Effect of Mono- and Polysaccharide on the Structure and Property of Soy Protein Isolate during Maillard Reaction
Maillard Reactions in Food Processing: Flavor-Enhancing and Risk-Reducing—a Look at the Structure and Function of Proteins and Polysaccharides
)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPI-Gal and SPI-βG
2.3. Characterization of SPI-Gal and SPI-βG
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Degree of Glycosylation (DG)
2.3.3. Fluorescence Spectra
2.4. Physicochemical Properties
2.4.1. Determination of Surface Hydrophobicity (H0)
2.4.2. Solubility Measurement
2.4.3. Determination of Emulsifying Activity (EA) and Emulsifying Stability (ES)
2.4.4. Determination of Foaming Capacity (FC) and Foam Stability (FS)
2.5. Viscosity Measurement
2.6. Physiological Properties
2.6.1. Swelling Power (SP)
2.6.2. Fat-Binding Capacity (FB)
2.6.3. Bile Acid-Binding Ability (BAB)
2.6.4. Glucose Availability (GA) in Chemical Digestion
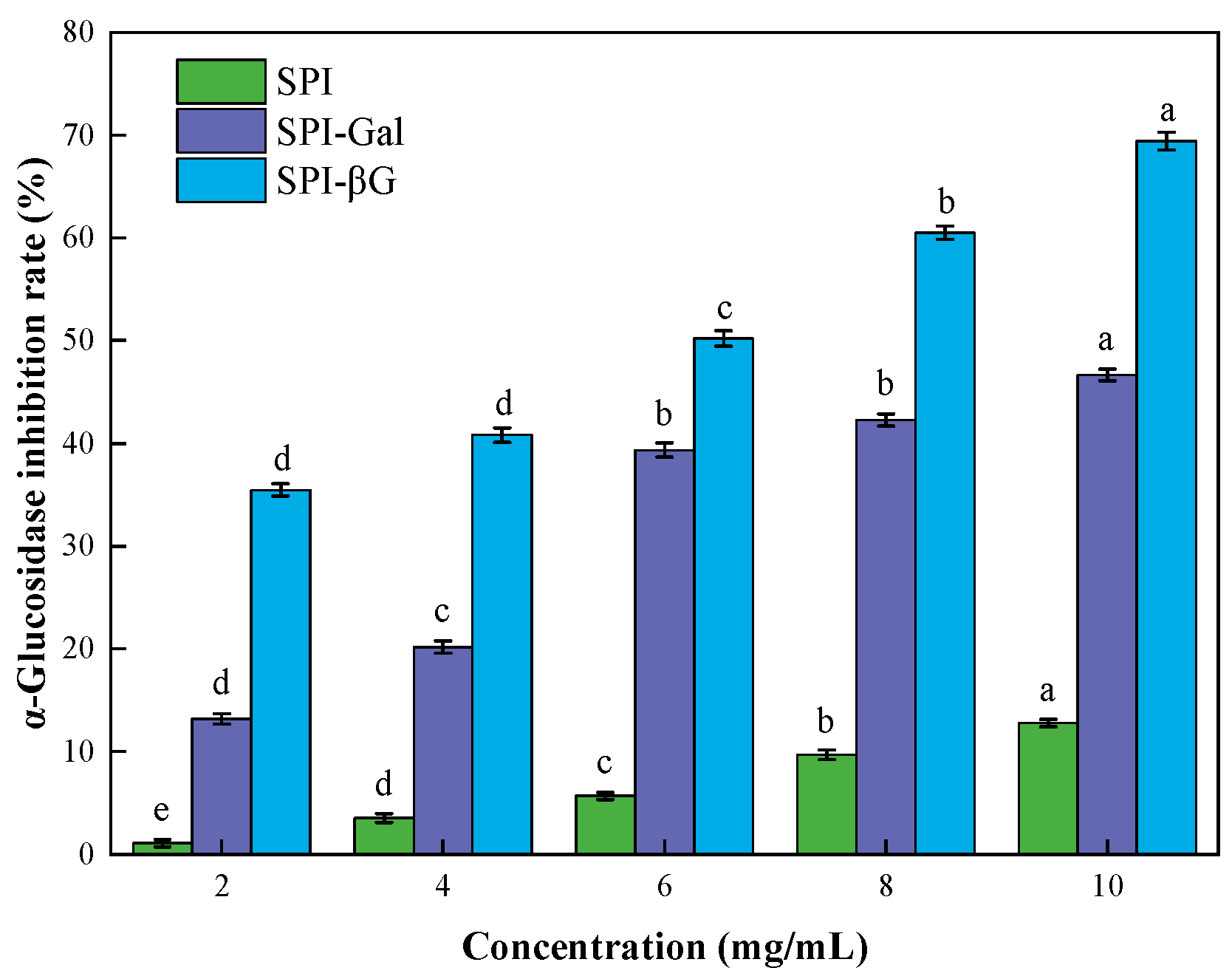
2.6.5. α-Glucosidase Inhibitory Activity
2.7. Antioxidant Activities
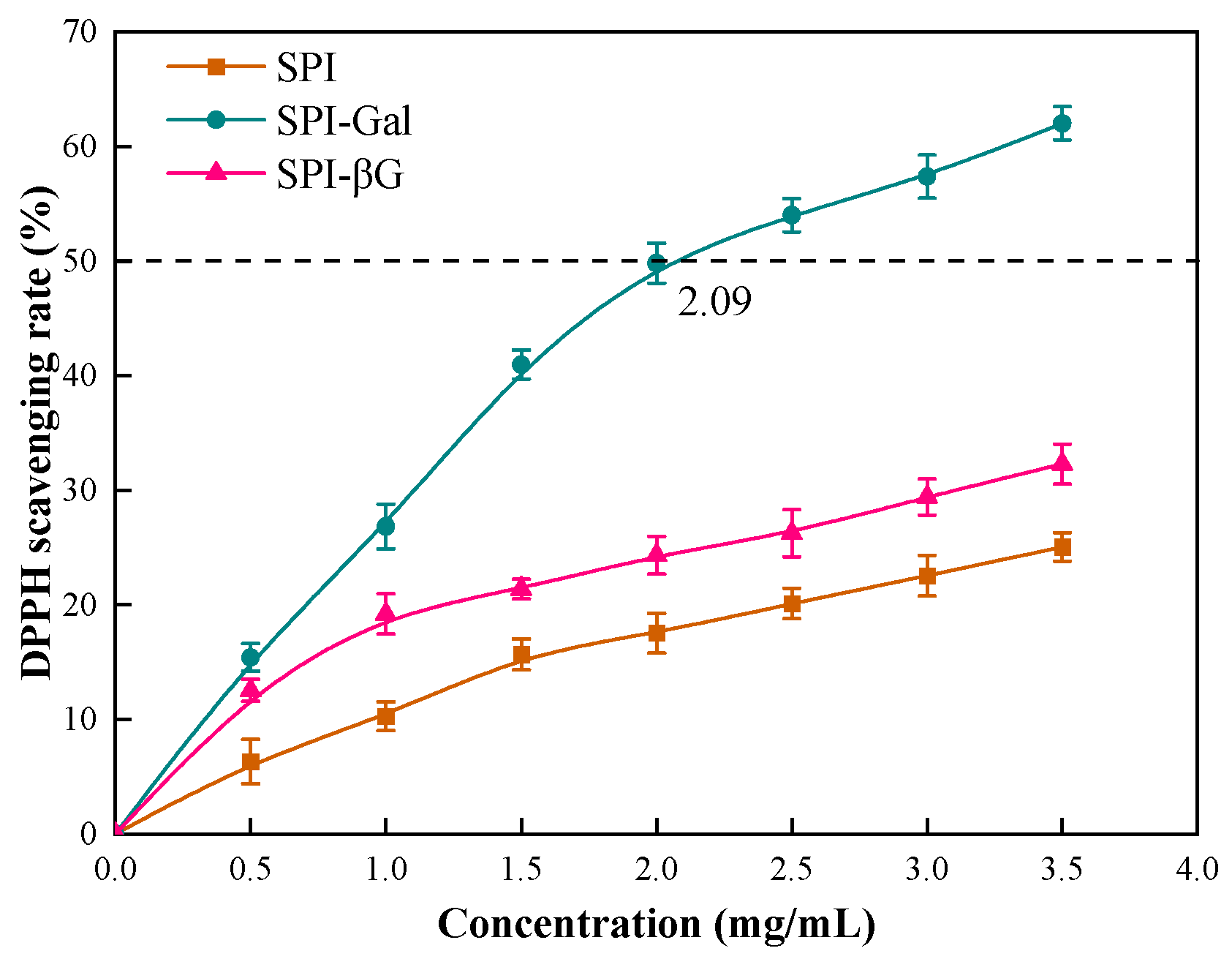
2.7.1. DPPH Scavenging Activity
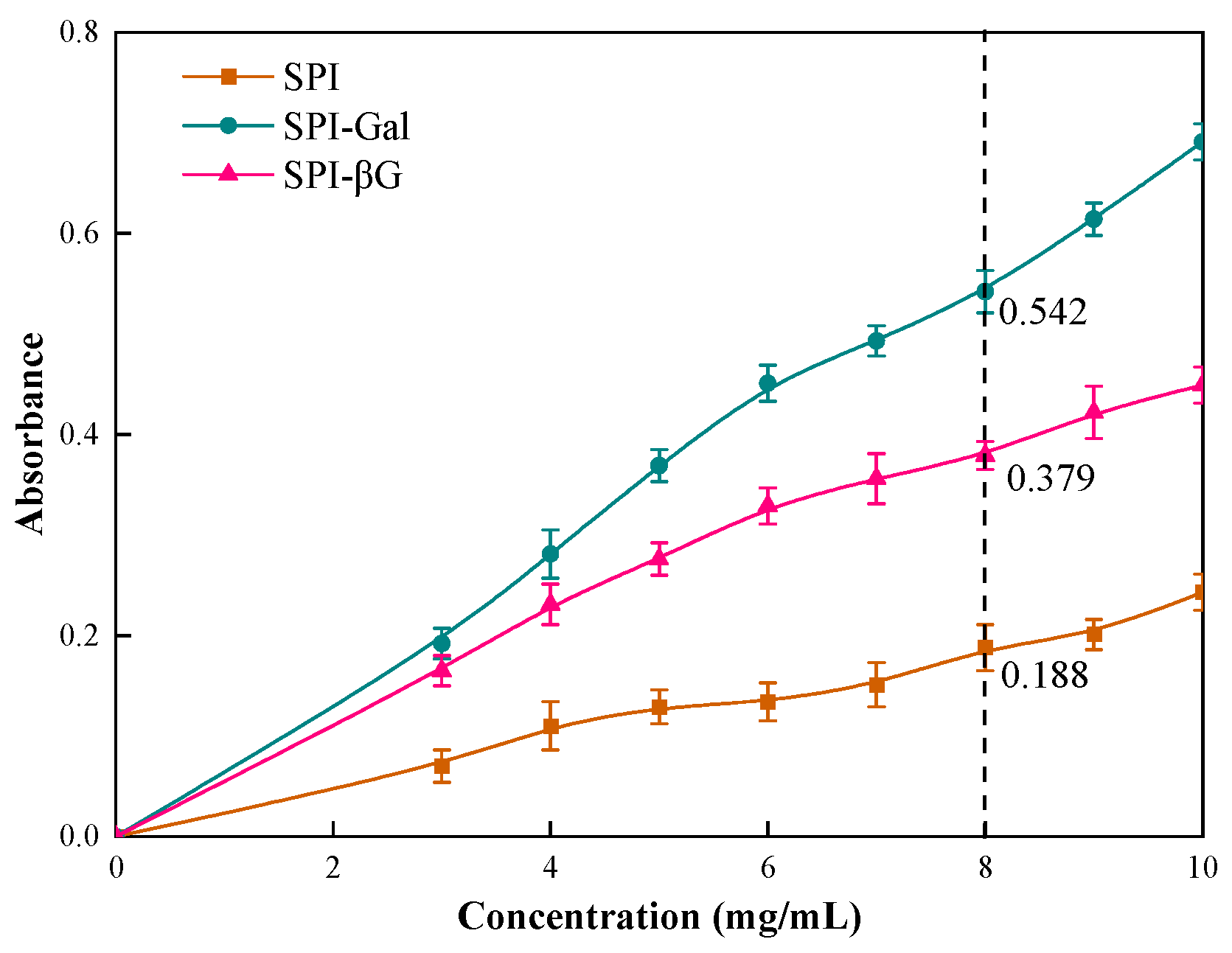
2.7.2. Determination of Reducing Power
2.7.3. Ferrous Ion (Fe2+)-Chelating Capacity
2.8. Statistical Analysis
3. Results and Discussion
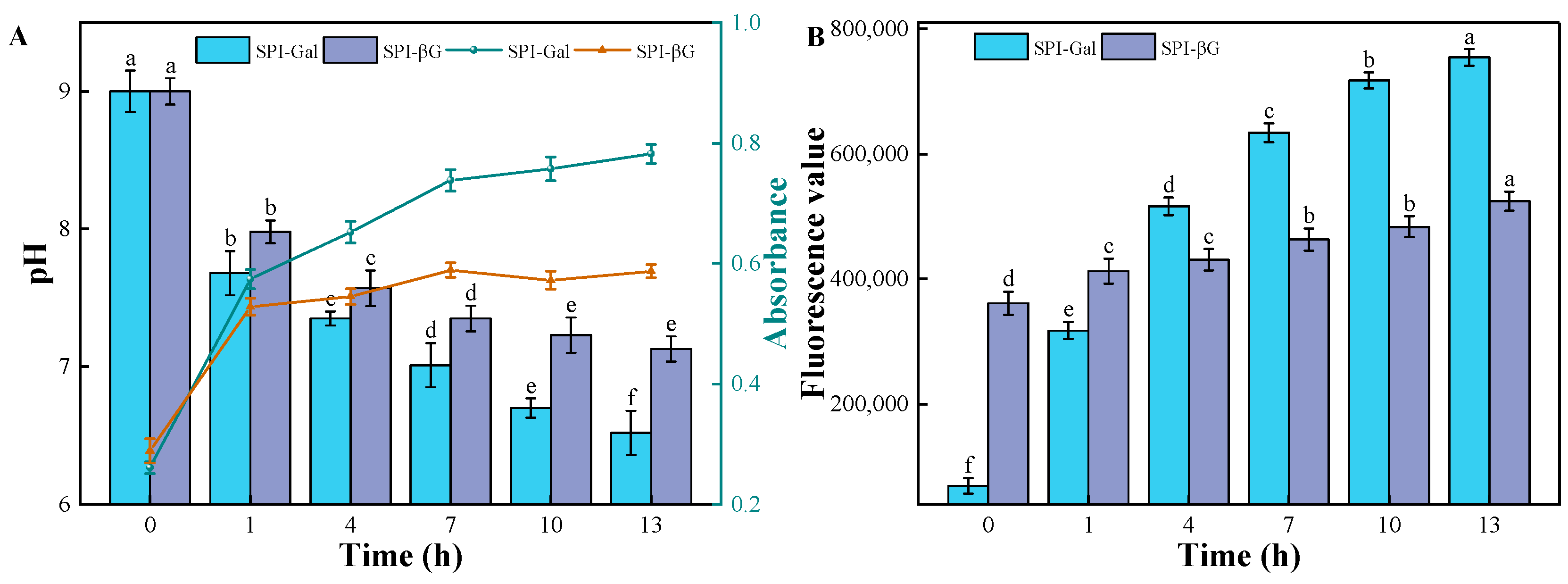
3.1. MR Process (pH, UV-Vis Absorbance, and Fluorescence Value)
3.2. Structural Characterization
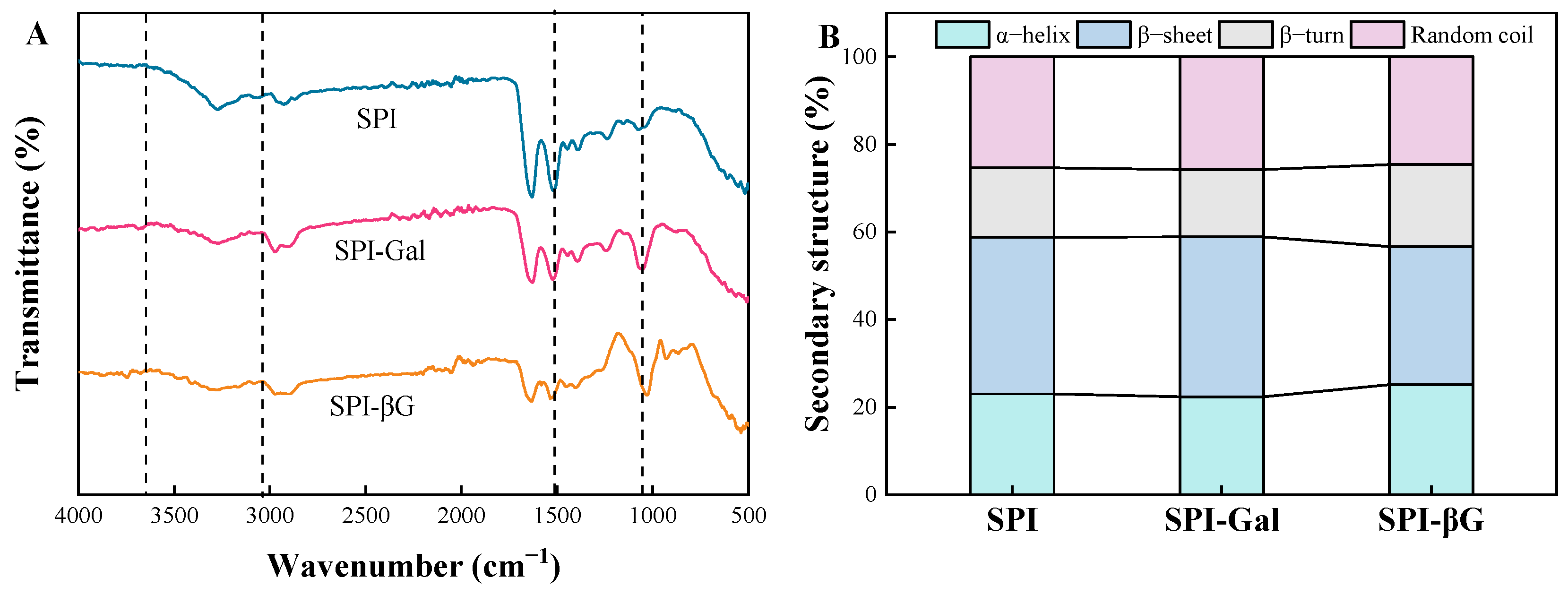
3.2.1. Infrared Spectral Characterization and Secondary Structure
3.2.2. The Degree of Glycosylation (DG)
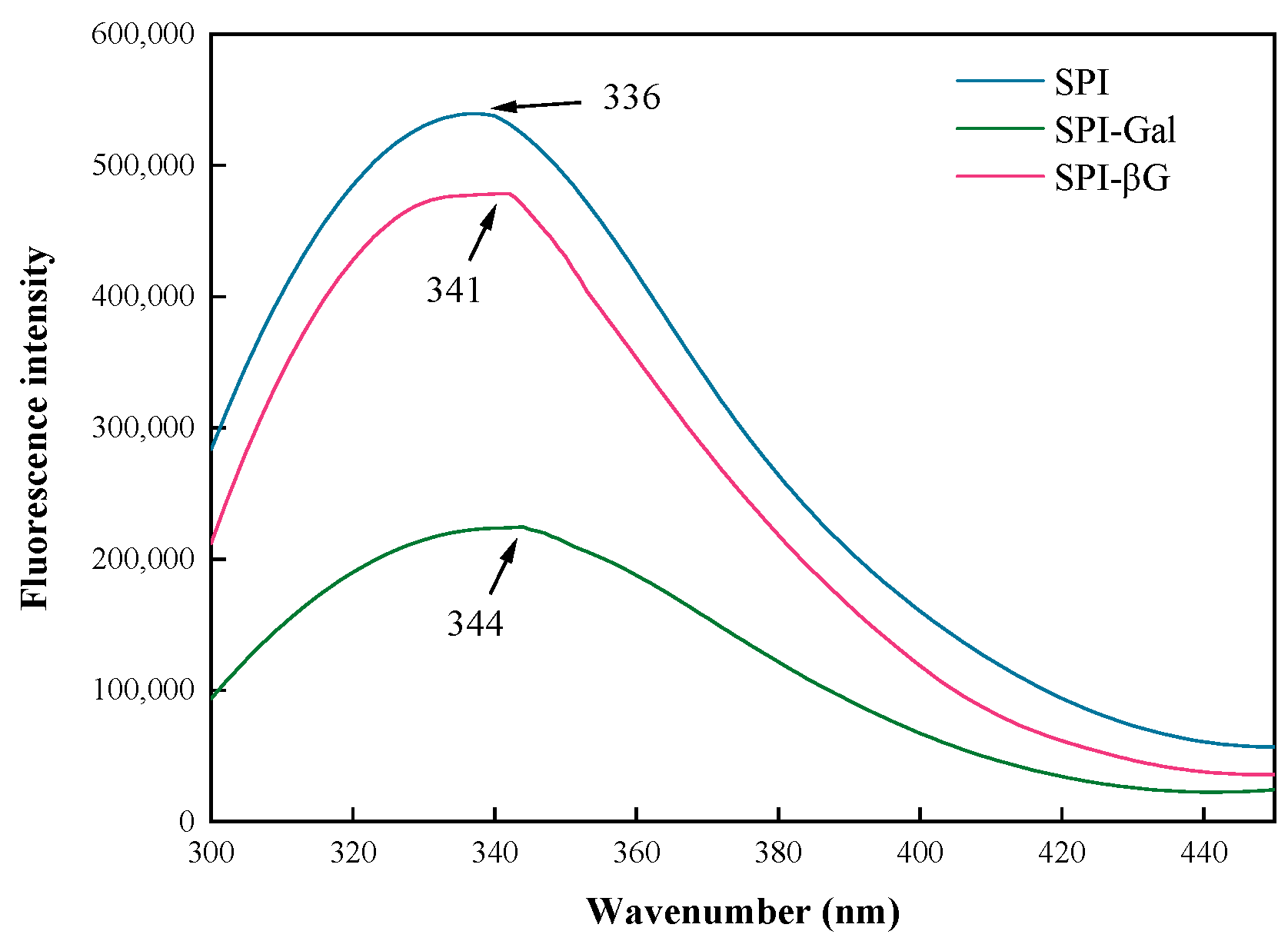
3.2.3. Fluorescence Spectral Analysis
3.3. Physicochemical Property
3.3.1. Surface Hydrophobicity (H0)
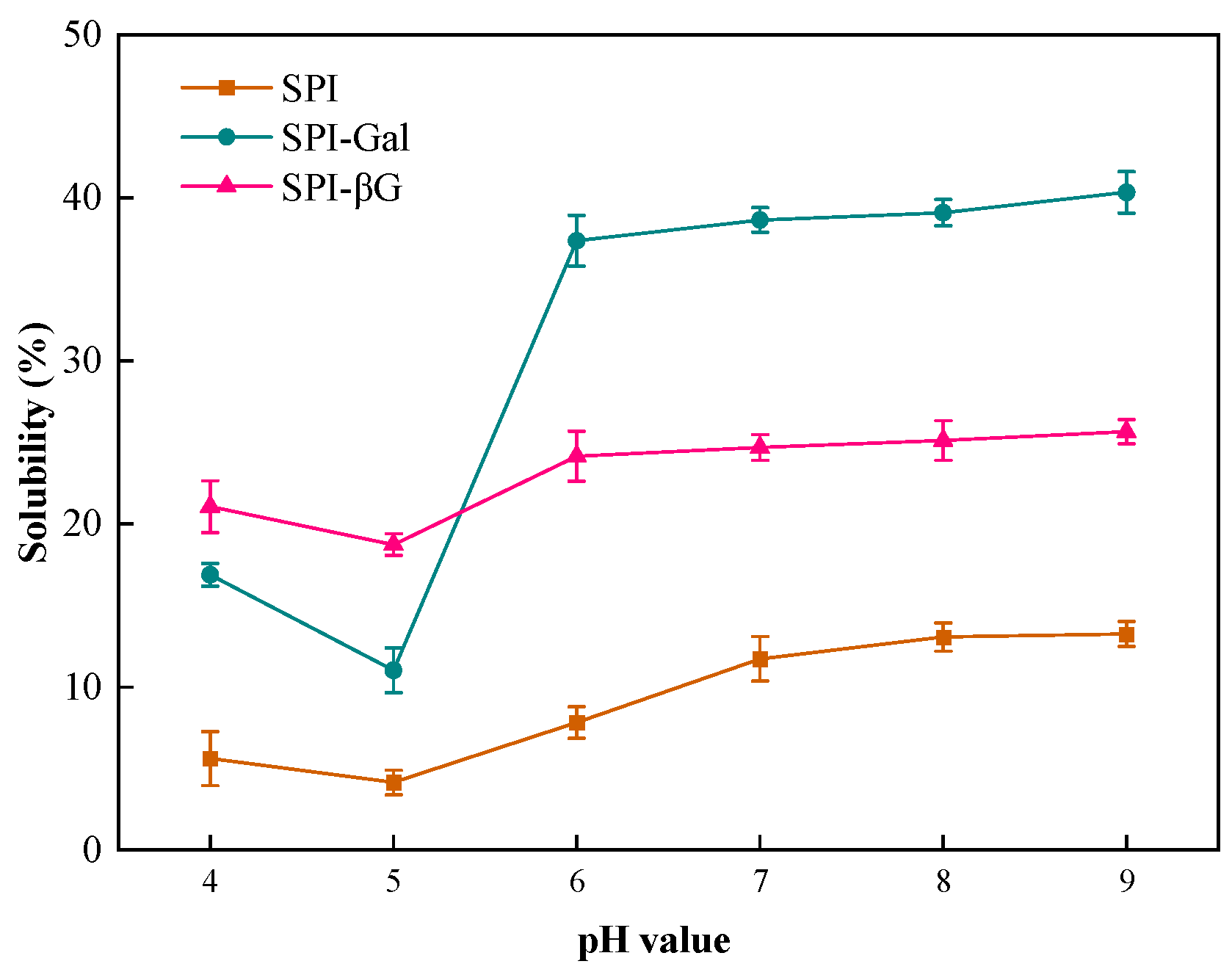
3.3.2. Solubility
3.3.3. Emulsifying Activity (EA) and Emulsifying Stability (ES)
3.3.4. Foaming Capacity (FC) and Foam Stability (FS)
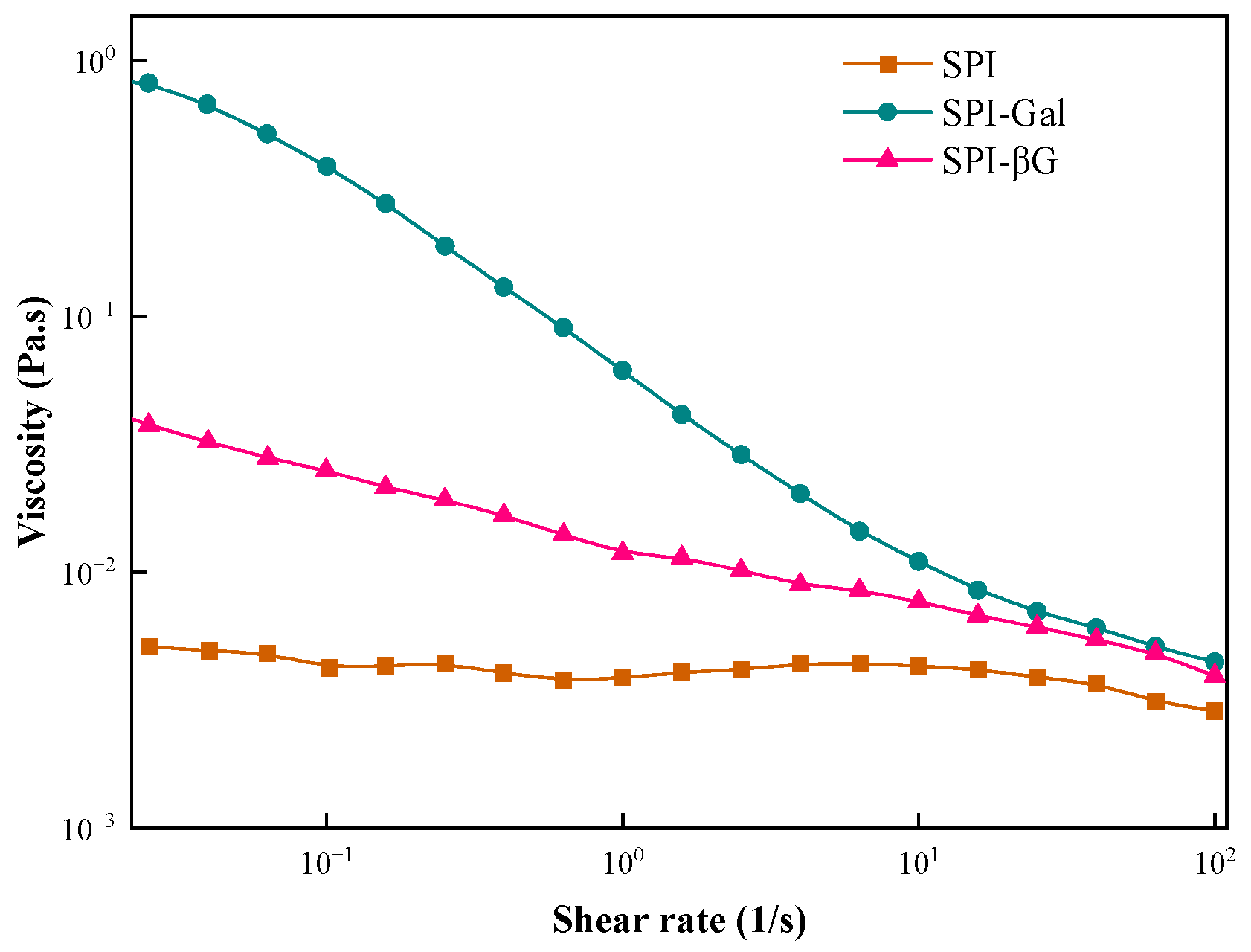
3.4. Viscosity
3.5. Physiological Activity
3.6. Antioxidant Activity
3.6.1. DPPH Scavenging Capacity
3.6.2. Reducing Power
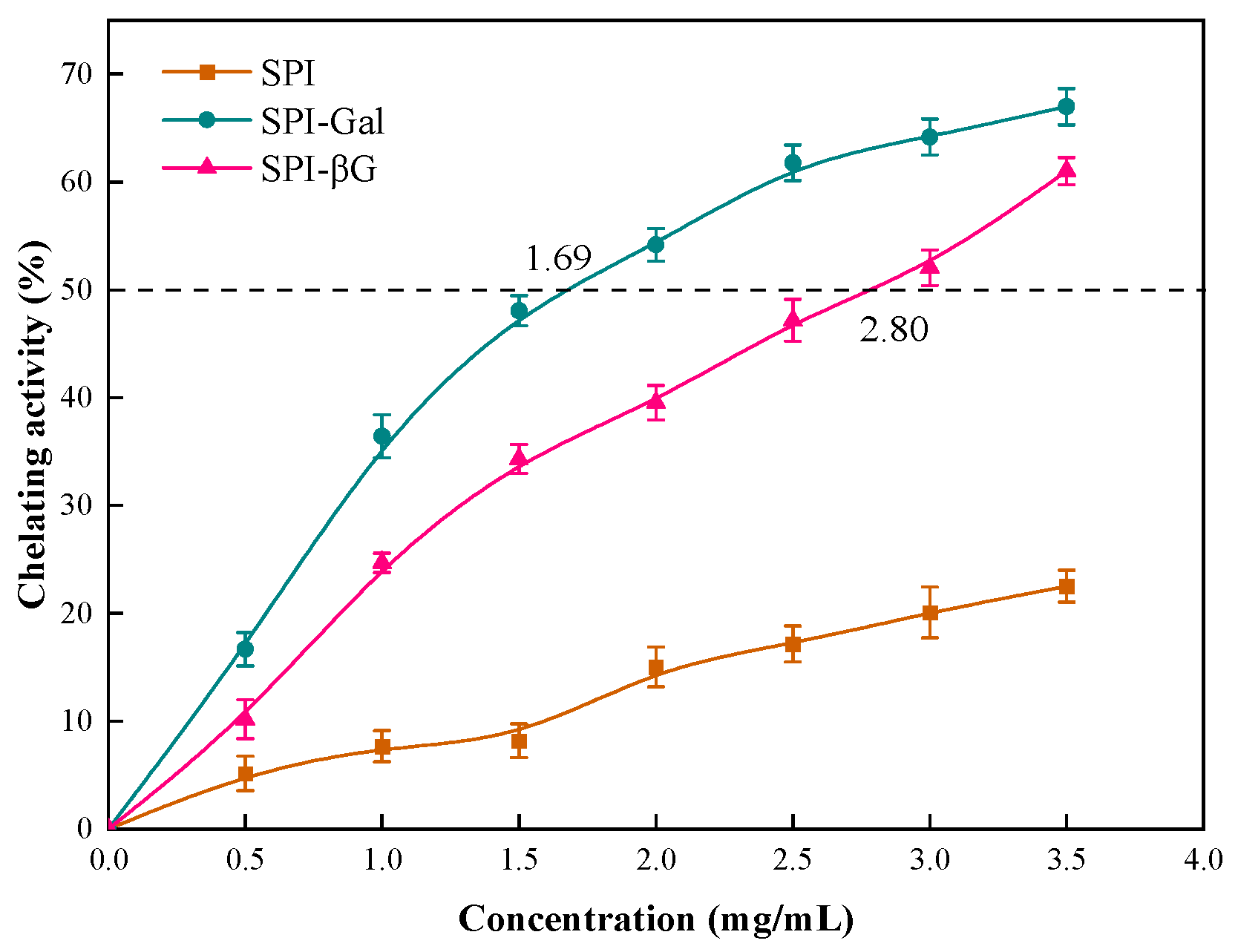
3.6.3. Ferrous Ion-Chelating Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Wu, X.; Gao, T.; Geng, M.; Teng, F.; Li, Y. Revealing the interaction mechanism and emulsion properties of carboxymethyl cellulose on soy protein isolate at different pH. Food Hydrocoll. 2024, 150, 109739. [Google Scholar] [CrossRef]
