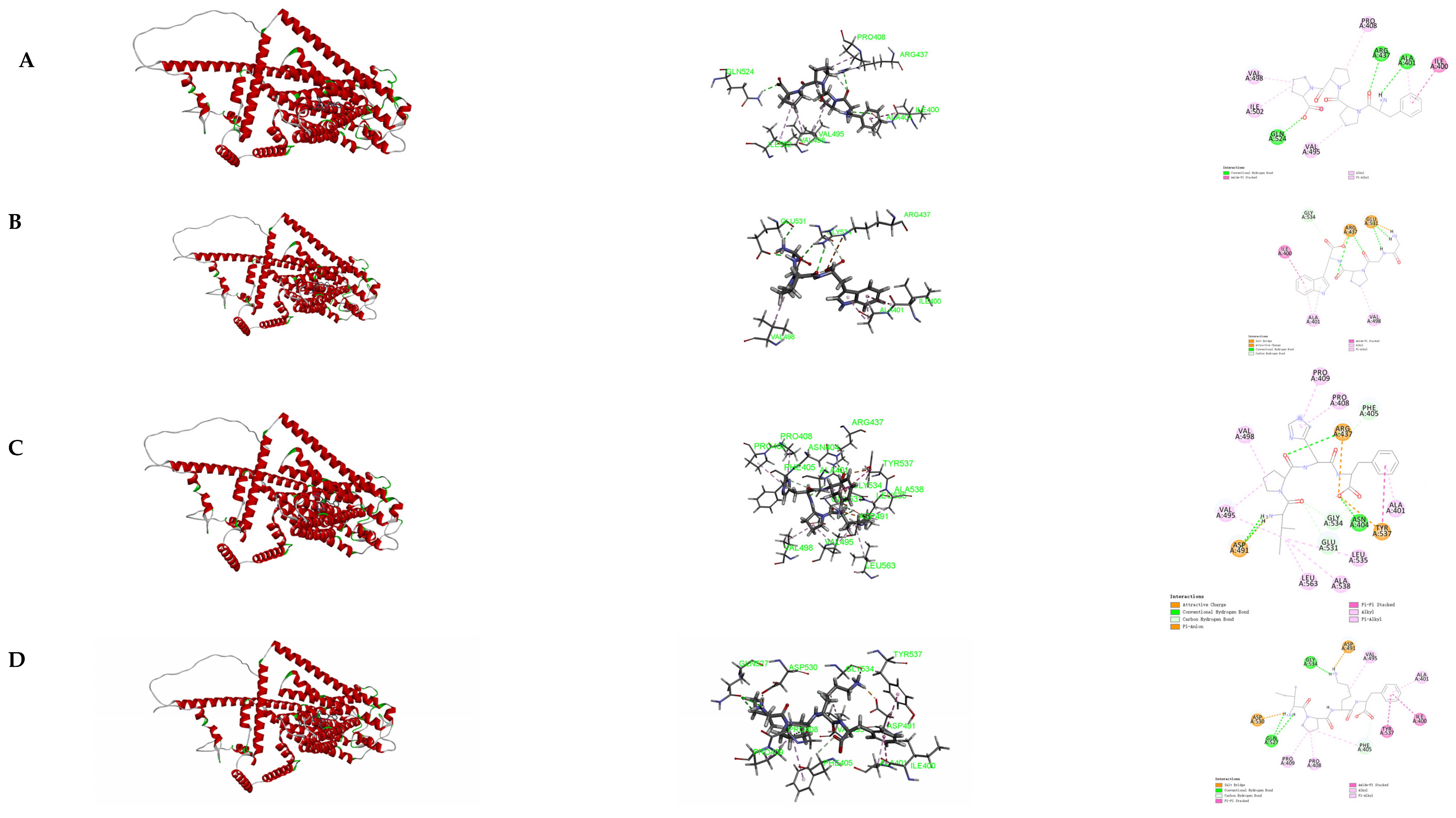
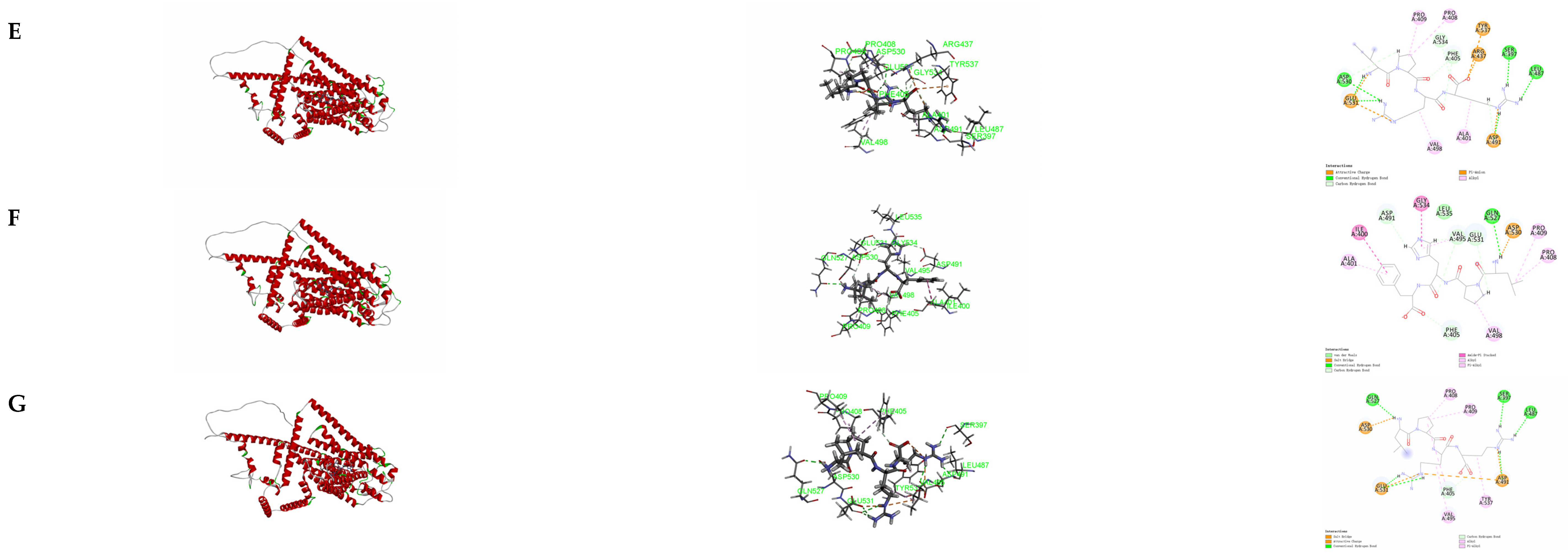
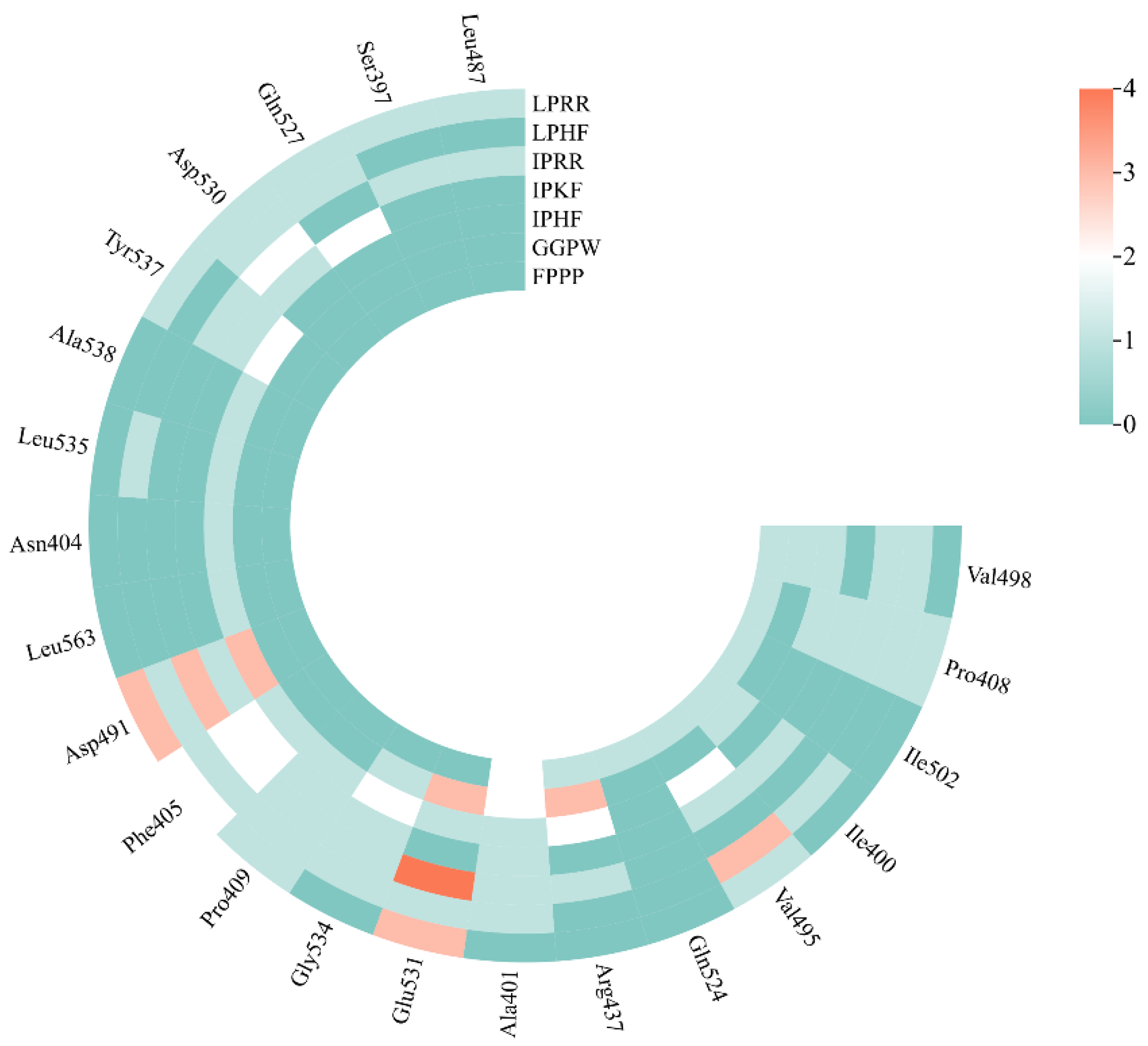
3.6. Molecular Docking of the Identified Peptide to the Salty Receptor TMC4
3.7. Molecular Docking of the Identified Peptide to the Salty Receptor TMC4 and Bioinformatics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Donnell, M.; Mente, A.; Alderman, M.H.; Brady, A.J.B.; Diaz, R.; Gupta, R.; López-Jaramillo, P.; Luft, F.C.; Lüscher, T.F.; Mancia, G.; et al. Salt and cardiovascular disease: Insufficient evidence to recommend low sodium intake. Eur. Heart J. 2020, 41, 3363–3373. [Google Scholar] [CrossRef]
- Chen, X.W.; Yang, D.X.; Guo, J.; Ruan, Q.J.; Yang, X.Q. Quillaja saponin-based hollow salt particles as solid carriers for enhancing sensory aroma with reduced sodium intake. Food Funct. 2018, 9, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kamleh, R.; Olabi, A.; Toufeili, I.; Daroub, H.; Younis, T.; Ajib, R. The effect of partial substitution of NaCl with KCl on the physicochemical, microbiological and sensory properties of Akkawi cheese. J Sci. Food Agric. 2015, 95, 1940–1948. [Google Scholar] [CrossRef]
- Silva, H.L.A.; Balthazar, C.F.; Silva, R.; Vieira, A.H.; Costa, R.G.B.; Esmerino, E.A.; Freitas, M.Q.; Cruz, A.G. Sodium reduction and flavor enhancer addition in probiotic Prato Cheese: Contributions of quantitative descriptive analysis and temporal dominance of sensations for sensory profiling. J. Dairy Sci. 2018, 101, 8837–8846. [Google Scholar] [CrossRef]
- Tada, M.; Shinoda, I.; Okai, H. L-Ornithyltaurine, a New Salty Peptide. J. Agric. Food Chem. 1984, 32, 992–996. [Google Scholar] [CrossRef]
