Valorization of Pig Brains for Prime Quality Oil: A Comparative Evaluation of Organic-Solvent-Based and Solvent-Free Extractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Pig Brains
2.3. Extraction of Pig Brain Oil Using Different Techniques
2.3.1. Wet Rendering Process
2.3.2. Aqueous Saline Process
2.3.3. Bligh and Dyer Process
2.4. Determination of Extraction Yield
2.5. Color Analysis
2.6. Determination of Total Phospholipid (PL), Cholesterol, Carotenoid, and Tocopherol Contents
2.7. Determination of Fatty Acid Profiles
2.8. Determination of Lipolysis and Lipid Oxidation
2.9. Fourier Transform Infrared (FTIR) Spectroscopy
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield
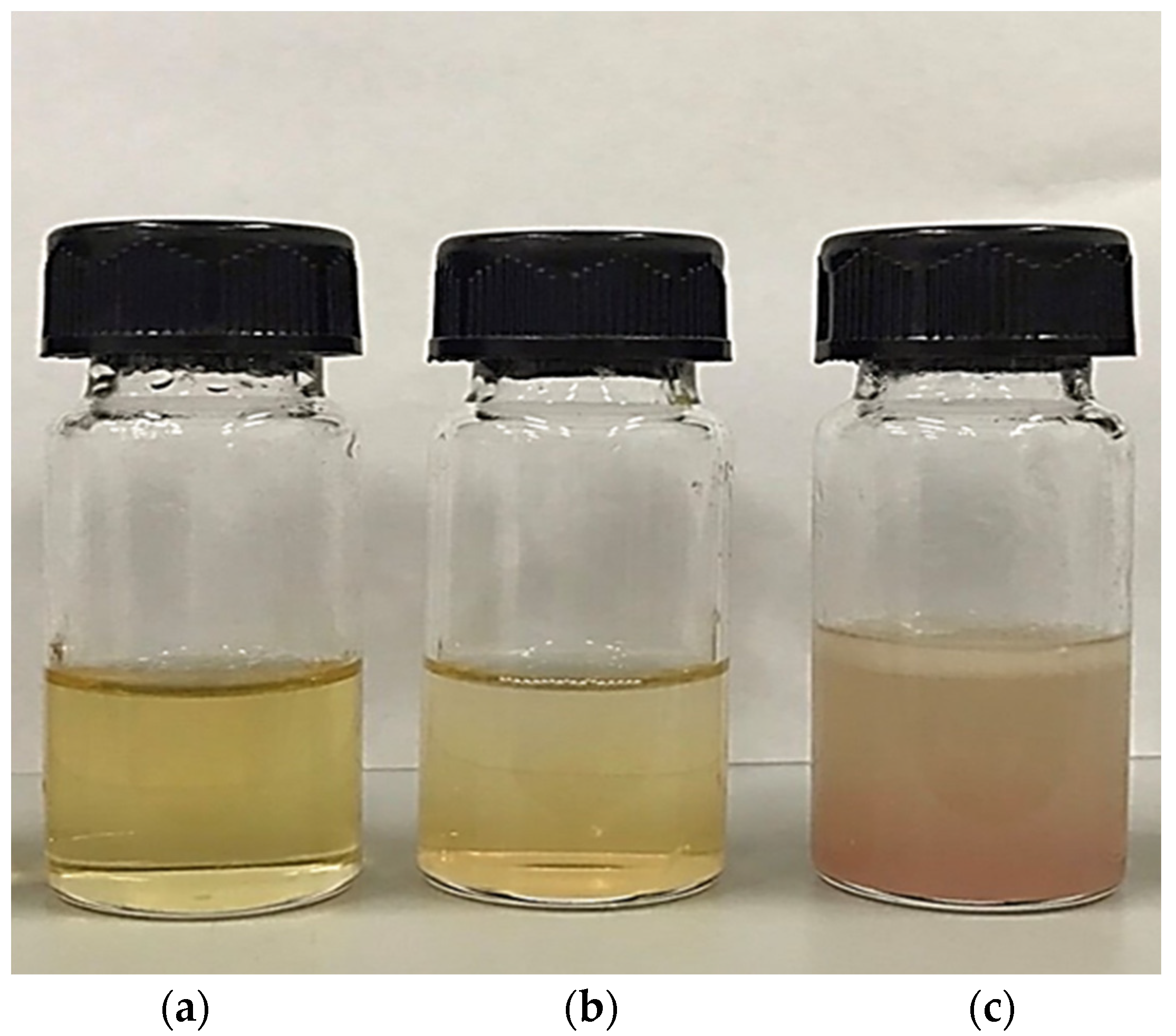
3.2. Color
3.3. Total PL, Cholesterol, Carotenoid, and Tocopherol Contents
3.4. Fatty Acid Profiles
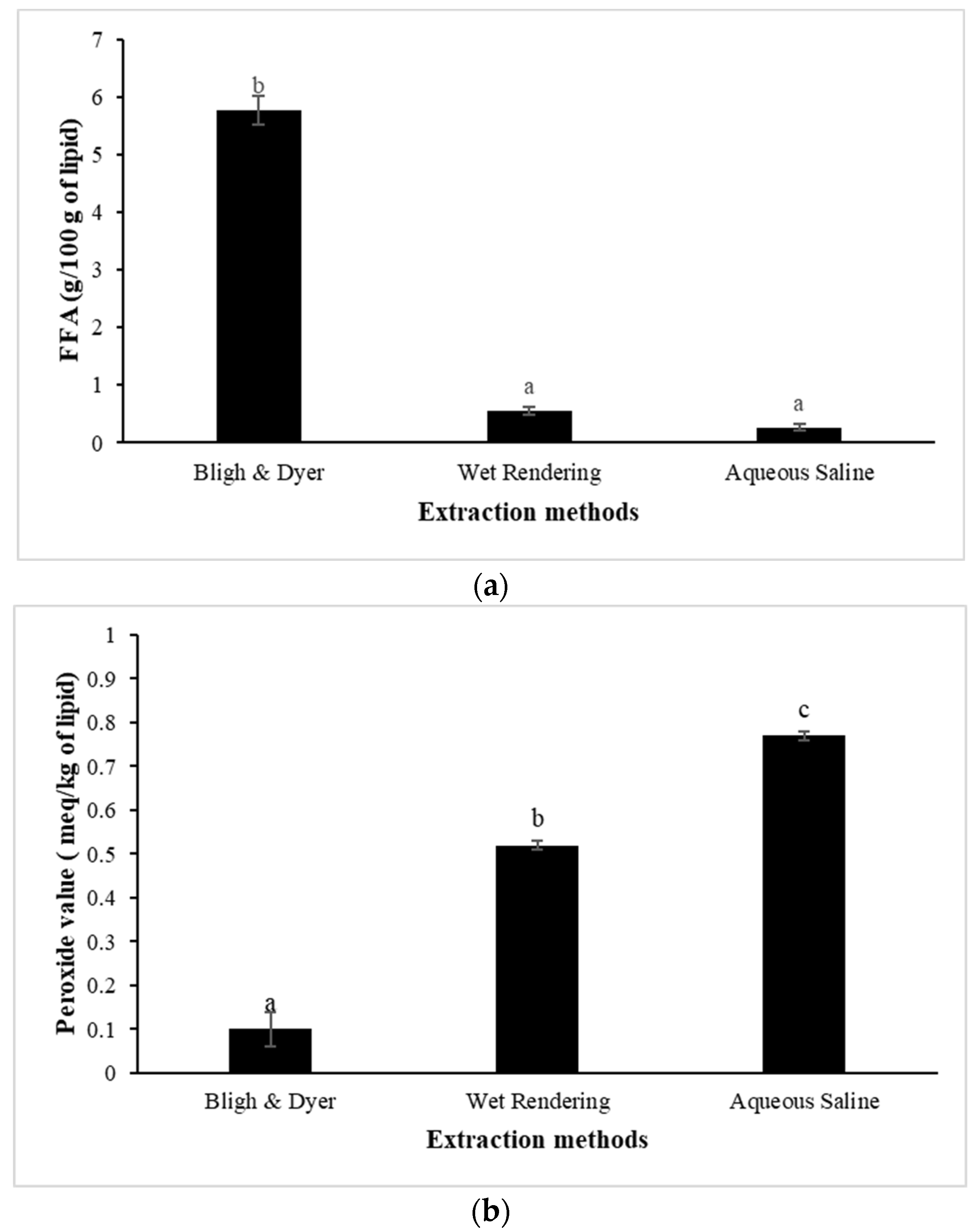
3.5. Lipolysis and Lipid Oxidation