- Liao, X.; Xu, J.; Lv, S.; Zhu, S.; Wang, W.; Zhou, Y.; Liu, Y.; Sui, X.; Xiao, Y. Theanine improves the gelation of soy protein isolate by modifying protein conformation and enhancing molecular interaction. Food Hydrocoll. 2024, 154, 110109. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Chen, Y.; Li, M.; Liu, C.; Wu, X. Effect of glycosylation modification on structure and properties of soy protein isolate: A review. J. Food Sci. 2024, 89, 4620–4637. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Li, C.; Adhikari, B. Physicochemical properties of soy protein isolates-cyanidin-3-galactoside conjugates produced using free radicals induced by ultrasound. Ultrason. Sonochem. 2020, 64, 104990. [Google Scholar] [CrossRef] [PubMed]
- Bu, G.; Ren, M.; Zuo, Y.; Zhao, C. Functional characteristics and structural properties of soybean protein isolate-maltose conjugates. Cereal Chem. 2022, 99, 100–110. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Mu, D.-C.; Feng, Y.-Y.; Xu, Z.; Wen, L.; Chen, M.-L.; Ye, J. Glycosylation of rice protein with dextran via the Maillard reaction in a macromolecular crowding condition to improve solubility. J. Cereal Sci. 2022, 103, 103374. [Google Scholar] [CrossRef]
- Tapal, A.; Tiku, P.K. Complexation of curcumin with soy protein isolate and its implications on solubility and stability of curcumin. Food Chem. 2012, 130, 960–965. [Google Scholar] [CrossRef]
- Wang, L.-H.; Sun, X.; Huang, G.-Q.; Xiao, J.-X. Conjugation of soybean protein isolate with xylose/fructose through wet-heating Maillard reaction. J. Food Meas. Charact. 2018, 12, 2718–2724. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, X.-H. Structure and property changes of the soy protein isolate glycated with maltose in an ionic liquid through the Maillard reaction. Food Funct. 2019, 10, 1948–1957. [Google Scholar] [CrossRef]
- Oliveira, F.C.d.; Coimbra, J.S.d.R.; Oliveira, E.B.d.; Zuniga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef]
- Li, R.; Hettiarachchy, N.; Rayaprolu, S.; Davis, M.; Eswaranandam, S.; Jha, A.; Chen, P. Improved functional properties of glycosylated soy protein isolate using D-glucose and xanthan gum. J. Food Sci. Technol. 2015, 52, 6067–6072. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Huang, G.-Q.; Xu, T.-C.; Liu, L.-N.; Xiao, J.-X. Comparative study on the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int. J. Biol. Macromol. 2019, 131, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Long, X.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Bian, X.; Sun, T. Effect of D-galactose on physicochemical and functional properties of soy protein isolate during Maillard reaction. Food Hydrocoll. 2022, 133, 107914. [Google Scholar] [CrossRef]
