Sustainable Production of Ulva Oligosaccharides via Enzymatic Hydrolysis: A Review on Ulvan Lyase
Abstract
:1. Introduction
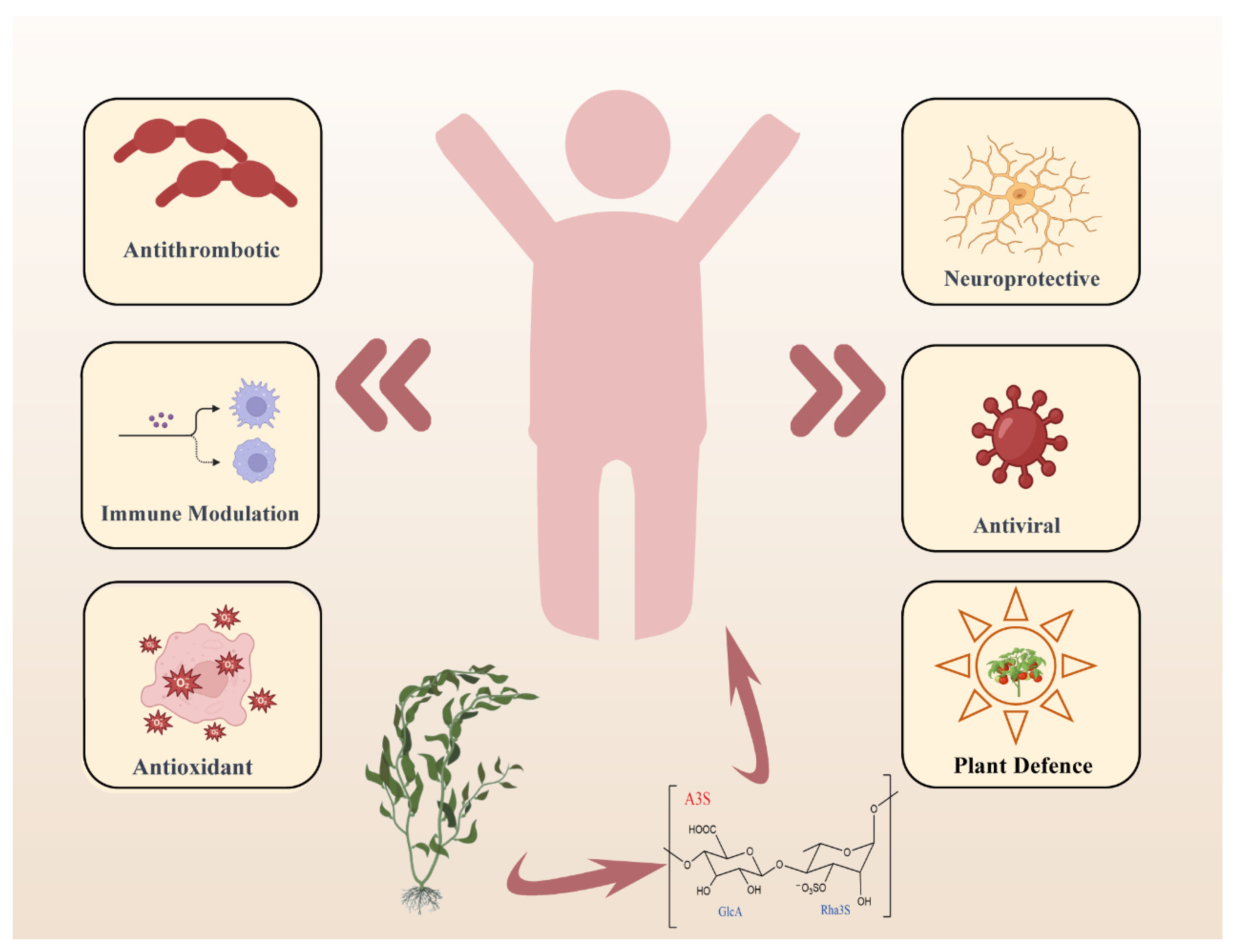
2. The Biological Activity of Ulvan Polysaccharides and Oligosaccharides
3. Source, Properties, Structures, and Catalytic Mechanism of the Ulvan Lyase
3.1. Sources and Categories of Ulvan Lyase
3.2. Enzymatic Properties of Ulvan Lyase
3.3. The Resolved 3D Structure of Ulvan Lyase
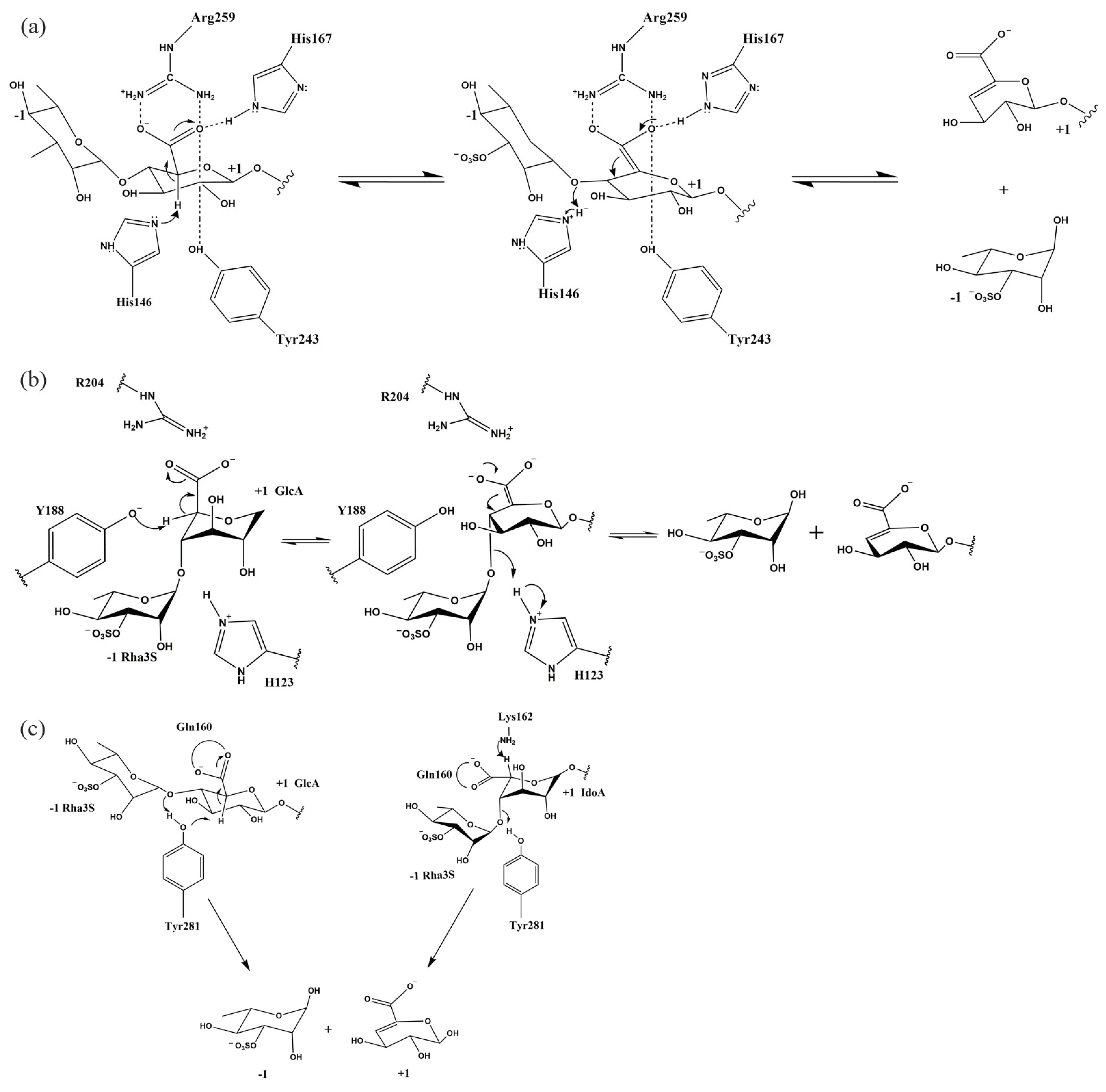
3.4. Catalytic Mechanism of Ulvan Lyase
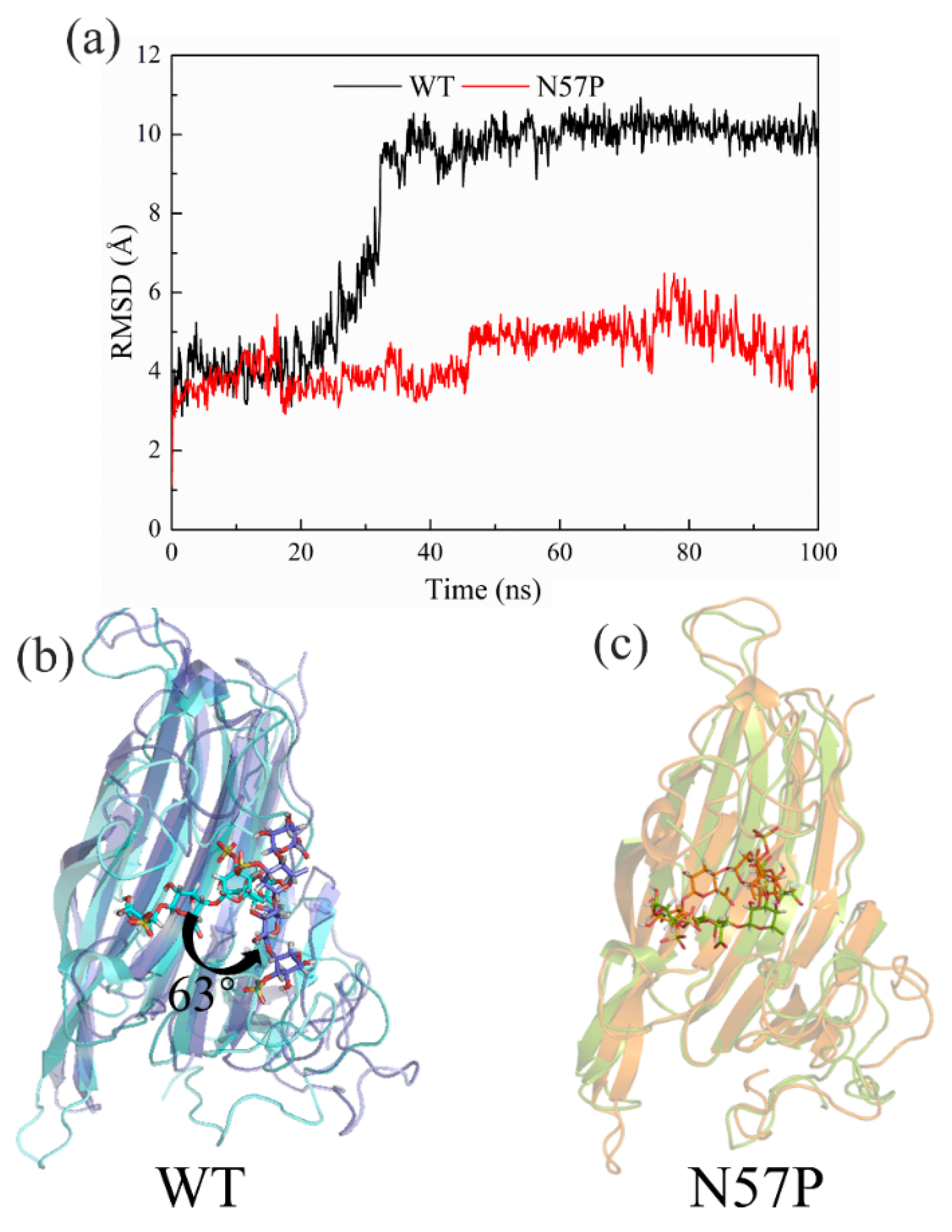
4. Molecular Modification of Ulvan Lyase
5. Production of Ulvan Lyase
6. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Kumar, M.; Soni, T.; Vivekanand, V.; Pareek, N. Insights into the genetic and metabolic engineering approaches to enhance the competence of microalgae as biofuel resource: A review. Bioresour. Technol. 2021, 339, 125597. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef]
- Chapman, R.L. Algae: The world’s most important “plants”—An introduction. Mitig. Adapt. Strateg. Glob. Chang. 2010, 18, 5–12. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Šubarić, D.; Roje, M.; Babić, J.; Jerković, I.; Jokić, S. Recent advances on macroalgal pigments and their biological activities (2016–2021). Algal Res. 2022, 65, 102748. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A practical perspective on ulvan extracted from green algae. J. Appl. Phycol. 2012, 25, 407–424. [Google Scholar] [CrossRef]
- Lahaye, M.; Axelos, M.A.V. Gelling properties of water-soluble polysaccharides from proliferating marine green seaweeds (Ulva spp.). Carbohydr. Polym. 1993, 22, 261–265. [Google Scholar] [CrossRef]
- Robic, A.; Gaillard, C.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Ultrastructure of ulvan: A polysaccharide from green seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Li, H.; Wang, P.; Du, C.; Ye, H.; Zuo, S.; Guan, H.; Wang, P. Structural characterization of ulvan extracted from Ulva clathrata assisted by an ulvan lyase. Carbohydr. Polym. 2020, 229, 115497. [Google Scholar] [CrossRef]
- Coste, O.; Malta, E.J.; López, J.C.; Fernández-Díaz, C. Production of sulfated oligosaccharides from the seaweed Ulva sp. using a new ulvan-degrading enzymatic bacterial crude extract. Algal Res. 2015, 10, 224–231. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. J. Appl. Phycol. 2012, 24, 715–723. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Li, Q.; Hu, F.; Zhu, B.; Ni, F.; Yao, Z. Insights into ulvan lyase: Review of source, biochemical characteristics, structure and catalytic mechanism. Crit. Rev. Biotechnol. 