Physicochemical, Nutritional, and Antioxidant Properties of Traditionally Fermented Thai Vegetables: A Promising Functional Plant-Based Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermented Vegetables
2.2. Physicochemical Properties
2.3. Nutritional Composition
2.4. Total Phenolic Content
2.5. DPPH Radical Scavenging Activity
2.6. ABTS Radical Scavenging Activity
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.2. Nutritional Properties
3.3. TPC and Antioxidant Activities
3.4. Comparison of Overall Profiles
3.5. Correlation Analysis among Different Chemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hyldelund, N.B.; Worck, S.; Olsen, A. Convenience may increase vegetable intake among young consumers. Food Qual. Prefer. 2020, 83, 103925. [Google Scholar] [CrossRef]
- Singh, B. Vegetables: Source of adequate health benefits. Ann. Hortic. 2020, 13, 124–130. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C.S. Tapping the economic and nutritional power of vegetables. Glob. Food Secur. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.-s. Fermented beverages of pre-and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Cuamatzin-García, L.; Rodríguez-Rugarcía, P.; El-Kassis, E.G.; Galicia, G.; Meza-Jiménez, M.L.; Baños-Lara, M.D.R.; Zaragoza-Maldonado, D.S.; Pérez-Armendáriz, B. Traditional Fermented Foods and Beverages from around the World and Their Health Benefits. Microorganisms 2022, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Küçükgöz, K.; Kołożyn-Krajewska, D. Traditional and New Microorganisms in Lactic Acid Fermentation of Food. Fermentation 2023, 9, 1019. [Google Scholar] [CrossRef]
- Saranraj, P.; Behera, S.S.; Ray, R.C. Chapter 7—Traditional Foods From Tropical Root and Tuber Crops: Innovations and Challenges. In Innovations in Traditional Foods; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 159–191. [Google Scholar]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented Fruits and Vegetables of Asia: A Potential Source of Probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- Viridiana, C.-R.; Lidia, D.-A.; Audry, P.-L.; Humberto, H.-S. Lactic Acid Bacteria Isolated From Vegetable Fermentations: Probiotic Characteristics. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Wang, Z.; Wu, J.; Tian, Z.; Si, Y.; Chen, H.; Gan, J. The Mechanisms of the Potential Probiotic Lactiplantibacillus plantarum against Cardiovascular Disease and the Recent Developments in its Fermented Foods. Foods 2022, 11, 2549. [Google Scholar] [CrossRef] [PubMed]
- Turreira-García, N.; Vilkamaa, A.; Byg, A.; Theilade, I. Diversity, Knowledge, and use of leafy vegetables in Northern Thailand—Maintenance and transmission of ethnobotanical knowledge during urbanisation. J. Siam Soc. 2017, 62, 85–105. [Google Scholar]
- Pakwan, C.; Chitov, T.; Chantawannakul, P.; Manasam, M.; Bovonsombut, S.; Disayathanoowat, T. Bacterial compositions of indigenous Lanna (Northern Thai) fermented foods and their potential functional properties. PLoS ONE 2020, 15, e0242560. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Thai Fermented Foods as a Versatile Source of Bioactive Microorganisms—A Comprehensive Review. Sci. Pharm. 2018, 86, 37. [Google Scholar] [CrossRef] [PubMed]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Surya, R. Fermented foods of Southeast Asia other than soybean- or seafood-based ones. J. Ethn. Foods 2024, 11, 27. [Google Scholar] [CrossRef]
