Tea’s Characteristic Components Eliminate Acrylamide in the Maillard Model System
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Glc-Asn Thermal Model Reaction
2.3. Elimination Evaluation of Effects of Tea’s Characteristic Components on Acrylamide in Maillard Model System
2.4. LC–Triple Quadrupole (QQQ) MS/MS Analysis of Acrylamide in the Maillard Model System
2.5. Free Radicals in the Maillard Model System
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Effects of Instant Green Tea on Acrylamide Levels in the Glc-Asn Model System
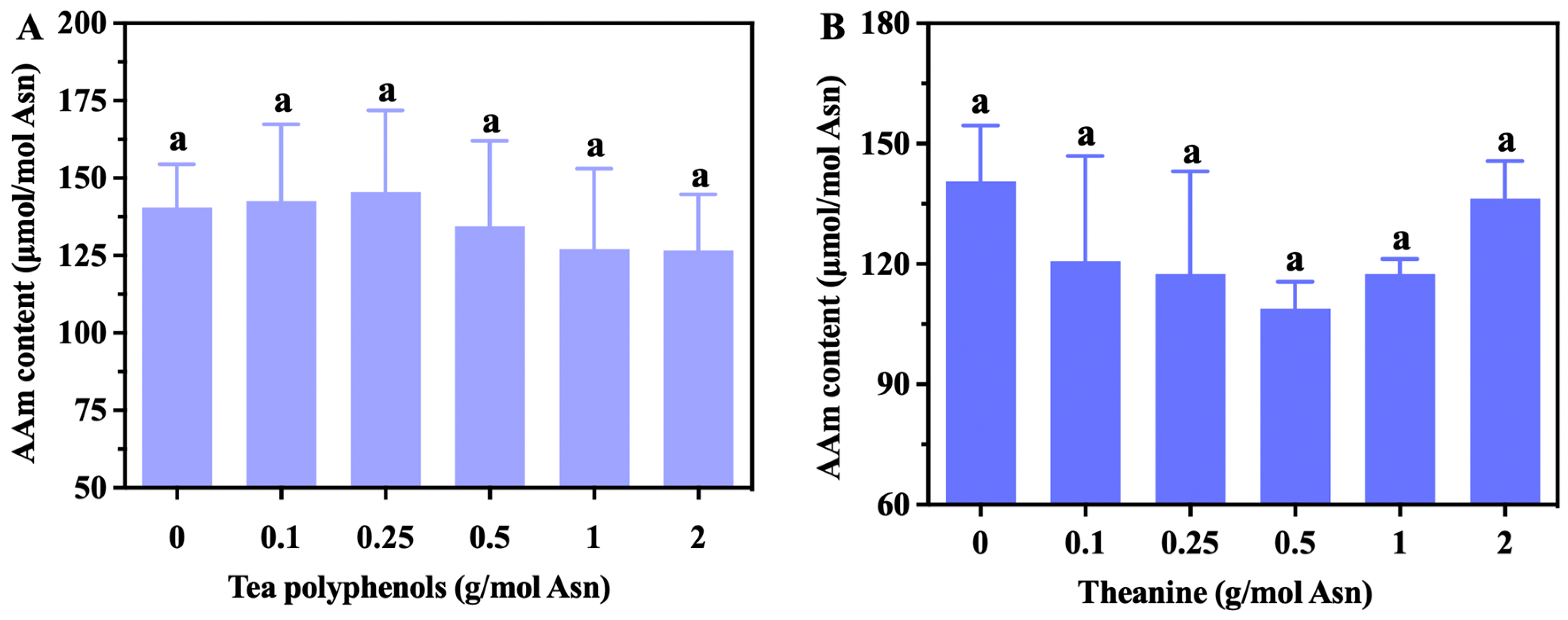
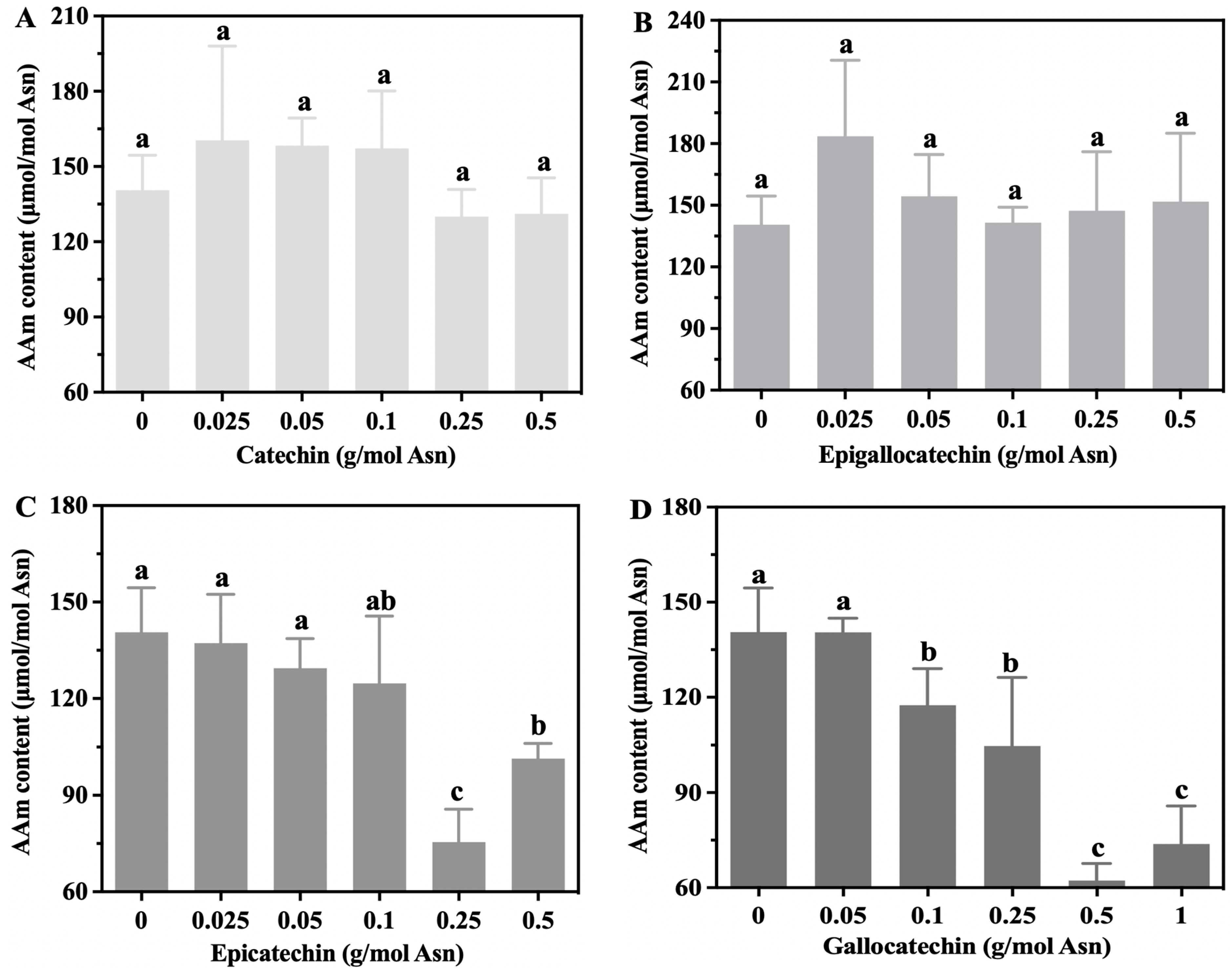
3.2. The Effects of Tea’s Characteristic Components on Acrylamide Levels in the Glc-Asn Model System
3.3. The Effects of Tea Polyphenol Oxides on Acrylamide Levels in the Glc-Asn Model System
3.4. Synergistic or Antagonistic Effects of Tea’s Characteristic Components on Acrylamide Formation
3.5. The Effects of Tea’s Characteristic Components on Free Radical Generation in the Glc-Asn Model System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Wang, P.; Chen, F.; Yuan, Y.; Zhu, Y.; Yan, H.; Hu, X. Role of plant polyphenols in acrylamide formation and elimination. Food Chem. 2015, 186, 46–53. [Google Scholar] [CrossRef]
- Maan, A.A.; Anjum, M.A.; Khan, M.K.I.; Nazir, A.; Saeed, F.; Afzaal, M.; Aadil, R.M. Acrylamide formation and different mitigation strategies during food processing—A review. Food Rev. Int. 2022, 38, 70–87. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Martinelli, E.; Mulazzi, A.; Rastelli, S. Acrylamide determination during an industrial roasting process of coffee and the influence of asparagine and low molecular weight sugars. Food Chem. 2020, 303, 125372. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- Commission, E. Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. 2017. Eur-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2017/2158 (accessed on 20 November 2017).