- Le, B.; Yu, B.; Amin, M.S.; Liu, R.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. Salt taste receptors and associated salty/salt taste-enhancing peptides: A comprehensive review of structure and function. Trends Food Sci. Tech. 2022, 129, 657–666. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, J.; Fu, H.; Feng, Q.; Wang, J.; Li, C.; Xia, G.; Shang, W.; He, Y. Identification and virtual screening of novel salty peptides from hydrolysate of Tilapia by-product by batch molecular docking. Front. Nutr. 2024, 10, 1343209. [Google Scholar] [CrossRef]
- Schindler, A.; Dunkel, A.; Stähler, F.; Backes, M.; Ley, J.; Meyerhof, W.; Hofmann, T. Discovery of salt taste enhancing arginyl dipeptides in protein digests and fermented fish sauces by means of a sensomics approach. J. Agric. Food Chem. 2011, 59, 12578–12588. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; Lu, W.; Dang, Y.; Liu, Z.; Chen, G.; Wang, B.; Zhang, C.; Xiao, C. Novel salty peptides derived from bovine bone: Identification, taste characteristic, and salt-enhancing mechanism. Food Chem. 2024, 447, 139035. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Wu, D.; Zhang, Z.; Liu, P.; Li, W.; Yang, Y. Saltiness enhancing peptides isolated from enzymolysis extract of Lentinula edodes and their taste enhancing action mechanisms. LWT 2023, 188, 115430. [Google Scholar] [CrossRef]
- Moore, A.; Luckett, C.R.; Munafo, J.P. Taste-active dipeptides from hydrolyzed mushroom protein enhance saltiness. J. Agric. Food Chem. 2021, 69, 11947–11959. [Google Scholar] [CrossRef]