- Waché, Y.; Do, T.-L.; Do, T.-B.-H.; Do, T.-Y.; Haure, M.; Ho, P.-H.; Kumar Anal, A.; Le, V.-V.-M.; Li, W.-J.; Licandro, H.; et al. Prospects for Food Fermentation in South-East Asia, Topics From the Tropical Fermentation and Biotechnology Network at the End of the AsiFood Erasmus+Project. Front. Microbiol. 2018, 9, 2278. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Bokelmann, W.; Huyskens-Keil, S.; Ferenczi, Z.; Stöber, S. The Role of Indigenous Vegetables to Improve Food and Nutrition Security: Experiences from the Project HORTINLEA in Kenya (2014–2018). Front. Sustain. Food Syst. 2022, 6, 806420. [Google Scholar] [CrossRef]
- Rodzi, N.A.R.M.; Lee, L.K. Traditional fermented foods as vehicle of non-dairy probiotics: Perspectives in South East Asia countries. Food Res. Int. 2021, 150, 110814. [Google Scholar] [CrossRef]
- Garza-Juárez, A.; Pérez-Carrillo, E.; Arredondo-Espinoza, E.U.; Islas, J.F.; Benítez-Chao, D.F.; Escamilla-García, E. Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases. Foods 2023, 12, 3262. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, P.; Chen, X. Bioactive peptides derived from fermented foods: Preparation and biological activities. J. Funct. Foods 2023, 101, 105422. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Gunawardena, S.; Nadeeshani, H.; Amarasinghe, V.; Liyanage, R. Bioactive properties and therapeutic aspects of fermented vegetables: A review. Food Prod. Process. Nutr. 2024, 6, 31. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An overview of fermentation in the food industry—Looking back from a new perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Pan-utai, W.; Atkonghan, J.; Onsamark, T.; Imthalay, W. Effect of Arthrospira microalga fortification on physicochemical properties of yogurt. Curr. Res. Nutr. Food Sci. 2020, 8, 531–540. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International Gaithersburg: Rockville, MD, USA, 2000; Volume 1. [Google Scholar]
- Pan-utai, W.; Pantoa, T.; Roytrakul, S.; Praiboon, J.; Kosawatpat, P.; Tamtin, M.; Thongdang, B. Ultrasonic-Assisted Extraction and Antioxidant Potential of Valuable Protein from Ulva rigida Macroalgae. Life 2023, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-H.R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T.V. Production of Ulvan Oligosaccharides with Antioxidant and Angiotensin-Converting Enzyme-Inhibitory Activities by Microbial Enzymatic Hydrolysis. Fermentation 2021, 7, 160. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Xiao, L.; Qu, L.; Zhang, X.; Wei, Y. Advancing Insights into Probiotics during Vegetable Fermentation. Foods 2023, 12, 3789. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Gao, J.; Mi, S.; Mao, K.; Zhang, T.; Wang, X.; Sang, Y. Chemical composition of naturally-fermented mixed fruit product and in vitro bioactivities. LWT 2023, 181, 114771. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, A.; Wu, Z.; Liu, C.; Zhang, W. Characterization of Microbial Community during the Fermentation of Chinese Homemade paocai, a Traditional Fermented Vegetable Food. Food Sci. Technol. Res. 2016, 22, 467–475. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation—The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Tan, X.; Cui, F.; Wang, D.; Lv, X.; Li, X.; Li, J. Fermented Vegetables: Health Benefits, Defects, and Current Technological Solutions. Foods 2024, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, Y.; El Abed, N.; Montanari, C.; Ozogul, F. Chapter Two—Contribution of polysaccharides from crustacean in fermented food products. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 102, pp. 47–92. [Google Scholar]
- Varzakas, T.; Zakynthinos, G.; Proestos, C.; Radwanska, M. Fermented vegetables. Minim. Process. Refrig. Fruits Veg. 2017, 537–584. [Google Scholar]
- Zannou, O.; Agossou, D.J.; Miassi, Y.; Agani, O.B.; Darino Aisso, M.; Chabi, I.B.; Euloge Kpoclou, Y.; Azokpota, P.; Koca, I. Traditional fermented foods and beverages: Indigenous practices of food processing in Benin Republic. Int. J. Gastron. Food Sci. 2022, 27, 100450. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, J.; Zhang, Y.; Yi, X.; Pang, X.; Li-Byarlay, H.; Gao, X. Differences in the bacterial profiles and physicochemical between natural and inoculated fermentation of vegetables from Shanxi Province. Ann. Microbiol. 2020, 70, 66. [Google Scholar] [CrossRef]
- Bernal-Castro, C.; Espinosa-Poveda, E.; Gutiérrez-Cortés, C.; Díaz-Moreno, C. Vegetable substrates as an alternative for the inclusion of lactic acid bacteria with probiotic potential in food matrices. J. Food Sci. Technol. 2024, 61, 833–846. [Google Scholar] [CrossRef]
- Fukudome, C.; Takisawa, R.; Nakano, R.; Kusano, M.; Kobayashi, M.; Motoki, K.; Nishimura, K.; Nakazaki, T. Analysis of mechanism regulating high total soluble solid content in the parthenocarpic tomato fruit induced by pat-k gene. Sci. Hortic. 2022, 301, 111070. [Google Scholar] [CrossRef]
- Giang, N.T.N.; Tan, N.D.; Khai, T.V.; Tuyen, V.T.X. Effect of initial total soluble solids and pH on the quality of fermented beverage from green asparagus roots (Asparagus officinalis L.). Food Res. 2024, 8, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, G.; Finley, J.W.; deMan, J.M. Carbohydrates. In Principles of Food Chemistry; deMan, J.M., Finley, J.W., Hurst, W.J., Lee, C.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 165–229. [Google Scholar]
- Medhe, S.V.; Kamble, M.T.; Kettawan, A.K.; Monboonpitak, N.; Kettawan, A. Effect of hydrothermal cooking and germination treatment on functional and physicochemical properties of Parkia timoriana bean flours: An underexplored legume Species of Parkia genera. Foods 2022, 11, 1822. [Google Scholar] [CrossRef]
- Medhe, S.V.; Kettawan, A.K.; Kamble, M.T.; Monboonpitak, N.; Thompson, K.D.; Kettawan, A.; Pirarat, N. Modification of Physiochemical and Techno-Functional Properties of Stink Bean (Parkia speciosa) by Germination and Hydrothermal Cooking Treatment. Foods 2023, 12, 4480. [Google Scholar] [CrossRef] [PubMed]
- Ziarno, M.; Bryś, J.; Parzyszek, M.; Veber, A. Effect of Lactic Acid Bacteria on the Lipid Profile of Bean-Based Plant Substitute of Fermented Milk. Microorganisms 2020, 8, 1348. [Google Scholar] [CrossRef]