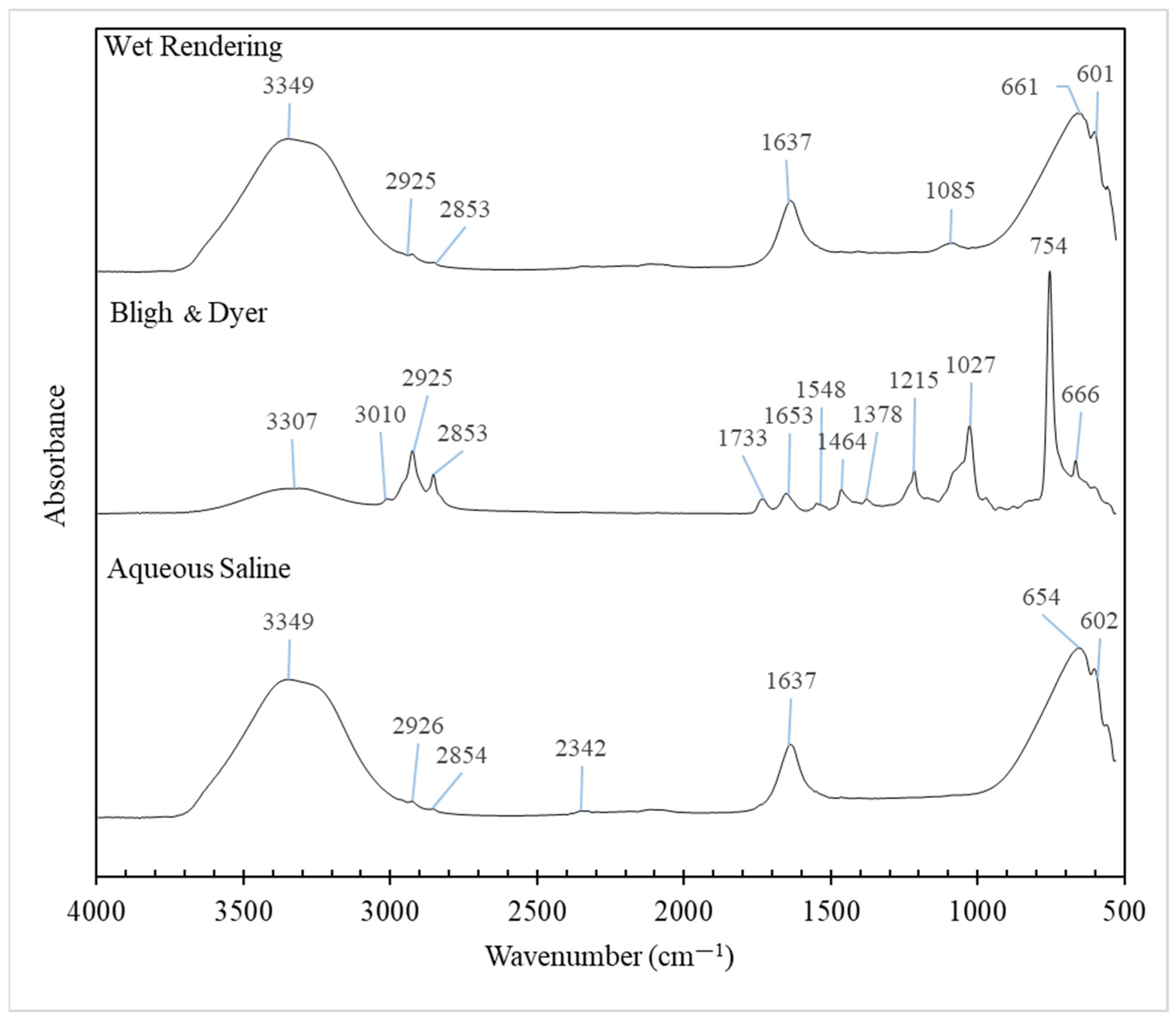
3.6. FTIR Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ockerman, H.W.; Basu, L. By-products. In Encyclopedia of Meat Sciences, 1st ed.; Jensen, W.K., Devine, C., Dikeman, M., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 104–112. [Google Scholar]
- Department of Livestock. Statistics Livestock in Thailand; Bureau of Livestock Development and Development, Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2024.