- Capuano, E.; Ferrigno, A.; Acampa, I.; Serpen, A.; Açar, Ö.; Gökmen, V.; Fogliano, V. Effect of flour type on Maillard reaction and acrylamide formation during toasting of bread crisp model systems and mitigation strategies. Food Res. Int. 2009, 42, 1295–1302. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. Reduction of acrylamide formation in potato slices during frying. LWT—Food Sci. Technol. 2004, 37, 679–685. [Google Scholar] [CrossRef]
- Lim, P.K.; Jinap, S.; Sanny, M.; Tan, C.P.; Khatib, A. The influence of deep frying using various vegetable oils on acrylamide formation in sweet potato (ipomoea batatas L. lam) chips. J. Food Sci. 2014, 79, T115–T121. [Google Scholar] [CrossRef]
- Tepe, T.K.; Kadakal, C. Temperature and slice size dependences of acrylamide in potato fries. J. Food Process. Preserv. 2019, 43, e14270. [Google Scholar] [CrossRef]
- Chan, D.S. Computer Simulation with a Temperature-Step Frying Approach to Mitigate Acrylamide Formation in French Fries. Foods 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Delgado, R.M.; Hidalgo, F.J. Amino phospholipids and lecithins as mitigating agents for acrylamide in asparagine/glucose and asparagine/2,4-decadienal model systems. Food Chem. 2011, 126, 104–108. [Google Scholar] [CrossRef]
- Ma, Y.J.; Huang, H.R.; Zhang, Y.; Li, F.; Gan, B.; Yu, Q.; Xie, J.H.; Chen, Y. Soluble dietary fiber from tea residues with inhibitory effects against acrylamide and 5-hydroxymethylfurfural formation in biscuits: The role of bound polyphenols. Food Res. Int. 2022, 159, 111595. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; Maertens, J.; Cucu, T.; Delporte, K.; Van Peteghem, C.; De Meulenaer, B. Impact of additives to lower the formation of acrylamide in a potato model system through pH reduction and other mechanisms. Food Chem. 2008, 107, 26–31. [Google Scholar] [CrossRef]
- Aiswarya, R.; Baskar, G. Enzymatic mitigation of acrylamide in fried potato chips using asparaginase from Aspergillus terreus. Int. J. Food Sci. Technol. 2018, 53, 491–498. [Google Scholar] [CrossRef]
- Liu, C.; Luo, L.J.; Lin, Q.L. Antitumor activity and ability to prevent acrylamide formation in fried foods of asparaginase from soybean root nodules. J. Food Biochem. 2019, 43, e12756. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Delgado, R.M.; Zamora, R. Role of mercaptans on acrylamide elimination. Food Chem. 2010, 122, 596–601. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.N.; Yuan, Y. Mitigation effect of sodium alginate on acrylamide formation in fried potato chips system based on response surface methodology. J. Food Sci. 2020, 85, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.W.; Zeng, X.H.; Tang, Y.S.; Wu, J.J.; Liu, Z.W.; Sze, K.H.; Chu, I.K.; Chen, F.; Wang, M.F. Inhibitory mechanism of naringenin against carcinogenic acrylamide formation and nonenzymatic browning in maillard model reactions. Chem. Res. Toxicol. 2009, 22, 1483–1489. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Sobral, M.M.C.; Viegas, O.; Cunha, S.C.; Alarcón-Enos, J.; Pinho, O.; Ferreira, I. Incorporation of avocado peel extract to reduce cooking-induced hazards in beef and soy burgers: A clean label ingredient. Food Res. Int. 2021, 147, 110434. [Google Scholar] [CrossRef]