- Widaningrum; Flanagan, B.M.; Williams, B.A.; Sonni, F.; Mikkelsen, D.; Gidley, M.J. Fruit and vegetable insoluble dietary fibre in vitro fermentation characteristics depend on cell wall type. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100223. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Fusco, V.; Cho, G.-S.; Kabisch, J.; Neve, H.; Bockelmann, W.; Huch, M.; Frommherz, L.; Trierweiler, B.; Becker, B.; et al. Produce from Africa’s Gardens: Potential for Leafy Vegetable and Fruit Fermentations. Front. Microbiol. 2016, 7, 981. [Google Scholar] [CrossRef]
- Willcox, J.K.; Catignani, G.L.; Lazarus, S. Tomatoes and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2003, 43, 1–18. [Google Scholar] [CrossRef]
- Polanowska, K.; Grygier, A.; Kuligowski, M.; Rudzińska, M.; Nowak, J. Effect of tempe fermentation by three different strains of Rhizopus oligosporus on nutritional characteristics of faba beans. LWT 2020, 122, 109024. [Google Scholar] [CrossRef]
- Mikulajová, A.; Matejčeková, Z.; Kohajdová, Z.; Mošovská, S.; Hybenová, E.; Valík, Ľ. Changes in phenolic composition, antioxidant, sensory and microbiological properties during fermentation and storage of maize products. Food Prod. Process. Nutr. 2024, 6, 9. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, Y.; Xu, Z. Evaluation of nitrite, ethyl carbamate, and biogenic amines in four types of fermented vegetables. Foods 2021, 10, 3150. [Google Scholar] [CrossRef]
- Thierry, A.; Baty, C.; Marché, L.; Chuat, V.; Picard, O.; Lortal, S.; Valence, F. Lactofermentation of vegetables: An ancient method of preservation matching new trends. Trends Food Sci. Technol. 2023, 139, 104112. [Google Scholar] [CrossRef]
- Thierry, A.; Madec, M.N.; Chuat, V.; Bage, A.S.; Picard, O.; Grondin, C.; Rué, O.; Mariadassou, M.; Marché, L.; Valence, F. Microbial communities of a variety of 75 homemade fermented vegetables. Front. Microbiol. 2023, 14, 1323424. [Google Scholar] [CrossRef]
- Martínez-Miranda, J.G.; Chairez, I.; Durán-Páramo, E. Mannitol production by heterofermentative lactic acid bacteria: A review. Appl. Biochem. Biotechnol. 2022, 194, 2762–2795. [Google Scholar] [CrossRef]

 ), pickled pak-sian (B;
), pickled pak-sian (B;  ), pickled bamboo shoots (C;
), pickled bamboo shoots (C;  ), pickled stink beans (D;
), pickled stink beans (D;  ), pickled shallot (E;
), pickled shallot (E;  ), pickled mustard greens (F;
), pickled mustard greens (F;  ), pickled ginger (G;
), pickled ginger (G;  ), fermented tea leaves (H;
), fermented tea leaves (H;  ), pickled scallions (I;
), pickled scallions (I;  ), and pickled bud rieng (J;
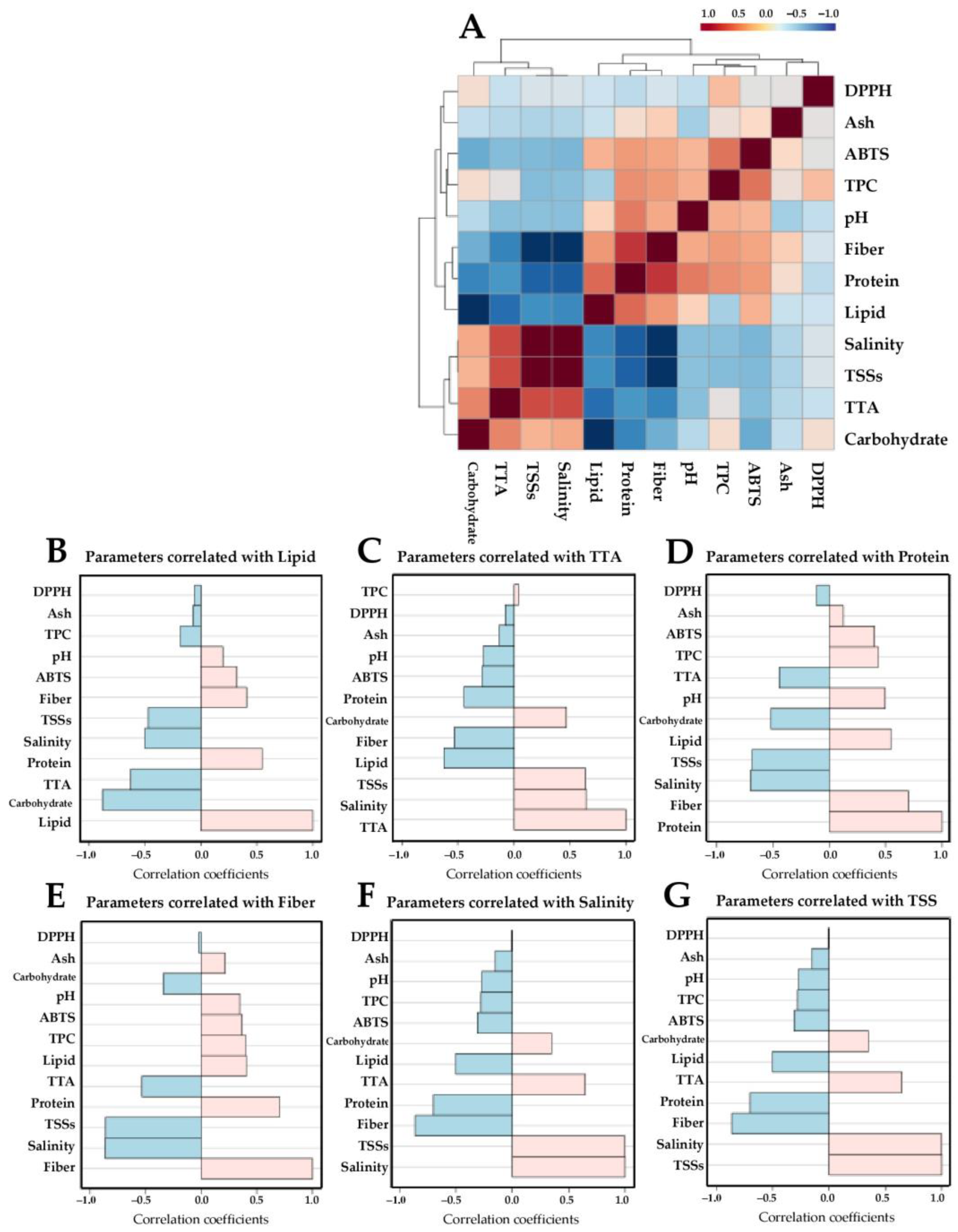
), and pickled bud rieng (J;  ) samples. The dendrogram represents sample clusters based on Pearson’s correlation coefficient with average linkage. Each square in the heatmap expresses normalized chemical abundance as the color range. The red color indicates a higher content of the corresponding chemical parameter. To better interpret the references to color in this figure, please see the statistical comparisons of the sample chemical properties in Table 3, Table 4 and Table 5.