- Toldrá, F.; Aristoy, M.-C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.E.; Kenney, P.B. Edible By-products from the Production and Processing of Muscle Foods. In Muscle Foods, 1st ed.; Campbell, R.E., Kenney, P.B., Eds.; Springer: Singapore, 1994; pp. 79–105. [Google Scholar]
- Nollet, L.M.L.; Toldrá, F. Introduction of offal meat: Definitions, regions, cultures, generalities. In Handbook of Analysis of Edible Animal By-Products, 1st ed.; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 3–11. [Google Scholar]
- Chanted, J.; Panpipat, W.; Panya, A.; Phonsatta, N.; Cheong, L.Z.; Chaijan, M. Compositional features and nutritional value of pig brain: Potential and challenges as a sustainable source of nutrients. Foods 2021, 10, 2943. [Google Scholar] [CrossRef]
- Chanted, J.; Panpipat, W.; Cheong, L.Z.; Chaijan, M. Recovery of functional proteins from pig brain using pH-shift processes. Foods. 2022, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, B.; Rui, H. Effects of microwave rendering on the yield and characteristics of chicken fat from broiler abdominal fat tissue. J. Food Sci. Technol. 2013, 50, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Soares Dias, A.P.; Ramos, M.; Rijo, B. Rendering of beef tallow for biodiesel production: Microwave versus boiling water and acetone fat extraction. Processes 2022, 10, 666. [Google Scholar] [CrossRef]
- Chantachum, S.; Benjakul, S.; Sriwirat, N. Separation and quality of fish oil from precooked and non-precooked tuna heads. Food Chem. 2000, 69, 289–294. [Google Scholar] [CrossRef]
- Pudtikajorn, K.; Benjakul, S. Simple wet rendering method for extraction of prime quality oil from skipjack tuna eyeballs. Eur. J. Lipid Sci. Technol. 2020, 122, 2000077. [Google Scholar] [CrossRef]
- Cho, M.J.; Kim, H.J. Effects of rendering and α-tocopherol addition on the oxidative stability of horse fat. Food Sci. Biotechnol. 2020, 29, 169–177. [Google Scholar] [CrossRef]
- Tangsanthatkun, J.; Peanparkdee, M.; Katekhong, W.; Harnsilawat, T.; Tan, C.P.; Klinkesorn, U. Application of aqueous saline process to extract silkworm pupae oil (Bombyx mori): Process optimization and composition analysis. Foods 2022, 11, 291. [Google Scholar] [CrossRef]
- Sui, Y.; Huang, W.-C.; Wu, Y.; Qi, X.; Mao, X. Lipid extraction from Greenland halibut (Reinhardtius hippoglossoides) by-product in low-voltage DC electric field and its mechanism. J. Clean. Prod. 2021, 283, 124673. [Google Scholar] [CrossRef]
- Cruz, S.; Yousfi, K.; Pérez, A.G.; Mariscal, C.; Garcia, J.M. Salt improves physical extraction of olive oil. Eur. Food Res. Technol. 2007, 225, 359–365. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Tzompa-Sosa, D.A.; Yi, L.; Van Valenberg, H.J.F.; Lakemond, C.M.M. Four insect oils as food ingredient: Physical and chemical characterisation of insect oils obtained by an aqueous oil extraction. J. Insects Food Feed. 2019, 5, 279–292. [Google Scholar] [CrossRef]
- Kadioglu, S.I.; Phan, T.T.; Sabatini, D.A. Surfactant-based oil extraction of corn germ. J. Am. Oil Chem. Soc. 2011, 88, 863–869. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Beyer, R.S.; Jensen, L.S. Overestimation of the cholesterol content of eggs. J. Agric. Food Chem. 1989, 37, 917–920. [Google Scholar] [CrossRef]
- de Carvalho, L.M.J.; Gomes, P.B.; de Oliveira Godoy, R.L.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.R.A.; Ramos, S.R.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Kayden, H.J.; Chow, C.K.; Bjornson, L.K. Spectrophotometric method for determination of tocopherol in red blood cells. J. Lipid Res. 1973, 14, 533–540. [Google Scholar] [CrossRef]