- Demirok, E.; Kolsarici, N. Effect of green tea extract and microwave pre-cooking on the formation of acrylamide in fried chicken drumsticks and chicken wings. Food Res. Int. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- NBSC (National Bureau of Statistics of China). Statistical Communiqué of the People’s Republic of China on The 2023 National Economic and Social Development. 2024. Available online: https://www.stats.gov.cn/english/PressRelease/202402/t20240228_1947918.html (accessed on 28 February 2023).
- Knol, J.J.; van Loon, W.A.M.; Linssen, J.P.H.; Ruck, A.L.; van Boekel, M.A.J.S.; Voragen, A.G.J. Toward a kinetic model for acrylamide formation in a glucose-asparagine reaction system. J. Agric. Food Chem. 2005, 53, 6133–6139. [Google Scholar] [CrossRef]
- GB 5009.204; Determination of Acrylamide in Food. SAPRC (Standardization Administration of the People’s Republic of China): Beijing, China, 2014.
- Morales, G.; Jimenez, M.; Garcia, O.; Mendoza, M.R.; Beristain, C.I. Effect of natural extracts on the formation of acrylamide in fried potatoes. LWT—Food Sci. Technol. 2014, 58, 587–593. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Zhang, Y.; Lu, B.; Jin, C.; Wu, X.; Zhang, Y. Study on mitigation of acrylamide formation in cookies by 5 antioxidants. J. Food Sci. 2012, 77, C1144–C1149. [Google Scholar] [CrossRef]
- Budryn, G.; Żyżelewicz, D.; Nebesny, E.; Oracz, J.; Krysiak, W. Influence of addition of green tea and green coffee extracts on the properties of fine yeast pastry fried products. Food Res. Int. 2013, 50, 149–160. [Google Scholar] [CrossRef]
- Peng, C.Y.; Ren, Y.F.; Ye, Z.H.; Zhu, H.Y.; Liu, X.Q.; Chen, X.T.; Hou, R.Y.; Granato, D.; Cai, H.M. A comparative UHPLC-Q/TOF-MS-based metabolomics approach coupled with machine learning algorithms to differentiate Keemun black teas from narrow-geographic origins. Food Res. Int. 2022, 158, 111512. [Google Scholar] [CrossRef]
- Xie, G.; Yan, J.; Lu, A.; Kun, J.; Wang, B.; Song, C.; Tong, H.; Meng, Q. Characterizing relationship between chemicals and in vitro bioactivities of teas made by six typical processing methods using a single Camellia sinensis cultivar, Meizhan. Bioengineered 2021, 12, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, X.; Zhao, S.; Zhang, Y. Antioxidant-capacity-based models for the prediction of acrylamide reduction by flavonoids. Food Chem. 2015, 168, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Oral, R.A.; Dogan, M.; Sarioglu, K. Effects of certain polyphenols and extracts on furans and acrylamide formation in model system, and total furans during storage. Food Chem. 2014, 142, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, R.V.; Granby, K.; Frandsen, H.; Thygesen, J.; Skibsted, L.H. Acrylamide in bread-Effect of prooxidants and antioxidants. Eur. Food Res. Technol. 