- Zheng, Y.; Tang, L.; Yu, M.; Li, T.; Song, H.; Li, P.; Li, K.; Xiong, J. Fractionation and identification of salty peptides from yeast extract. J. Food Sci. Technol. 2021, 58, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Song, S.; Zhou, T.; Zhang, H.; Cui, H.; Zhang, F.; Hayat, K.; Zhang, X.; Ho, C.T. Preparation of saltiness-enhancing enzymatic hydrolyzed pea protein and identification of the functional small peptides of salt reduction. J. Agric. Food Chem. 2023, 71, 8140–8149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Wang, M.; Blank, I.; Xu, J.; Chung, H.Y. Saltiness-enhancing peptides isolated from the Chinese commercial fermented soybean curds with potential applications in salt reduction. J. Agric. Food Chem. 2021, 69, 10272–10280. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Shiga, K.; Kodama, Y.; Imamura, M.; Uchida, R.; Obata, A.; Bamba, T.; Fukusaki, E. Analysis of the correlation between dipeptides and taste differences among soy sauces by using metabolomics-based component profiling. J. Biosci. Bioeng. 2014, 118, 56–63. [Google Scholar] [CrossRef]
- Kasahara, Y.; Narukawa, M.; Takeuchi, A.; Tominaga, M.; Abe, K.; Asakura, T. Molecular logic of salt taste reception in special reference to transmembrane Channel-like 4 (TMC4). J. Physiol. Sci. 2022, 72, 31. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Narukawa, M.; Ishimaru, Y.; Kanda, S.; Umatani, C.; Takayama, Y.; Tominaga, M.; Oka, Y.; Kondo, K.; Kondo, T.; et al. TMC4 is a novel chloride channel involved in high-concentration salt taste sensation. J. Physiol. Sci. 2021, 71, 23. [Google Scholar] [CrossRef] [PubMed]
- Freer, R.J.; Day, A.R.; Radding, J.A.; Schiffmann, E.; Aswanikumar, S.; Showell, H.J.; Becker, E.L. Further studies on the structural requirements for synthetic peptide chemoattractants. Biochemistry 1980, 19, 2404–2410. [Google Scholar] [CrossRef]