- Chinarak, K.; Panpipat, W.; Summpunn, P.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Chaijan, M. Insights into the effects of dietary supplements on the nutritional composition and growth performance of sago palm weevil (Rhynchophorus ferrugineus) larvae. Food Chem. 2021, 363, 130279. [Google Scholar] [CrossRef]
- Lowry, R.R.; Tinsley, I.J. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef]
- Low, L.K.; Ng. Determination of peroxide value. In Laboratory Manual on Analytical Methods and Procedures for Fish and Fish Products, 1st ed.; Hasegawa, H., Ed.; Marine Fisheries Research Department, Southeast Asian Fisheries Development Centre: Singapore, 1978; pp. C7.1–C7.3. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Method Enzymol. 1978, 52, 302–310. [Google Scholar]
- Chaijan, M.; Panpipat, W. Feasibility of a pH driven method for maximizing protein recovery of over-salted albumen. Food Biosci. 2018, 24, 89–94. [Google Scholar] [CrossRef]
- Yusoh, N.A.M.; Man, R.C.; Azman, N.A.M.; Shaarani, S.M.; Mudalip, S.K.A.; Sulaiman, S.Z.; Arshad, Z.I.M. Recovery of antioxidant from Decapterus macarellus waste using wet rendering method. Mater. Today 2022, 57, 1382–1388. [Google Scholar] [CrossRef]
- Bullmore, E.; Barnes, A.; Bassett, D.S.; Fornito, A.; Kitzbichler, M.; Meunier, D.; Suckling, J. Generic aspects of complexity in brain imaging data and other biological systems. Neuroimage 2009, 47, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.E.; Barboza, J.C.S.; Da Silva, M.L.C.P. Production of ethylic biodiesel from tilapia visceral oil. Renew. Energy Power Qual. J. 2013, 13, 412–415. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F. Role of lipids in brain injury and diseases. Future Lipidol. 2007, 2, 403–422. [Google Scholar] [CrossRef]
- Pichot, R.; Watson, R.L.; Norton, I.T. Phospholipids at the interface: Current trends and challenges. Int. J. Mol. Sci. 2013, 14, 11767–11794. [Google Scholar] [CrossRef]
- Lu, F.S.H.; Nielsen, N.S.; Baron, C.P.; Jensen, L.H.S.; Jacobsen, C. Physico-chemical properties of marine phospholipid emulsions. J. Am. Oil Chem. Soc. 2012, 89, 2011–2024. [Google Scholar] [CrossRef]
- Pan, Y.; Tikekar, R.V.; Nitin, N. Effect of antioxidant properties of lecithin emulsifier on oxidative stability of encapsulated bioactive compounds. Int. J. Pharm. 2013, 450, 129–137. [Google Scholar] [CrossRef]
- Chinarak, K.; Chaijan, M.; Panpipat, W. Farm-raised sago palm weevil (Rhynchophorus ferrugineus) larvae: Potential and challenges for promising source of nutrients. J. Food Compos. Anal. 2020, 92, 103542. [Google Scholar] [CrossRef]
- Albalat, A.; Nadler, L.E.; Foo, N.; Dick, J.R.; Watts, A.J.; Philp, H.; Monroig, O. Lipid composition of oil extracted from wasted Norway lobster (Nephrops norvegicus) heads and comparison with oil extracted from Antarctic krill (Euphasia superba). Marine Drugs 2016, 14, 219. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Basic composition, antioxidant activity and nanoemulsion behavior of oil from mantis shrimp (Oratosquilla nepa). Food Biosci. 2019, 31, 100448. [Google Scholar] [CrossRef]
- Rahmati-Ahmadabad, S.; Broom, D.R.; Ghanbari-Niaki, A.; Shirvani, H. Effects of exercise on reverse cholesterol transport: A systemized narrative review of animal studies. Life Sci. 2019, 224, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.L.; Burrin, D.G.; Mensink, R.P. Plant-based sterols and stanols in health & disease: “Consequences of human development in a plant-based environment?”. Prog. Lipid Res. 2019, 74, 87–102. [Google Scholar] [PubMed]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar]