2007, 227, 519–525. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, T.; Zhang, Y. Reduction of acrylamide and its kinetics by addition of antioxidant of bamboo leaves (AOB) and extract of green tea (EGT) in asparagine–Glucose Microwave Heating System. J. Food Sci. 2008, 73, C60–C66. [Google Scholar] [CrossRef]
- Kotsiou, K.; Tasioula-Margari, M.; Kukurová, K.; Ciesarová, Z. Impact of oregano and virgin olive oil phenolic compounds on acrylamide content in a model system and fresh potatoes. Food Chem. 2010, 123, 1149–1155. [Google Scholar] [CrossRef]
- Cheng, K.W.; Shi, J.J.; Ou, S.Y.; Wang, M.; Jiang, Y. Effects of fruit extracts on the formation of acrylamide in model reactions and fried potato crisps. J. Agric. Food Chem. 2010, 58, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z. Studies on Reduction Mechanism and Structure-Activity Relationship of Acrylamide in Foods by Bio-Flavonoids. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2008. [Google Scholar]
- Salazar, R.; Arámbula-Villa, G.; Hidalgo, F.J.; Zamora, R. Mitigating effect of piquin pepper (Capsicum annuum L. var. Aviculare) oleoresin on acrylamide formation in potato and tortilla chips. LWT—Food Sci. Technol. 2012, 48, 261–267. [Google Scholar] [CrossRef]
- Huang-you, L. Inhibition of Acrylamide and 5-Hydroxymethylfurfural Formation by Natural Plant Flavonoids in the Maillard Systems. Ph.D. Thesis, Jilin University, Changchun, China, 2018. [Google Scholar]
- Li, X.D.; Teng, W.D.; Liu, G.M.; Guo, F.Y.; Xing, H.Z.; Zhu, Y.H.; Li, J.W. Allicin promoted reducing effect of garlic powder through acrylamide formation stage. Foods 2022, 11, 2394. [Google Scholar] [CrossRef] [PubMed]



| Combination | Ingredient | AAm Content (μmol/mol Asn) | Reduction Rate (%) | ||||
|---|---|---|---|---|---|---|---|
| Theaflavins | Thearubigins | EC | GC | CG | |||
| Control | - | - | - | - | - | 140.58 ± 13.92 a | ND |
| 1 | + | + | + | + | + | 113.14 ± 10.80 bc | 19.52 |
| 2 | + | + | + | + | - | 74.39 ± 14.61 ghij | 47.08 |
| 3 | + | + | + | - | + | 55.97 ± 19.74 jkl | 60.19 |
| 4 | + | + | - | + | + | 51.14 ± 5.22 kl | 63.62 |
| 5 | + | - | + | + | + | 61.66 ± 3.35 ijk | 56.14 |
| 6 | - | + | + | + | + | 36.56 ± 1.79 l | 73.99 |
| 7 | - | - | + | + | + | 50.28 ± 3.93 kl | 64.24 |
| 8 | - | + | - | + | + | 77.78 ± 13.13 fghi | 44.67 |
| 9 | - | + | + | - | + | 38.42 ± 3.71 l | 72.67 |
| 10 | - | + | + | + | - | 45.53 ± 3.64 kl | 67.62 |
| 11 | + | - | - | + | + | 40.73 ± 2.34 l | 71.03 |
| 12 | + | - | + | - | + | 125.61 ± 17.96 ab | 10.65 |