- Meng, H.; Cui, Z.; Yu, Y.; Li, Y.; Jiang, S.; Liu, Y. From molecular dynamics to taste sensory perception: A comprehensive study on the interaction of umami peptides with the T1R1/T1R3-VFT receptor. J. Agric. Food Chem. 2024, 72, 6533–6543. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Li, H.; Cao, W.; Chen, Z.; Zheng, H.; Gao, J.; Lin, H.; Zhu, G. Recent advances in taste transduction mechanism, analysis methods and strategies employed to improve the taste of taste peptides. Crit. Rev. Food Sci. Nutr. 2023, 1–20, Online ahead of print. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, H.; Zhang, Q.; Hayat, K.; Yu, J.; Hussain, S.; Tahir, M.U.; Zhang, X.; Ho, C.T. Taste improvement of Maillard reaction intermediates derived from enzymatic hydrolysates of pea protein. Food Res. Int. 2021, 140, 109985. [Google Scholar] [CrossRef]
- Fu, B.; Xu, X.; Zhang, X.; Cheng, S.; El-Seedi, H.R.; Du, M. Identification and characterisation of taste-enhancing peptides from oysters (Crassostrea gigas) via the Maillard reaction. Food Chem. 2023, 424, 136412. [Google Scholar] [CrossRef]
- Amin, M.N.G.; Kusnadi, J.; Hsu, J.-L.; Doerksen, R.J.; Huang, T.C. Identification of a novel umami peptide in Tempeh (Indonesian fermented soybean) and its binding mechanism to the umami receptor T1R. Food Chem. 2020, 333, 127411. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, L.; Miao, X.; Li, Y.; Liu, Y. The structure features of umami hexapeptides for the T1R1/T1R3 receptor. Food Chem. 2017, 221, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, J.; Niu, Y.; Sun, B.; Liu, Z.; Mao, X.; Zhang, Y. Virtual screening and characteristics of novel umami peptides from porcine type I collagen. Food Chem. 2024, 434, 137386. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, X.C.; Xiang, J.; Sun, W.; Tomasevic, I. Prospects and challenges for the application of salty and saltiness-enhancing peptides in low-sodium meat products. Meat Sci. 2023, 204, 109261. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Huang, X.; Chen, X.; Cai, X.; Huang, J.; Vincent, G.; Wang, S. Advances in flavor peptides with sodium-reducing ability: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–17, Online ahead of print. [Google Scholar] [CrossRef]
- Xie, X.; Dang, Y.; Pan, D.; Sun, Y.; Zhou, C.; He, J.; Gao, X. The enhancement and mechanism of the perception of saltiness by umami peptide from Ruditapes philippinarum and ham. Food Chem. 2023, 405, 134886. [Google Scholar] [CrossRef]
- Shan, Y.; Pu, D.; Zhang, J.; Zhang, L.; Huang, Y.; Li, P.; Xiong, J.; Li, K.; Zhang, Y. Decoding of the saltiness enhancement taste peptides from the yeast extract and molecular docking to the taste receptor T1R1/T1R3. J. Agric. Food Chem. 2022, 70, 14898–14906. [Google Scholar] [CrossRef]
- Bu, Y.; Zhou, Y.; Sun, C.; Zhu, W.; Li, X.; Li, J. Identification novel salty-enhancing peptides from sea cucumber collagen: AlphaFold2 modeling and molecular simulation. Food Bioprocess Technol. 2024, 17, 2435–2445. [Google Scholar] [CrossRef]
- Shen, D.-Y.; Pan, F.; Yang, Z.-C.; Song, H.L.; Zou, T.; Xiong, J.; Li, K.; Li, P.; Hu, N.; Xue, D. Identification of novel saltiness-enhancing peptides from yeast extract and their mechanism of action for transmembrane channel-like 4 (TMC4) protein through experimental and integrated computational modeling. Food Chem. 2022, 388, 132993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, X.; Pan, D.; Zhang, Z.; Zhou, T.; Dang, Y. Isolation, characterization and molecular docking of novel umami and umami-enhancing peptides from Ruditapes philippinarum. Food Chem. 2021, 343, 128522. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, X.; Wang, J.; Xu, Y.; Yi, S.; Zhu, W.; Mi, H.; Li, T.; Li, J. Identification, taste characteristics and molecular docking study of novel umami peptides derived from the aqueous extract of the Clam meretrix meretrix Linnaeus. Food Chem. 2020, 312, 126053. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Tirado–Rives, J. Molecular modeling of organic and biomolecular systems using BOSS and MCPRO. J. Comput. Chem. 2005, 26, 1689–1700. [Google Scholar] [CrossRef]
- Wang, R.; Lu, Y.; Wang, S. Comparative evaluation of 11 scoring functions for molecular docking. J. Med. Chem. 2003, 46, 2287–2303. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef]
| Molecular Weight | Percent (%) |
|---|---|
| >5 kDa | 17 |
| 3–5 kDa | 10 |
| 1–3 kDa | 20 |
| 100 Da–1 kDa | 49 |
| <100 Da | 4 |
| Species | Content (%) |
|---|---|
| Glu | 17.0 |
| Asp | 9.49 |
| Leu | 7.00 |
| Arg | 6.30 |
| Lys | 5.20 |
| Ser | 4.38 |
| Phe | 4.33 |
| Pro | 4.10 |
| Ile | 3.90 |
| Val | 3.83 |
| Ala | 3.55 |
| Gly | 3.41 |
| Tyr | 3.17 |
| Thr | 3.10 |
| His | 2.14 |
| Met | 1.15 |
| Total | 82.1 |
| Peptide Sequence | Peptide Score (−10LgP) | m/z (kg/C) |
|---|---|---|
| KPII | 56.97 | 470.3322 |
| KPIL | 56.97 | 470.3322 |
| KPLI | 56.97 | 470.3322 |
| KPLL | 56.97 | 470.3322 |
| APSI | 55.55 | 387.2217 |
| APSL | 55.55 | 387.2217 |
| GGSW | 55.14 | 406.1700 |
| IGGY | 54.74 | 409.2053 |
| LGGY | 54.74 | 409.2053 |
| SGGW | 54.73 | 406.1700 |
| AASF | 54.46 | 395.1902 |
| GSGW | 54.37 | 406.1700 |
| VGGI | 54.25 | 345.2112 |
| VGGL | 54.25 | 345.2112 |
| IAGY | 54.12 | 423.2215 |
| LAGY | 54.12 | 423.2215 |