- Naviglio, D.; Gallo, M.; Le Grottaglie, L.; Scala, C.; Ferrara, L.; Santini, A. Determination of cholesterol in Italian chicken eggs. Food Chem. 2012, 132, 701–708. [Google Scholar] [CrossRef]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W. Chemical compositions of the roes from skipjack, tongol and bonito. Food Chem. 2011, 124, 1328–1334. [Google Scholar] [CrossRef]
- Sweeney, J.P.; WeBirauch, J.L. Summary of available data for cholesterol in foods and methods for its determination. Crit. Rev. Food Sci. Nutr. 1976, 8, 131–159. [Google Scholar] [CrossRef]
- Pihl, A. Cholesterol studies I. The cholesterol content of foods. Scand. J. Clin. Lab. Investig. 1952, 4, 115–121. [Google Scholar] [CrossRef]
- Ellis, G.W.; Gardner, J.A. The origin and destiny of cholesterol in the animal organism. Part IX.—On the cholesterol content of the tissues, other than liver, of rabbits under various diets and during inanition. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. 1912, 85, 385–393. [Google Scholar]
- Kritchevsky, D.; Tepper, S.A. The free and ester sterol content of various foodstuffs. J. Nutr. 1961, 74, 441–444. [Google Scholar] [CrossRef]
- Stromer, M.H.; Goll, D.E.; Roberts, J.H. Cholesterol in subcutaneous and intramuscular lipid depots from bovine carcasses of different maturity and fatness. J. Anim. Sci. 1966, 25, 1145–1147. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef] [PubMed]
- Mawazi, S.M.; Ann, T.J.; Widodo, R.T. Application of niosomes in cosmetics: A systematic review. Cosmetics 2022, 9, 127. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Palozza, P.; Krinsky, N.I. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch. Biochem. Biophys. 1992, 297, 291–295. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; Santoso, J.; Trilaksani, W.; Nurilmala, M. Extraction and stability of carotenoid-containing lipids from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). J. Food Process. Preserv. 2015, 39, 10–18. [Google Scholar] [CrossRef]
- Nukala, U.; Thakkar, S.; Krager, K.J.; Breen, P.J.; Compadre, C.M.; Aykin-Burns, N. Antioxidant tocols as radiation countermeasures (challenges to be addressed to use tocols as radiation countermeasures in humans). Antioxidants 2018, 7, 33. [Google Scholar] [CrossRef]
- Suárez-Jiménez, G.M.; López-Saiz, C.M.; Ramírez-Guerra, H.E.; Ezquerra-Brauer, J.M.; Ruiz-Cruz, S.; Torres-Arreola, W. Role of endogenous and exogenous tocopherols in the lipid stability of marine oil systems: A review. Int. J. Mol. Sci. 2016, 17, 1968. [Google Scholar] [CrossRef] [PubMed]
- Abbey, M.; Nestel, P.J. Plasma cholesteryl ester transfer protein activity is increased when trans-elaidic acid is substituted for cis-oleic acid in the diet. Atherosclerosis 1994, 106, 99–107. [Google Scholar] [CrossRef]
- Tardy, A.L.; Morio, B.; Chardigny, J.M.; Malpuech-Brugere, C. Ruminant and industrial sources of trans-fat and cardiovascular and diabetic diseases. Nutr. Res. Rev. 2011, 24, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Zsombok, A.; Eckel, R.H. Lipid processing in the brain: A key regulator of systemic metabolism. Front. Endocrinol. 2017, 8, 258596. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Huang, H.; Sacks, F.M.; Rimm, E.B.; Wang, M.; Siscovick, D.S. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: A cohort study. Ann. Intern. Med. 2013, 158, 515–525. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, X.; Zeng, Q.; Zhong, R.; Guo, Y.; Shi, F.; Liang, P. The inhibitory effect of large yellow croaker roe phospholipid as a potential antioxidant on fish oil oxidation stability. Food Biosci. 2023, 56, 103291. [Google Scholar] [CrossRef]
- CXS 211-1999; Standard for Named Animal Fats. Codex Alimentarius Commission: Rome, Italy, 1999.