| 13 | + | - | + | + | - | 82.79 ± 2.70 efgh | 41.11 |
| 14 | + | + | - | - | + | 96.47 ± 5.59 cdef | 31.38 |
| 15 | + | + | - | + | - | 96.75 ± 4.03 cdef | 31.18 |
| 16 | + | + | + | - | - | 100.96 ± 14.17 cde | 28.19 |
| 17 | - | - | - | + | + | 73.94 ± 0.96 hij | 47.40 |
| 18 | - | - | + | - | + | 91.08 ± 3.86 defg | 35.21 |
| 19 | - | - | + | + | - | 72.32 ± 1.99 ghij | 48.56 |
| 20 | - | + | - | - | + | 72.65 ± 13.71 ghij | 48.32 |
| 21 | - | + | - | + | - | 70.87 ± 11.83 hij | 49.59 |
| 22 | - | + | + | - | - | 110.30 ± 24.53 bcd | 21.54 |
| 23 | + | - | - | - | + | 92.51 ± 9.22 defg | 34.19 |
| 24 | + | - | - | + | - | 91.70 ± 11.91 defgh | 34.77 |
| 25 | + | - | + | - | - | 86.39 ± 17.54 efgh | 38.55 |
| 26 | + | + | - | - | - | 48.165 ± 5.3 kl | 65.74 |
| Plant Polyphenols | Matrix | Amount | Elimination Rate (%) | Reference |
|---|---|---|---|---|
| Tea characteristic components | Glc-Asn model system | 0.25–0.5 g/mol Asn | 37.36–55.73 | This study |
| Combinations | 1 g/mol Asn | 65.74 | ||
| 67.62 | ||||
| 71.03 | ||||
| 72.67 | ||||
| 73.99 | ||||
| Naringin | 11.6 mg/mol Asn | 20 | [18] | |
| 22.3 mg/mol Asn | 40 | |||
| 58.1 mg/mol Asn | 60 | |||
| Apigenin | 18.9 g/mol Asn | 67.17 | [35] | |
| Cyanidenon | 20.02 g/mol Asn | 84.17 | ||
| Quercetin | 2.11 g/mol Asn | 80.11 | ||
| Glycyrrhizin | 0.18 g/mol Asn | 88.77 | ||
| Liquiritin | 0.29 g/mol Asn | 81.65 | ||
| Genistein | 1.89 g/mol Asn | 86.51 | ||
| Silymarin | 3.37 mg/mol Asn | 83.99 | ||
| Garlic powder (freeze-dry) | 41.67 g/mol Asn | 41 | [36] | |
| Garlic powder (oven-dry) | 37.3 | |||
| Garlicin | 9.1 mg/mol Asn | 71.3 | ||
| Antioxidant of bamboo leaves | Potato chips | 0.1% (w/w) | 74.1 | [37] |
| French fries | 0.01% (w/w) | 76.1 | ||
| Chinese fried dough stick | 0.1% (w/w) | 82.9 | ||
| Fried chicken wings | 0.5% (w/w) | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Xu, H.; Xie, Y.; Peng, Z.; Li, H.; Hou, R.; Cai, H.; Song, W.; Peng, C.; Li, D. Tea’s Characteristic Components Eliminate Acrylamide in the Maillard Model System. Foods 2024, 13, 2836. https://doi.org/10.3390/foods13172836
Ye Z, Xu H, Xie Y, Peng Z, Li H, Hou R, Cai H, Song W, Peng C, Li D. Tea’s Characteristic Components Eliminate Acrylamide in the Maillard Model System. Foods. 2024; 13(17):2836. https://doi.org/10.3390/foods13172836
Chicago/Turabian StyleYe, Zhihao, Haojie Xu, Yingying Xie, Ziqi Peng, Hongfang Li, Ruyan Hou, Huimei Cai, Wei Song, Chuanyi Peng, and Daxiang Li. 2024. "Tea’s Characteristic Components Eliminate Acrylamide in the Maillard Model System" Foods 13, no. 17: 2836. https://doi.org/10.3390/foods13172836
APA StyleYe, Z., Xu, H., Xie, Y., Peng, Z., Li, H., Hou, R., Cai, H., Song, W., Peng, C., & Li, D. (2024). Tea’s Characteristic Components Eliminate Acrylamide in the Maillard Model System. Foods, 13(17), 2836. https://doi.org/10.3390/foods13172836