2020, 40, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.C.; Cao, S.S.; Zhu, B.W.; Li, Q. Ulvan polysaccharide-degrading enzymes: An updated and comprehensive review of sources category, property, structure, and applications of ulvan lyases. Algal Res. 2021, 60, 102477. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedi, J.; Rodenas, S.; Sanchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef]
- Castro-González, M.; Pérez-Gil, F.; Pérez-Estrella, S.; Carrillo-Domínguez, S. Chemical composition of the green alga Ulva lactuca. Cienc. Mar. 1996, 22, 205–213. [Google Scholar] [CrossRef]
- Agarwal, S.; Singh, V.; Chauhan, K. Antidiabetic potential of seaweed and their bioactive compounds: A review of developments in last decade. Crit. Rev. Food Sci. Nutr. 2023, 63, 5739–5770. [Google Scholar] [CrossRef]
- Nova, P.; Pimenta-Martins, A.; Laranjeira Silva, J.; Silva, A.M.; Gomes, A.M.; Freitas, A.C. Health benefits and bioavailability of marine resources components that contribute to health—what’s new? Crit. Rev. Food Sci. Nutr. 2020, 60, 3680–3692. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhaiyya, R.; Khandare, K.; Tingirikari, J.M.R. Macroalgal dietary glycans: Potential source for human gut bacteria and enhancing immune system for better health. Crit. Rev. Food Sci. Nutr. 2022, 62, 1674–1695. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.T.; Cheong, K.L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 7703–7717. [Google Scholar] [CrossRef]
- Anisha, G.S.; Augustianath, T.; Padmakumari, S.; Singhania, R.R.; Pandey, A.; Patel, A.K. Ulvan from green macroalgae: Bioactive properties advancing tissue engineering, drug delivery systems, food industry, agriculture and water treatment. Bioresour. Technol. Rep. 2023, 22, 101457. [Google Scholar] [CrossRef]
- Kaeffer, B.; Benard, C.; Lahaye, M.; Blottiere, H.M.; Cherbut, C. Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial cells. Planta Med. 1999, 65, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Schutz, M.; Mukherjee, S.; Jana, S.; Ray, S.; Marschall, M. Exploiting the Amazing Diversity of Natural Source-Derived Polysaccharides: Modern Procedures of Isolation, Engineering, and Optimization of Antiviral Activities. Polymers 2020, 13, 136. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]