- Zhang, Q.; Dou, L.; Sun, T.; Li, X.; Xue, B.; Xie, J.; Bian, X.; Shao, Z.; Gan, J. Physicochemical and functional property of the Maillard reaction products of soy protein isolate with L-arabinose/D-galactose. J. Sci. Food Agric. 2023, 103, 7040–7049. [Google Scholar] [CrossRef]
- Bai, J.; Ren, Y.; Li, Y.; Fan, M.; Qian, H.; Wang, L.; Wu, G.; Zhang, H.; Qi, X.; Xu, M.; et al. Physiological functionalities and mechanisms of β-glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Wan, Y.; Wang, L.; Zhou, T. Preparation, characterization and releasing property of antibacterial nano-capsules composed of e-PL-EGCG and sodium alginate-chitosan. Int. J. Biol. Macromol. 2022, 204, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hou, F.; Zhao, H.; Wang, D.; Chen, W.; Miao, S.; Liu, D. Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocoll. 2020, 108, 106056. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Zhang, H.; Liu, G.; Qin, X. Preparation, Characterization and Antioxidant Activity of Glycosylated Whey Protein Isolate/Proanthocyanidin Compounds. Foods. 2023, 12, 12153. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Wang, Y.; Wang, X.; Xu, N.; Jiang, L. Effects of irradiation on the structure and properties of glycosylated soybean proteins. Food Funct. 2020, 11, 1635–1646. [Google Scholar] [CrossRef]
- Lu, F.; Chi, Y.; Chi, Y. High-temperature glycosylation of saccharides to modify molecular conformation of egg white protein and its effect on the stability of high internal phase emulsions. Food Res. Int. 2024, 176, 113825. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Du, S.; Deng, Q.; Tang, H.; Yang, C.; Wei, F.; Chen, H.; Quek, S.Y.; Zhou, A.; Liu, L. Study on the antioxidant activity and emulsifying properties of flaxseed gum-whey protein isolate conjugates prepared by Maillard reaction. Int. J. Biol. Macromol. 2020, 153, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Peng, G.; Pang, X.; Wen, Z.; Yi, J. Physicochemical, emulsifying, and interfacial properties of different whey protein aggregates obtained by thermal treatment. LWT-Food Sci. Technol. 2021, 149, 111904. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; Zhang, S.; Wang, T.; Yu, D. Non-covalent interaction between soybean protein isolate and naringenin: Focused on binding mechanism, interface behavior, and functional properties. Food Hydrocoll. 2024, 153, 109975. [Google Scholar] [CrossRef]