- Borompichaichartkul, C.; Chinprahast, N.; Devahastin, S.; Wiset, L.; Poomsa-Ad, N.; Ratchapo, T. Multistage heat pump drying of macadamia nut under modified atmosphere. Int. Food Res. J. 2013, 20, 2199–2203. [Google Scholar]
- Mei, W.S.C.; Ismail, A.; Mohd Esa, N.; Akowuah, G.A.; Wai, H.C.; Seng, Y.H. The effectiveness of rambutan (Nephelium lappaceum L.) extract in stabilization of sunflower oil under accelerated conditions. Antioxidants 2014, 3, 371–386. [Google Scholar] [CrossRef]
- Jo, H.G.; Chilakala, R.; Kim, M.J.; Sin, Y.S.; Lee, K.S.; Cheong, S.H. Assessment of the effects of salt and Salicornia herbacea L. on physiochemical, nutritional, and quality parameters for extending the shelf-life of semi-dried mullets (Chelon haematocheilus). Foods 2022, 11, 597. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Krafft, C.; Neudert, L.; Simat, T.; Salzer, R. Near infrared Raman spectra of human brain lipids. Spectrochim Acta A Mol. Biomol. Spectrosc. 2005, 61, 1529–1535. [Google Scholar] [CrossRef]
- Dreissig, I.; Machill, S.; Salzer, R.; Krafft, C. Quantification of brain lipids by FTIR spectroscopy and partial least squares regression. Spectrochim Acta A Mol. Biomol. Spectrosc. 2009, 71, 2069–2075. [Google Scholar] [CrossRef]

| Parameters | Extraction Method | ||
|---|---|---|---|
| Bligh and Dyer | Wet Rendering | Aqueous Saline | |
| Extraction yield (%) | 6.61 ± 1.03 b | 13.09 ± 0.18 c | 2.43 ± 0.30 a |
| Color | |||
| L* | 11.45 ± 0.69 c | 7.42 ± 0.16 a | 10.61 ± 0.14 b |
| a* | −0.20 ± 0.07 b | −0.45 ± 0.28 a | −0.06 ± 0.45 c |
| b* | 0.28 ± 0.20 b | 0.27 ± 0.15 b | −0.66 ± 1.39 a |
| Redness index (a*/b*) | −0.71 ± 0.14 b | −1.67 ± 0.21 a | 0.09 ± 0.12 c |
| Compositions | Extraction Method | ||
|---|---|---|---|
| Bligh and Dyer | Wet Rendering | Aqueous Saline | |
| Total phospholipid (g/100 g lipid) | 3.22 ± 0.05 b | 0.08 ± 0.01 a | 0.11 ± 0.01 a |
| Total cholesterol (mg /100 g lipid) | 4305.70 ± 0.05 c | 45.65 ± 0.01 a | 173.72 ± 0.01 b |
| Total carotenoids (mg/100 g lipid) | 0.09 ± 0.90 c | 0.05 ± 0.88 b | 0.02 ± 0.27 a |
| Total tocopherols (mg/100 g lipid) | 66.24 ± 0.01 c | 33.07 ± 0.00 b | 27.35 ± 0.00 a |
| Fatty Acids (% of Total Fatty Acid) | Extraction Method | ||
|---|---|---|---|
| Bligh and Dyer | Wet Rendering | Aqueous Saline | |
| Saturated fatty acid (SFA) | |||
| Butyric acid (C4:0) | 0.05 ± 0.02 c | 11.68 ± 0.40 a | 6.77 ± 1.44 b |
| Caprylic acid (C8:0) | 0.01 ± 0.01 b | 1.63 ± 0.24 a | 1.40 ± 0.38 a |
| Capric acid (C10:0) | 0.004 ± 0.00 b | 0.27 ± 0.16 a | 0.22 ± 0.03 a |
| Undecylic acid (C11:0) | 0.02 ± 0.00 b | 3.56 ± 0.78 a | 2.88 ± 0.41 a |
| Lauric acid (C12:0) | 0.005 ± 0.00 c | 0.38 ± 0.10 b | 0.51 ± 0.03 a |
| Myristic acid (C14:0) | 0.44 ± 0.30 b | 5.27 ± 0.43 a | 4.58 ± 0.79 a |
| Pentadecanoic acid (C15:0) | 0.08 ± 0.01 b | 0.87 ± 0.11 a | 0.74 ± 0.09 a |