- Maray, S.O.; Abdel-Kareem, M.S.M.; Mabrouk, M.E.M.; El-Halmouch, Y.; Makhlof, M.E.M. In Vitro Assessment of Antiviral, Antimicrobial, Antioxidant and Anticancer Activities of Ulvan Extracted from the Green Seaweed. Thalassas 2023, 39, 779–790. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, M.; Wang, X.; Fu, X.; Guan, H.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2006, 17, 527–534. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Qi, H.; Sun, Y. Antioxidant activity of high sulfate content derivative of ulvan in hyperlipidemic rats. Int. J. Biol. Macromol. 2015, 76, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Arockiaraj, J.; Jagruthi, C. Efficacy of ulvan on immune response and immuno-antioxidant gene modulation in Labeo rohita against columnaris disease. Fish. Shellfish. Immunol. 2021, 117, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Bhuyan, P.P.; Ki, J.S. Immunomodulatory, Antioxidant, Anticancer, and Pharmacokinetic Activity of Ulvan, a Seaweed-Derived Sulfated Polysaccharide: An Updated Comprehensive Review. Mar. Drugs 2023, 21, 300. [Google Scholar] [CrossRef]
- El-Shafei, R.; Hegazy, H.; Acharya, B. A Review of Antiviral and Antioxidant Activity of Bioactive Metabolite of Macroalgae within an Optimized Extraction Method. Energies 2021, 14, 3092. [Google Scholar] [CrossRef]
- Abd-Ellatef, G.F.; Ahmed, O.M.; Abdel-Reheim, E.S.; Abdel-Hamid, A.Z. Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer 2017, 9, 67–83. [Google Scholar]
- Qi, H.M.; Huang, L.Y.; Liu, X.L.; Liu, D.M.; Zhang, Q.B.; Liu, S.M. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Ning, L.; Xiguang, L.; Gefei, Z.; Quanbin, Z.; Pengcheng, L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar] [CrossRef]
- Qi, H.; Sheng, J. The antihyperlipidemic mechanism of high sulfate content ulvan in rats. Mar. Drugs 2015, 13, 3407–3421. [Google Scholar] [CrossRef]
- Jiang, N.; Li, B.; Wang, X.; Xu, X.; Liu, X.; Li, W.; Chang, X.; Li, H.; Qi, H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef]
- Qi, H.; Liu, X.; Zhang, J.; Duan, Y.; Wang, X.; Zhang, Q. Synthesis and antihyperlipidemic activity of acetylated derivative of ulvan from Ulva pertusa. Int. J. Biol. Macromol. 2012, 50, 270–272. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, W.; Zhao, F.; Dong, Q.; Wang, Y.; Wei, W.; Jia, L.; Li, L.; Lu, F. Dual Effect of the Acidic Polysaccharose Ulvan on the Inhibition of Amyloid-beta Protein Fibrillation and Disintegration of Mature Fibrils. ACS Appl. Mater. Interface 2020, 12, 41167–41176. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Gao, W.; Cui, Z.; Zhang, H.; Lu, F.; Liu, F. Ulvan inhibits alpha-synuclein fibrillation and disrupts the mature fibrils: In vitro and in vivo studies. Int. J. Biol. Macromol. 