- Long, X.; Xie, J.; Xue, B.; Li, X.; Sun, T. Effect of oxidative modification on physicochemical and functional properties of soybean polysaccharides. J. Mol. Struct. 2022, 1262, 133047. [Google Scholar] [CrossRef]
- Moura, F.A.d.; Pereira, J.M.; Silva, D.O.d.; Zavareze, E.R.; Silveira Moreira, A.d.; Helbig, E.; Dias, A.R.G. Effects of oxidative treatment on the physicochemical, rheological and functional properties of oat beta-glucan. Food Chem. 2011, 128, 982–987. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, G.; Liu, J. Effect of preparation methods on physiochemical and functional properties of yeastβ-glucan. LWT-Food Sci. Technol. 2022, 160, 113284. [Google Scholar] [CrossRef]
- Zhang, C.-C.; Geng, C.-A.; Huang, X.-Y.; Zhang, X.-M.; Chen, J.-J. Antidiabetic stilbenes from peony seeds with PTP1B, alpha-glucosidase, and DPPIV inhibitory activities. J. Agric. Food Chem. 2019, 67, 6765–6772. [Google Scholar] [CrossRef]
- Yuan, M.; Cao, Y.; Zheng, H.Y.; Chen, K.L.; Lu, Y.P.; Wang, J.; Zhu, L.Q.; Chen, M.; Cai, Z.P.; Shen, Y.G. Structural and functional properties of Maillard-reacted casein phosphopeptides with different carbohydrates. Food Sci. Biotechnol. 2024, 33, 1603–1614. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, Y.; He, X.; Hu, S.; Li, S.; Liu, Y. A study of the tyramine/glucose Maillard reaction: Variables, characterization, cytotoxicity and preliminary application. Food Chem. 2018, 239, 377–384. [Google Scholar] [CrossRef]
- Chen, S.L.; Jin, S.Y.; Chen, C.S. Relative reactivities of glucose and galactose in browning and pyruvaldehyde formation in sugar/glycine model systems. Food Chem. 2005, 92, 597–605. [Google Scholar] [CrossRef]
- Wang, W.-D.; Li, C.; Bin, Z.; Huang, Q.; You, L.-J.; Chen, C.; Fu, X.; Liu, R.H. Physicochemical properties and bioactivity of whey protein isolate-inulin conjugates obtained by Maillard reaction. Int. J. Biol. Macromol. 2020, 150, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Bao, Z.; Xu, W.; Chi, Y. Influence of glycation extent on the physicochemical and gelling properties of soybean β-conglycinin. Eur. Food Res. Technol. 2015, 240, 399–411. [Google Scholar] [CrossRef]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, G.; Wang, X.; Xu, Y.; Chen, S.; Wang, X.; Jiang, L. Improving the freeze-thaw stability of soy protein emulsions via combing limited hydrolysis and Maillard-induced glycation. LWT-Food Sci. Technol. 