| Palmitic acid (C16:0) | 27.26 ± 0.97 a | 21.50 ± 1.49 b | 19.17 ± 1.21 b |
| Heptadecanoic acid (C17:0) | 0.35 ± 0.02 | nd * | nd |
| Stearic acid (C18:0) | 16.03 ± 0.59 b | 39.79 ± 1.69 a | 35.95 ± 3.58 a |
| Arachidic acid (C20:0) | 0.19 ± 0.01 b | 1.02 ± 0.22 a | nd |
| Heneicosanoic acid (C21:0) | 0.01 ± 0.00 | nd | nd |
| Behenic acid (C22:0) | 0.08 ± 0.01 | nd | nd |
| Tricosylic acid (C23:0) | 0.47 ± 0.03 | nd | nd |
| Lignoceric acid (C24:0) | 0.06 ± 0.00 | nd | nd |
| Total SFA | 45.04 ± 1.31 c | 86.57 ± 2.26 a | 77.58 ± 3.70 b |
| Monounsaturated fatty acid (MUFA) | |||
| cis-10-pentadecenoic acid (C15:1) | 12.93 ± 0.91 | nd | nd |
| Palmitoleic acid (C16:1 n-7) | 0.91 ± 0.04 | nd | nd |
| Cis-10-heptadecenoic acid (C17:1) | 0.23 ± 0.02 | nd | nd |
| Elaidic acid (C18:1 n-9 trans) | 20.64 ± 0.60 a | 11.51 ± 2.87 b | 18.22 ± 2.77 a |
| Cis-11-eicosenoic acid (C20:1 n-11) | 1.64 ± 0.07 | nd | nd |
| Cis-13-docosenoate (Erucate) (C22:1) | 0.33 ± 0.03 | nd | nd |
| Cis-15-tetracosenoate (Nervonate) (C24:1) | 1.74 ± 0.14 | nd | nd |
| Total MUFA | 38.41 ± 1.40 a | 11.51 ± 2.87 c | 18.22 ± 2.77 b |
| Polyunsaturated fatty acid (PUFA) | |||
| Cis-9,12-octadecadienoic acid (C18:2 n-6) | 1.67 ± 0.06 b | 2.12 ± 1.92 b | 5.51 ± 4.20 a |
| Cis-9,12,15-octadecatrienoic acid (C18:3 n-3) | 0.03 ± 0.01 | nd | nd |
| Cis-6,9,12-octadecatrienoic acid (C18:3 n-6) | 0.04 ± 0.00 | nd | nd |
| Cis-11, 14-eicosadienoic acid (C20:2 n-6) | 0.57 ± 0.04 | nd | nd |
| Cis-8, 11, 14-eicosatrienoic acid (C20:3 n-6) | 0.67 ± 0.05 | nd | nd |
| Cis-5, 8, 11, 14, 17-eicosapentaenoic acid (C20:5 n-3, EPA) | 0.04 ± 0.01 | nd | nd |
| Cis-13,16-docosadienoic acid (C22:2 n-6) | 0.31 ± 0.39 | nd | nd |
| Cis-4,7,10,13,16,19-docosahexaenoic acid (C22:6 n-3, DHA) | 13.21 ± 0.97 | nd | nd |
| Total PUFA | 16.54 ± 0.66 a | 2.12 ± 1.92 c | 5.51 ± 4.20 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanted, J.; Anantawat, V.; Wongnen, C.; Aewsiri, T.; Panpipat, W.; Panya, A.; Phonsatta, N.; Cheong, L.-Z.; Chaijan, M. Valorization of Pig Brains for Prime Quality Oil: A Comparative Evaluation of Organic-Solvent-Based and Solvent-Free Extractions. Foods 2024, 13, 2818. https://doi.org/10.3390/foods13172818
Chanted J, Anantawat V, Wongnen C, Aewsiri T, Panpipat W, Panya A, Phonsatta N, Cheong L-Z, Chaijan M. Valorization of Pig Brains for Prime Quality Oil: A Comparative Evaluation of Organic-Solvent-Based and Solvent-Free Extractions. Foods. 2024; 13(17):2818. https://doi.org/10.3390/foods13172818
Chicago/Turabian StyleChanted, Jaruwan, Visaka Anantawat, Chantira Wongnen, Tanong Aewsiri, Worawan Panpipat, Atikorn Panya, Natthaporn Phonsatta, Ling-Zhi Cheong, and Manat Chaijan. 2024. "Valorization of Pig Brains for Prime Quality Oil: A Comparative Evaluation of Organic-Solvent-Based and Solvent-Free Extractions" Foods 13, no. 17: 2818. https://doi.org/10.3390/foods13172818