2022, 211, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.T.; Wan, X.Z.; Wang, D.Y.; Zhao, C.; Liu, D.; Gao, L.Y.; Wang, M.F.; Wu, C.J.; Nabavid, S.M.; Daglia, M.; et al. Polysaccharides from Marine: Structure and function. Trends Food Sci. Technol. 2020, 99, 11–20. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Costa, M.M.; Trevisi, P.; Carvalho, D.F.P.; Correa, F.; Martins, C.F.; Pinho, M.; Mourato, M.; de Almeida, A.M.; Freire, J.P.B.; et al. Piglets performance, nutrient digestibility and gut health in response to feeding Ulva lactuca seaweed supplemented with a recombinant ulvan lyase or a commercial carbohydrase mixture. J. Anim. Physiol. Anim. Nutr. 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.M.; Coelho, D.; Costa, M.; Carvalho, D.F.P.; Leclercq, C.C.; Renaut, J.; Freire, J.P.B.; Almeida, A.M.; Prates, J.A.M. Integrated transcriptomics and proteomics analysis reveals muscle metabolism effects of dietary Ulva lactuca and ulvan lyase supplementation in weaned piglets. Sci. Rep. 2024, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- El Modafar, C.; Elgadda, M.; El Boutachfaiti, R.; Abouraicha, E.; Zehhar, N.; Petit, E.; El Alaoui-Talibi, Z.; Courtois, B.; Courtois, J. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hortic. 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerre-Tugaye, M.T.; Dumas, B. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J. Biomed. Biotechnol. 2010, 2010, 525291. [Google Scholar] [CrossRef]
- Lahaye, M.; Brunel, M.; Bonnin, E. Fine chemical structure analysis of oligosaccharides produced by an ulvan-lyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp. (Ulvales, Chlorophyta). Carbohydr. Res. 1997, 304, 325–333. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Delattre, C.; Michaud, P.; Keller, C.; Elboutachfaiti, R.; Beven, L.; Courtois, B.; Courtois, J. Purification and characterization of a novel glucuronan lyase from Trichoderma sp. GL2. Appl. Microbiol. Biotechnol. 2006, 70, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Nyvall Collen, P.; Sassi, J.F.; Rogniaux, H.; Marfaing, H.; Helbert, W. Ulvan lyases isolated from the Flavobacteria Persicivirga ulvanivorans are the first members of a new polysaccharide lyase family. J. Biol. Chem. 2011, 286, 42063–42071. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Muramatsu, H.; Kato, S.I.; Ohnishi, K. Characterization of an Alteromonas long-type ulvan lyase involved in the degradation of ulvan extracted from Ulva ohnoi. Biosci. Biotechnol. Biochem. 2017, 81, 2145–2151. [Google Scholar] [CrossRef]