2018, 91, 63–69. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Liu, J.; Cui, Q.; Wang, X.; Chen, S.; Jiang, L. Relationship between Molecular Flexibility and Emulsifying Properties of Soy Protein Isolate-Glucose Conjugates. J. Agric. Food Chem. 2019, 67, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Feng-Lin, G.; Jin Moon, K.; Shabbar, A.; Xiao-Ming, Z.; Shu-Qin, X.; Zheng-Xing, C. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein-glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Chu, Z.; Zhang, Q.; Li, X.; Xue, B.; Sun, T.; Xie, J. Effect of Oat β-Glucan on the Structure and Properties of Soybean Protein Isolate During Maillard Reaction. Plant Food Hum. Nutr. 2023, 78, 552–556. [Google Scholar] [CrossRef]
- Song, Y.; Huang, D.; Guo, W.; Gao, Y.; Xue, F.; Xiong, X.; Li, C. Physicochemical and Structural Properties of Gluten-Konjac glucomannan Conjugates Prepared by Maillard Reaction. Polymers 2023, 15, 631. [Google Scholar] [CrossRef]
- Meng, Y.; Liang, Z.; Zhang, C.; Hao, S.; Han, H.; Du, P.; Li, A.; Shao, H.; Li, C.; Liu, L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT-Food Sci. Technol. 2021, 152, 112272. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Ren, J.; Zhao, M. Effects of Ultrasound Pretreatment on the Enzymatic Hydrolysis of Soy Protein Isolates and on the Emulsifying Properties of Hydrolysates. J. Agric. Food Chem. 2011, 59, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Mangavel, C.; Barbot, J.; Popineau, Y.; Guéguen, J. Evolution of wheat gliadins conformation during film formation:: A Fourier transform infrared study. J. Agric. Food Chem. 2001, 49, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Han, F.F.; Sui, X.N.; Qi, B.K.; Yang, Y.; Zhang, H.; Wang, R.; Li, Y.; Jiang, L.Z. Effect of ultrasound treatment on the wet heating Maillard reaction between mung bean [Vigna radiate (L.)] protein isolates and glucose and on structural and physico-chemical properties of conjugates. J. Sci. Food Agric. 2016, 96, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.J.; Qiu, A.Y.; Liu, X.Y.; Hua, Y.F.; Ma, Y.H. Microwave improvement of soy protein isolate-saccharide graft reactions. Food Chem. 2006, 97, 577–585. [Google Scholar] [CrossRef]