| IVGY | 54.09 | 451.2529 |
| LVGY | 54.09 | 451.2529 |
| FGGP | 53.85 | 377.1799 |
| NGVF | 53.82 | 436.2166 |
| IGHI | 53.51 | 439.2643 |
| IGHL | 53.51 | 439.2643 |
| LGHI | 53.51 | 439.2643 |
| LGHL | 53.51 | 439.2643 |
| IPKF | 53.43 | 504.3156 |
| LPKF | 53.43 | 504.3156 |
| GGMI | 53.32 | 377.1833 |
| GGML | 53.32 | 377.1833 |
| GGPW | 53.32 | 416.1907 |
| IPRR | 53.17 | 541.3558 |
| LPRR | 53.17 | 541.3558 |
| AAGY | 52.95 | 381.1743 |
| IYGG | 52.89 | 409.2061 |
| LYGG | 52.89 | 409.2061 |
| PIAP | 52.84 | 397.2425 |
| PLAP | 52.84 | 397.2425 |
| IPAV | 52.73 | 399.2579 |
| LPAV | 52.73 | 399.2579 |
| VIYI | 52.71 | 507.3153 |
| VIYL | 52.71 | 507.3153 |
| VLYI | 52.71 | 507.3153 |
| VLYL | 52.71 | 507.3153 |
| GGSY | 52.66 | 383.1505 |
| TPAF | 52.31 | 435.2211 |
| IPHF | 52.29 | 513.2806 |
| LPHF | 52.29 | 513.2806 |
| ITFI | 52.24 | 493.2996 |
| ITFL | 52.24 | 493.2996 |
| LTFI | 52.24 | 493.2996 |
| LTFL | 52.24 | 493.2996 |
| KGII | 52.17 | 430.3005 |
| KGIL | 52.17 | 430.3005 |
| KGLI | 52.17 | 430.3005 |
| KGLL | 52.17 | 430.3005 |
| AGPY | 52.06 | 407.1905 |
| IAIT | 52.03 | 417.2683 |
| IALT | 52.03 | 417.2683 |
| LAIT | 52.03 | 417.2683 |
| LALT | 52.03 | 417.2683 |
| KIFN | 52.00 | 521.3060 |
| KLFN | 52.00 | 521.3060 |
| GGCF | 51.91 | 383.1327 |
| GGAW | 51.78 | 390.1743 |
| IHIP | 51.57 | 479.2962 |
| IHLP | 51.57 | 479.2962 |
| LHIP | 51.57 | 479.2962 |
| LHLP | 51.57 | 479.2962 |
| KGPR | 51.52 | 457.2856 |
| Model Name | PLDDT | PTM |
|---|---|---|
| RANK1 | 78.3 | 0.773 |
| RANK2 | 75.6 | 0.750 |
| RANK3 | 75.3 | 0.743 |
| RANK4 | 73.1 | 0.723 |
| RANK5 | 71.2 | 0.705 |
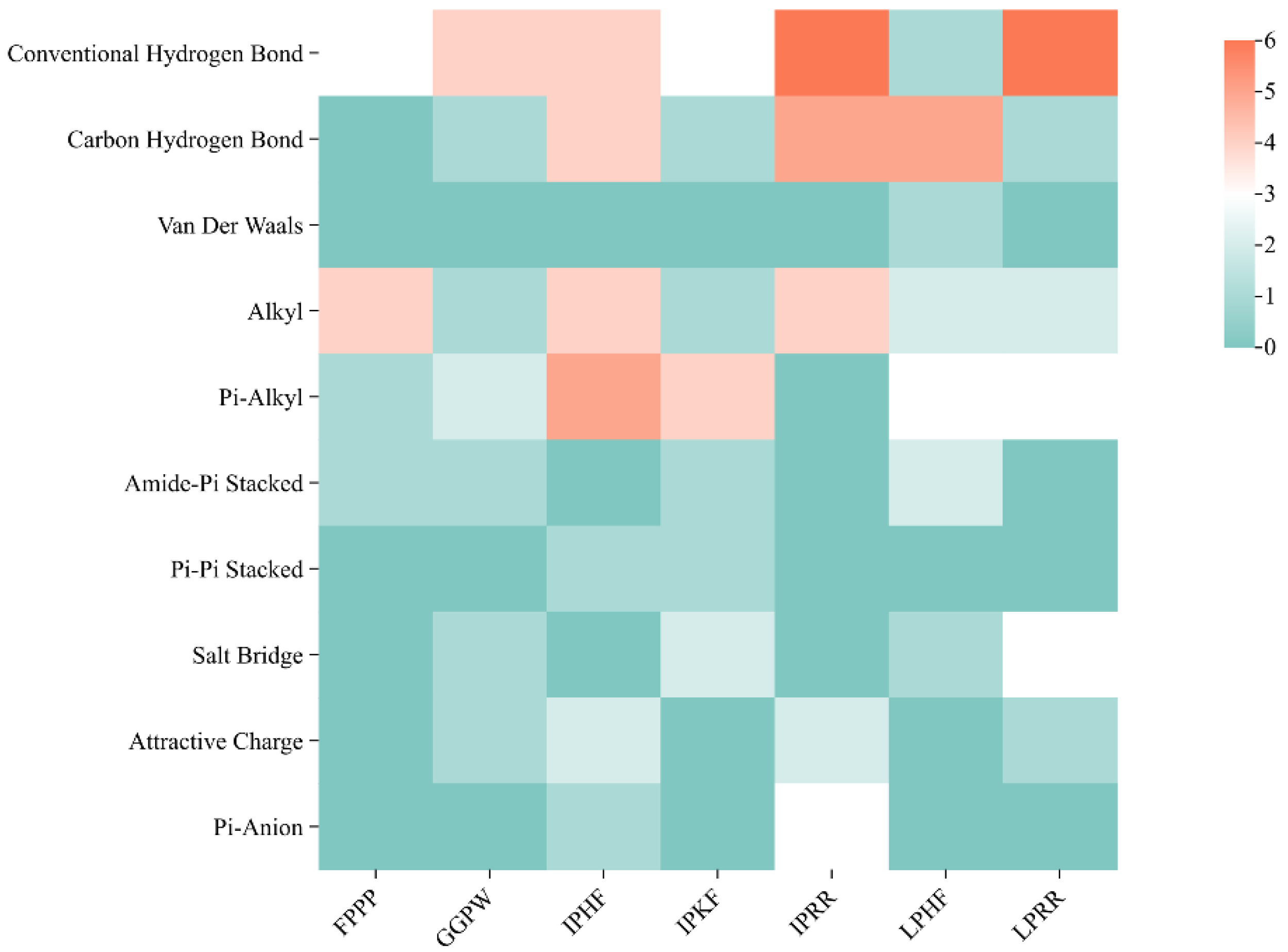
| Interaction Type | FPPP | GGPW | IPHF | IPKF | IPRR | LPHF | LPRR |