- Kopel, M.; Helbert, W.; Belnik, Y.; Buravenkov, V.; Herman, A.; Banin, E. New Family of Ulvan Lyases Identified in Three Isolates from the Alteromonadales Order. J. Biol. Chem. 2016, 291, 5871–5878. [Google Scholar] [CrossRef]
- He, C.; Ohnishi, K. Efficient renaturation of inclusion body proteins denatured by SDS. Biochem. Biophys. Res. Commun. 2017, 490, 1250–1253. [Google Scholar] [CrossRef]
- Qin, H.M.; Xu, P.P.; Guo, Q.Q.; Cheng, X.T.; Gao, D.K.; Sun, D.Y.; Zhu, Z.L.; Lu, F.P. Biochemical characterization of a novel ulvan lyase from Pseudoalteromonas sp strain PLSV. RSC Adv. 2018, 8, 2610–2615. [Google Scholar] [CrossRef]
- Gao, J.; Du, C.; Chi, Y.; Zuo, S.; Ye, H.; Wang, P. Cloning, Expression, and Characterization of a New PL25 Family Ulvan Lyase from Marine Bacterium Alteromonas sp. A321. Mar. Drugs 2019, 17, 568. [Google Scholar] [CrossRef]
- Ulaganathan, T.; Boniecki, M.T.; Foran, E.; Buravenkov, V.; Mizrachi, N.; Banin, E.; Helbert, W.; Cygler, M. New Ulvan-Degrading Polysaccharide Lyase Family: Structure and Catalytic Mechanism Suggests Convergent Evolution of Active Site Architecture. ACS Chem. Biol. 2017, 12, 1269–1280. [Google Scholar] [CrossRef]
- Foran, E.; Buravenkov, V.; Kopel, M.; Mizrahi, N.; Shoshani, S.; Helbert, W.; Banin, E. Functional characterization of a novel “ulvan utilization loci” found in Alteromonas sp LOR genome. Algal Res. 2017, 25, 39–46. [Google Scholar] [CrossRef]
- Reisky, L.; Stanetty, C.; Mihovilovic, M.D.; Schweder, T.; Hehemann, J.H.; Bornscheuer, U.T. Biochemical characterization of an ulvan lyase from the marine flavobacterium Formosa agariphila KMM 3901(T). Appl. Microbiol. Biotechnol. 2018, 102, 6987–6996. [Google Scholar] [CrossRef]
- Ulaganathan, T.; Banin, E.; Helbert, W.; Cygler, M. Structural and functional characterization of PL28 family ulvan lyase NLR48 from Nonlabens ulvanivorans. J. Biol. Chem. 2018, 293, 11564–11573. [Google Scholar] [CrossRef] [PubMed]
- Konasani, V.R.; Jin, C.; Karlsson, N.G.; Albers, E. A novel ulvan lyase family with broad-spectrum activity from the ulvan utilisation loci of Formosa agariphila KMM 3901. Sci. Rep. 2018, 8, 14713. [Google Scholar] [CrossRef]
- Reisky, L.; Prechoux, A.; Zuhlke, M.K.; Baumgen, M.; Robb, C.S.; Gerlach, N.; Roret, T.; Stanetty, C.; Larocque, R.; Michel, G.; et al. A marine bacterial enzymatic cascade degrades the algal polysaccharide ulvan. Nat. Chem. Biol. 2019, 15, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.M.; Gao, D.; Zhu, M.; Li, C.; Zhu, Z.; Wang, H.; Liu, W.; Tanokura, M.; Lu, F. Biochemical characterization and structural analysis of ulvan lyase from marine Alteromonas sp. reveals the basis for its salt tolerance. Int. J. Biol. Macromol. 