- Lavaei, Y.; Varidi, M.; Nooshkam, M. Gellan gum conjugation with soy protein via Maillard-driven molecular interactions and subsequent clustering lead to conjugates with tuned technological functionality. Food Chem. X 2022, 15, 100408. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yu, X.; Jing, Y. Optimized preparation, characterization, and antioxidant activity of chitooligosaccharide-glycine Maillard reaction products. J. Food Sci. Technol. 2018, 55, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Ao, L.; Liu, P.; Wu, A.; Zhao, J.; Hu, X. Characterization of Soybean Protein Isolate-Food Polyphenol Interaction via Virtual Screening and Experimental Studies. Foods 2021, 10, 2813. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Hayakawa, S.; Nakai, S. Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins. J. Food Sci. 1985, 50, 486–491. [Google Scholar] [CrossRef]
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate-polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res. Int. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll. 2013, 30, 53–60. [Google Scholar] [CrossRef]
- Wang, T.; Yi, K.; Li, Y.; Wang, H.; Fan, Z.; Jin, H.; Xu, J. Esterified Soy Proteins with Enhanced Antibacterial Properties for the Stabilization of Nano-Emulsions under Acidic Conditions. Molecules 2023, 28, 3078. [Google Scholar] [CrossRef]
- Martinez-Alvarenga, M.S.; Martinez-Rodriguez, E.Y.; Garcia-Amezquita, L.E.; Olivas, G.I.; Zamudio-Flores, P.B.; Acosta-Muniz, C.H.; Sepulveda, D.R. Effect of Maillard reaction conditions on the degree of glycation and functional properties of whey protein isolate—Maltodextrin conjugates. Food Hydrocoll. 2014, 38, 110–118. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, R.; Zhang, C.; Liu, D.; Han, J. Structural Characteristics and Emulsifying Properties of Soy Protein Isolate Glycated with Galacto-Oligosaccharides under High-Pressure Homogenization. Foods 2022, 11, 3505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gong, S.; Wang, Y.; Liu, D.; Han, J. Structural and Emulsifying Properties of Soybean Protein Isolate-Sodium Alginate Conjugates under High Hydrostatic Pressure. Foods 2021, 10, 2829. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Moreno, S.; Bialystok, E.; Barac, R.; Schellenberg, E.G.; Cepeda, N.J.; Chau, T. Short-term music training enhances verbal intelligence and executive function. Psychol. Sci. 2011, 22, 1425–1433. [Google Scholar] [CrossRef]
- Bazzano, L.A. Effects of Soluble Dietary Fiber on Low-Density Lipoprotein Cholesterol and Coronary Heart Disease Risk. Curr. Atheroscleros. Rep. 2008, 10, 473–477. [Google Scholar] [CrossRef]