|---|---|---|---|---|---|---|---|
| Hydrogen Bonding | 3 | 5 | 8 | 4 | 11 | 6 | 7 |
| Hydrophobic Interactions | 6 | 4 | 10 | 7 | 4 | 7 | 5 |
| Electrostatic Interactions | 0 | 2 | 3 | 2 | 5 | 1 | 4 |
| List | Binding Energy (kcal/mol) | Toxicity | Sensitization | Stability |
|---|---|---|---|---|
| FPPP | −9.4 | − | − | − |
| IPHF | −9.1 | − | − | + |
| LPHF | −9.1 | − | − | + |
| GGPW | −9 | − | − | + |
| IPKF | −8.6 | − | − | + |
| IPRR | −8.8 | − | − | − |
| LPRR | −8.6 | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Yang, M.; Li, Z.; Tang, H.; Song, X.; Wang, X. Enzymatic Preparation, Identification by Transmembrane Channel-like 4 (TMC4) Protein, and Bioinformatics Analysis of New Salty Peptides from Soybean Protein Isolate. Foods 2024, 13, 2798. https://doi.org/10.3390/foods13172798
Zhao Z, Yang M, Li Z, Tang H, Song X, Wang X. Enzymatic Preparation, Identification by Transmembrane Channel-like 4 (TMC4) Protein, and Bioinformatics Analysis of New Salty Peptides from Soybean Protein Isolate. Foods. 2024; 13(17):2798. https://doi.org/10.3390/foods13172798
Chicago/Turabian StyleZhao, Ziying, Mingzhe Yang, Zhijiang Li, Huacheng Tang, Xuejian Song, and Xinhui Wang. 2024. "Enzymatic Preparation, Identification by Transmembrane Channel-like 4 (TMC4) Protein, and Bioinformatics Analysis of New Salty Peptides from Soybean Protein Isolate" Foods 13, no. 17: 2798. https://doi.org/10.3390/foods13172798
APA StyleZhao, Z., Yang, M., Li, Z., Tang, H., Song, X., & Wang, X. (2024). Enzymatic Preparation, Identification by Transmembrane Channel-like 4 (TMC4) Protein, and Bioinformatics Analysis of New Salty Peptides from Soybean Protein Isolate. Foods, 13(17), 2798. https://doi.org/10.3390/foods13172798