2020, 147, 1309–1317. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; An, L.; Qiu, Y.K.; Mao, A.H.; He, Z.X.; Guo, J.L.; Yan, H.B.; Li, H.; Hu, Z. Biochemical Characterization of a Novel Thermostable Ulvan Lyase from Tamlana fucoidanivorans CW2-9. J. Agric. Food Chem. 2024, 72, 11773–11781. [Google Scholar] [CrossRef]
- Mann, A.J.; Hahnke, R.L.; Huang, S.; Werner, J.; Xing, P.; Barbeyron, T.; Huettel, B.; Stuber, K.; Reinhardt, R.; Harder, J.; et al. The genome of the alga-associated marine flavobacterium Formosa agariphila KMM 3901T reveals a broad potential for degradation of algal polysaccharides. Appl. Environ. Microbiol. 2013, 79, 6813–6822. [Google Scholar] [CrossRef]
- Li, G.; Rabe, K.S.; Nielsen, J.; Engqvist, M.K.M. Machine Learning Applied to Predicting Microorganism Growth Temperatures and Enzyme Catalytic Optima. ACS Synth. Biol. 2019, 8, 1411–1420. [Google Scholar] [CrossRef]
- Ulaganathan, T.; Helbert, W.; Kopel, M.; Banin, E.; Cygler, M. Structure-function analyses of a PL24 family ulvan lyase reveal key features and suggest its catalytic mechanism. J. Biol. Chem. 2018, 293, 4026–4036. [Google Scholar] [CrossRef]
- Xu, F.; Dong, F.; Sun, X.H.; Cao, H.Y.; Fu, H.H.; Li, C.Y.; Zhang, X.Y.; McMinn, A.; Zhang, Y.Z.; Wang, P.; et al. Mechanistic Insights into Substrate Recognition and Catalysis of a New Ulvan Lyase of Polysaccharide Lyase Family 24. Appl. Environ. Microbiol. 2021, 87, e0041221. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wei, T.D.; Chen, X.L.; Li, C.Y.; Wang, P.; Xie, B.B.; Qin, Q.L.; Zhang, X.Y.; Pang, X.H.; Zhou, B.C.; et al. Molecular insight into the role of the N-terminal extension in the maturation, substrate recognition, and catalysis of a bacterial alginate lyase from polysaccharide lyase family 18. J. Biol. Chem. 2014, 289, 29558–29569. [Google Scholar] [CrossRef] [PubMed]
- Yip, V.L.; Withers, S.G. Breakdown of oligosaccharides by the process of elimination. Curr. Opin. Chem. Biol. 2006, 10, 147–155. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Guo, Z.; Tang, T.; Zhu, B. Alginate degrading enzymes: An updated comprehensive review of the structure, catalytic mechanism, modification method and applications of alginate lyases. Crit. Rev. Biotechnol. 2021, 41, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Garron, M.L.; Cygler, M. Structural and mechanistic classification of uronic acid-containing polysaccharide lyases. Glycobiology 2010, 20, 1547–1573. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Li, Q.; Xu, Y.; Tang, T.; Ning, L.; Zhu, B. Evolving strategies for marine enzyme engineering: Recent advances on the molecular modification of alginate lyase. Mar. Life Sci. Technol. 2022, 4, 106–116. [Google Scholar] [CrossRef]