- Allen, K.G.D.; Bristow, S.J.; Yu, L.L. Hypolipidemic effects of modified psyllium preparations. J. Agric. Food Chem. 2004, 52, 4998–5003. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Rokita, B.; Ulanski, P.; Rosiak, J.M. Radiation-induced and sonochemical degradation of chitosan as a way to increase its fat-binding capacity. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 2005, 236, 383–390. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Xie, J.; Xue, B.; Li, X.H.; Gan, J.H.; Zhu, T.; Sun, T. Effect of acid and oxidative degradation on the structural, rheological, and physiological properties of oat β-glucan. Food Hydrocoll. 2021, 112, 106284. [Google Scholar] [CrossRef]
- Fang, H.C.; Li, J.Y.; Huo, T.Y.; Niu, Y.G.; Yu, L.L. Novel double cross-linked gels of soybean protein isolates and soluble dietary fiber from soybean coats with their functionalities. Food Hydrocoll. 2021, 113, 106474. [Google Scholar] [CrossRef]
- Ragot, F.I.J.; Russell, G.F.; Schneeman, B.O. Effect of Maillard reaction products on bile acid binding, plasma and hepatic lipids, and weight of gastrointestinal organs. J. Agric. Food Chem. 1992, 40, 1634–1640. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. Interactional Effects of β-Glucan, Starch, and Protein in Heated Oat Slurries on Viscosity and In Vitro Bile Acid Binding. J. Agric. Food Chem. 2012, 60, 6217–6222. [Google Scholar] [CrossRef] [PubMed]
- Regand, A.; Chowdhury, Z.; Tosh, S.M.; Wolever, T.M.S.; Wood, P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef]
- Guo, H.; Wu, H.; Kong, X.; Zhang, N.; Li, H.; Dong, X.; Li, Z. Oat, B-glucan ameliorates diabetes in high fat diet and streptozotocin-induced mice by regulating metabolites. J. Nutr. Biochem. 2023, 113, 109251. [Google Scholar] [CrossRef]
- Mohan, S.; Pinto, B.M. Zwitterionic glycosidase inhibitors: Salacinol and related analogues. Carbohydr. Res. 2007, 342, 1551–1580. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Hong, J.T.; Hwang, B.Y.; Jung, J.K.; Lee, J.; Jeong, H.S. Isolation and characterisation of an α-glucosidase inhibitory substance from fructose-tyrosine Mail lard reaction products. Food Chem. 2011, 127, 122–126. [Google Scholar] [CrossRef]
- Pedrali, D.; Barbarito, S.; Lavelli, V. Encapsulation of grape seed phenolics from winemaking byproducts in hydrogel microbeads-impact of food matrix and processing on the inhibitory activity towards alpha-glucosidase. LWT-Food Sci. Technol. 2020, 133, 109952. [Google Scholar] [CrossRef]