- Li, L.; Cao, S.; Zhu, B.; Yao, Z.; Zhu, B.; Qin, Y.; Jiang, J. Efficient Degradation of Alginate and Preparation of Alginate Oligosaccharides by a Novel Biofunctional Alginate Lyase with High Activity and Excellent Thermophilic Features. Mar. Drugs 2023, 21, 180. [Google Scholar] [CrossRef]
- Yang, M.; Yang, S.X.; Liu, Z.M.; Li, N.N.; Li, L.; Mou, H.J. Rational Design of Alginate Lyase from Microbulbifer sp. Q7 to Improve Thermal Stability. Mar. Drugs 2019, 17, 378. [Google Scholar] [CrossRef]
- Hu, F.; Li, Q.; Zhu, B.; Ni, F.; Sun, Y.; Yao, Z. Effects of module truncation on biochemical characteristics and products distribution of a new alginate lyase with two catalytic modules. Glycobiology 2019, 29, 876–884. [Google Scholar] [CrossRef]
- Yan, J.; Chen, P.; Zeng, Y.; Men, Y.; Mu, S.; Zhu, Y.; Chen, Y.; Sun, Y. The Characterization and Modification of a Novel Bifunctional and Robust Alginate Lyase Derived from Marinimicrobium sp. H1. Mar. Drugs 2019, 17, 545. [Google Scholar] [CrossRef]
- Huang, A.; Chai, C.; Zhang, J.; Zhao, L.; Lu, F.; Liu, F. Engineered N57P Variant of Ulvan Lyase with Improvement of Catalytic Efficiency and Thermostability via Reducing Loop Flexibility and Anchoring Substrate. ACS Sustain. Chem. Eng. 2021, 9, 16415–16423. [Google Scholar] [CrossRef]
- Huang, A.; Chen, Z.; Wu, X.; Yan, W.; Lu, F.; Liu, F. Improving the thermal stability and catalytic activity of ulvan lyase by the combination of FoldX and KnowVolution campaign. Int. J. Biol. Macromol. 2024, 257, 128577. [Google Scholar] [CrossRef]
- Konasani, V.R.; Jin, C.S.; Karlsson, N.G.; Albers, E. Ulvan lyase from Formosa agariphila and its applicability in depolymerisation of ulvan extracted from three different Ulva species. Algal Res. 2018, 36, 106–114. [Google Scholar] [CrossRef]
- Qiao, L.K.; Yang, X.K.; Xie, R.Z.; Du, C.Y.; Chi, Y.Z.; Zhang, J.L.; Wang, P. Efficient production of ulvan lyase from Ulva prolifera by Catenovulum sp. LP based on stage-controlled fermentation strategy. Algal Res. 2020, 46, 8. [Google Scholar] [CrossRef]
- Saha, S.; Navid, M.H.; Bandyopadhyay, S.S.; Schnitzler, P.; Ray, B. Sulfated polysaccharides from Laminaria angustata: Structural features and in vitro antiviral activities. Carbohydr. Polym. 2012, 87, 123–130. [Google Scholar] [CrossRef]

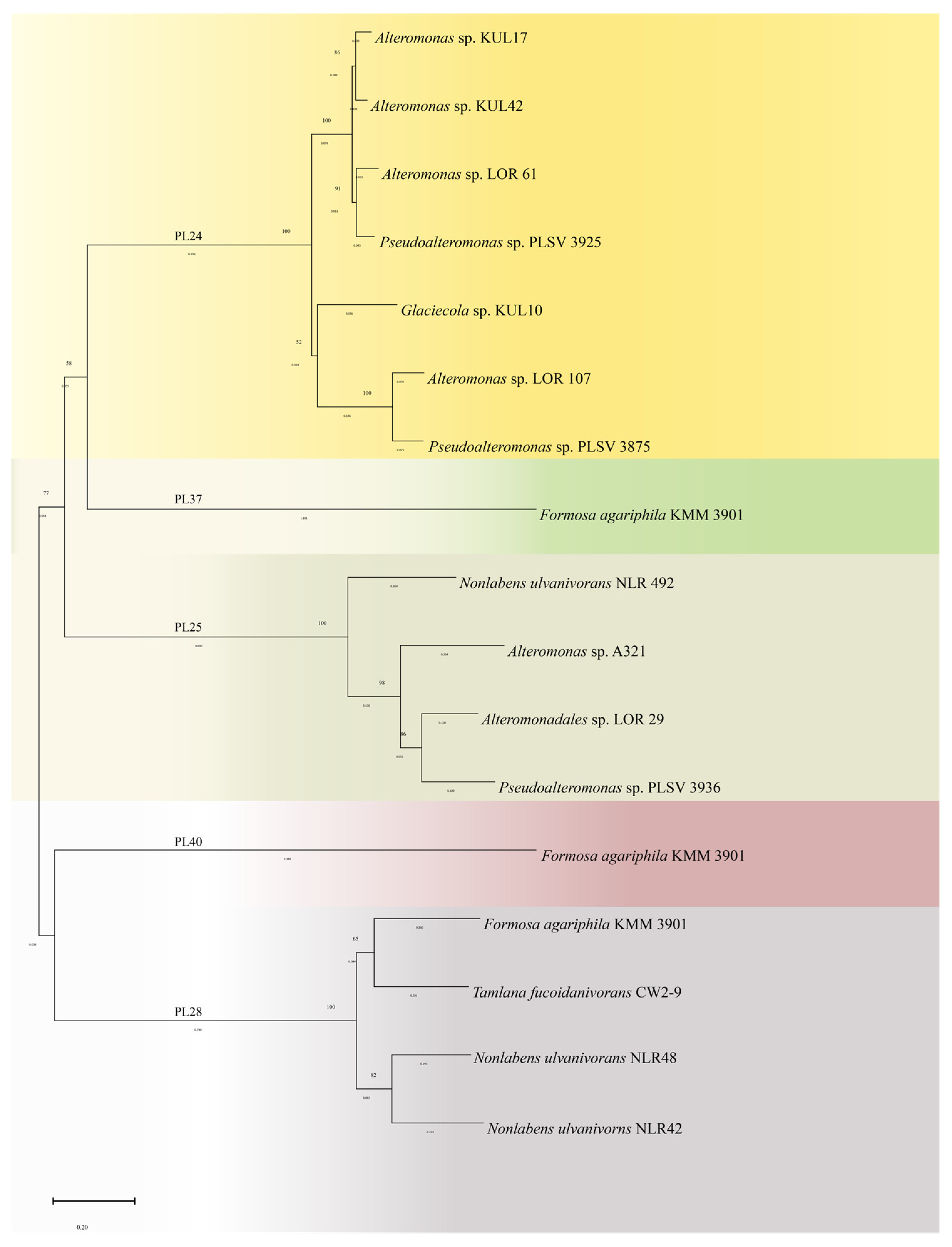

| Family | GeneBank | Bacteria | Topt/°C | Optimal pH | MW/kDa 1 | Ref. |
|---|---|---|---|---|---|---|
| PL24 | BAY00694.1 | Alteromonas sp. KUL17 | - | - | 55 | [54] |
| BAY00695.1 | Alteromonas sp. KUL42 | - | - | 111.23 | [54] | |
| AMA19991.1 | Alteromonas sp. LOR_107 | 40 | 8.0 | 59.64 | [55,66] | |
| WP_032096165.1 | Alteromonas sp. LOR_61 | - | - | 110.9 | [55] | |
| BAY00693.1 | Glaciecola sp. KUL10 | - | - | 112.74 | [54,56] | |
| AMA19992.1 | Pseudoalteromonas sp. PLSV_3875 | 35 | 8.0 | 59 | [55,57] | |
| WP_033186955.1 | Pseudoalteromonas sp. PLSV_3925 | - | - | 111.4 | [55] | |
| PL25 | QFR04505.1 | Alteromonas sp. A321 | 50 | 8.0 | 53 | [58] |
| WP_033186995.1 | Pseudoalteromonas sp. PLSV_3936 | - | - | 54.28 | [59] | |
| WP_052010178.1 | Alteromonadales sp. LOR_29 | 45 | 7.5 | 52 | [60] | |
| WP_036580476.1 | Nonlabens ulvanivorans NLR_492 | - | - | 55 | [60] | |
| WP_129740764.1 | Alteromonas sp. 76–1 | 45 | 9.0 | |||
| PL28 | CDF79931.1 | Formosa agariphila KMM 3901 | 45 | 8.5 | 54.73 | [61] |
| KEZ94336.1 | Nonlabens ulvanivorans NLR48 | - | - | 30 | [62] | |
| AEN28574.1 | Nonlabens ulvanivorns NLR42 | 50 | 9.0 | 46 | [53] | |
| WP_139698591 | Tamlana fucoidanivorans CW2-9 | 40 | 9.0 | 52 | [67] | |
| PL37 | CDF79930.1 | Formosa agariphila KMM 3901 | 40 | 8.0 | 69.31 | [63] |
| PL40 | CDF79911.1 | Formosa agariphila KMM 3901 | 35 | 8.0 | 30 | [68] |
| Uncategorized | Alteromonas sp. GIIUL2 | 35 | 8.0 | [10] |
| Enzymes | Monosaccharides Contained in the Substrate | Catalytic Base | Catalytic Acid | Neutralization of Negative Charge in the Substrate |
|---|---|---|---|---|
| PL24 | GlcA | His146 | His146 | Arg259 His167 Tyr243 |
| PL25 | GlcA | Tyr188 | His123 | Arg204 |
| PL28 | GlcA | Tyr281 | Tyr281 | Gln160 Arg117 |
| PL28 | IdoA | Lys162 | Tyr281 | Gln160 Arg117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, A.; Wu, X.; Lu, F.; Liu, F. Sustainable Production of Ulva Oligosaccharides via Enzymatic Hydrolysis: A Review on Ulvan Lyase. Foods 2024, 13, 2820. https://doi.org/10.3390/foods13172820
Huang A, Wu X, Lu F, Liu F. Sustainable Production of Ulva Oligosaccharides via Enzymatic Hydrolysis: A Review on Ulvan Lyase. Foods. 2024; 13(17):2820. https://doi.org/10.3390/foods13172820
Chicago/Turabian StyleHuang, Ailan, Xinming Wu, Fuping Lu, and Fufeng Liu. 2024. "Sustainable Production of Ulva Oligosaccharides via Enzymatic Hydrolysis: A Review on Ulvan Lyase" Foods 13, no. 17: 2820. https://doi.org/10.3390/foods13172820