- Thi Ngoc, T.; Chien Thang, D.; Van Bon, N.; Anh Dzung, N.; Wang, S.-L. Anti-Oxidant and Anti-Diabetes Potential of Water-Soluble Chitosan-Glucose Derivatives Produced by Maillard Reaction. Polymers 2019, 11, 1714. [Google Scholar] [CrossRef] [PubMed]
- Kotora, P.; Sersen, F.; Filo, J.; Loos, D.; Gregan, J.; Gregan, F. The Scavenging of DPPH, Galvinoxyl and ABTS Radicals by Imine Analogs of Resveratrol. Molecules 2016, 21, 21010127. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Goldman, V.M.; Bistrian, B.R.; Arnold, A.H.; Ostroff, G.; Forse, R.A. Effect of β-glucan from oats and yeast on serum lipids. Crit. Rev. Food Sci. Nutr. 1999, 39, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
| Sample | DG (%) | H0 Values |
|---|---|---|
| SPI | - | 246.22 ± 3.90 c |
| SPI-Gal | 18.84 ± 0.38 a | 986.60 ± 7.01 a |
| SPI-βG | 10.61 ± 0.16 b | 600.41 ± 3.96 b |
| Sample | Emulsifying Activity (m2/g) | Emulsifying Stability (min) | Foaming Capacity (%) | Foam Stability (%) |
|---|---|---|---|---|
| SPI | 21.17 ± 0.37 c | 11.17 ± 0.26 c | 15.01 ± 0.27 c | 11.67 ± 0.29 c |
| SPI-Gal | 47.92 ± 0.42 b | 19.18 ± 0.28 a | 62.50 ± 0.34 a | 52.09 ± 0.43 a |
| SPI-βG | 57.69 ± 0.35 a | 13.26 ± 0.41 b | 37.07 ± 0.32 b | 38.78 ± 0.57 b |
| Sample | Swelling Power (g/g) | Fat-Binding Capacity (g/g) | Bile Acid-Binding Capacity (%) | Glucose Availability (mmol/L) |
|---|---|---|---|---|
| SPI | 9.12 ± 0.19 a | 3.89 ± 0.13 c | 10.89 ± 0.28 c | 20.33 ± 0.27 a |
| SPI-Gal | 2.05 ± 0.07 b | 10.67 ± 0.18 a | 20.78 ± 0.32 b | 18.90 ± 0.14 b |
| SPI-βG | 1.10 ± 0.03 c | 7.50 ± 0.15 b | 48.96 ± 1.43 a | 10.05 ± 0.12 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, K.; Zhang, Q.; Xie, J.; Xue, B.; Li, X.; Bian, X.; Sun, T. Effect of Mono- and Polysaccharide on the Structure and Property of Soy Protein Isolate during Maillard Reaction. Foods 2024, 13, 2832. https://doi.org/10.3390/foods13172832
Wen K, Zhang Q, Xie J, Xue B, Li X, Bian X, Sun T. Effect of Mono- and Polysaccharide on the Structure and Property of Soy Protein Isolate during Maillard Reaction. Foods. 2024; 13(17):2832. https://doi.org/10.3390/foods13172832
Chicago/Turabian StyleWen, Kun, Qiyun Zhang, Jing Xie, Bin Xue, Xiaohui Li, Xiaojun Bian, and Tao Sun. 2024. "Effect of Mono- and Polysaccharide on the Structure and Property of Soy Protein Isolate during Maillard Reaction" Foods 13, no. 17: 2832. https://doi.org/10.3390/foods13172832