) samples. The dendrogram represents sample clusters based on Pearson’s correlation coefficient with average linkage. Each square in the heatmap expresses normalized chemical abundance as the color range. The red color indicates a higher content of the corresponding chemical parameter. To better interpret the references to color in this figure, please see the statistical comparisons of the sample chemical properties in Table 3, Table 4 and Table 5.
 ), pickled pak-sian (B;
), pickled pak-sian (B;  ), pickled bamboo shoots (C;
), pickled bamboo shoots (C;  ), pickled stink beans (D;
), pickled stink beans (D;  ), pickled shallot (E;
), pickled shallot (E;  ), pickled mustard greens (F;
), pickled mustard greens (F;  ), pickled ginger (G;
), pickled ginger (G;  ), fermented tea leaves (H;
), fermented tea leaves (H;  ), pickled scallions (I;
), pickled scallions (I;  ), and pickled bud rieng (J;
), and pickled bud rieng (J;  ) samples. The dendrogram represents sample clusters based on Pearson’s correlation coefficient with average linkage. Each square in the heatmap expresses normalized chemical abundance as the color range. The red color indicates a higher content of the corresponding chemical parameter. To better interpret the references to color in this figure, please see the statistical comparisons of the sample chemical properties in Table 3, Table 4 and Table 5.
) samples. The dendrogram represents sample clusters based on Pearson’s correlation coefficient with average linkage. Each square in the heatmap expresses normalized chemical abundance as the color range. The red color indicates a higher content of the corresponding chemical parameter. To better interpret the references to color in this figure, please see the statistical comparisons of the sample chemical properties in Table 3, Table 4 and Table 5.
 ), pickled pak-sian (B;
), pickled pak-sian (B;  ), pickled bamboo shoots (C;
), pickled bamboo shoots (C;  ), pickled stink beans (D;
), pickled stink beans (D;  ), pickled shallot (E;
), pickled shallot (E;  ), pickled mustard greens (F;
), pickled mustard greens (F;  ), pickled ginger (G;
), pickled ginger (G;  ), fermented tea leaves (H;
), fermented tea leaves (H;  ), pickled scallions (I;
), pickled scallions (I;  ), and pickled bud rieng (J;
), and pickled bud rieng (J;  ) samples. Important features are organized in descending order of variable importance in projection (VIP) scores (B). Squares in the VIP score panel express normalized chemical abundance with respect to the color range. The red color indicates a higher content of the corresponding chemical parameter. To better interpret the references to color in this figure, please see the statistical comparisons of the sample chemical properties in Table 2, Table 3 and Table 4.
) samples. Important features are organized in descending order of variable importance in projection (VIP) scores (B). Squares in the VIP score panel express normalized chemical abundance with respect to the color range. The red color indicates a higher content of the corresponding chemical parameter. To better interpret the references to color in this figure, please see the statistical comparisons of the sample chemical properties in Table 2, Table 3 and Table 4.
 ), pickled pak-sian (B;
), pickled pak-sian (B;  ), pickled bamboo shoots (C;
), pickled bamboo shoots (C;  ), pickled stink beans (D;
), pickled stink beans (D;  ), pickled shallot (E;
), pickled shallot (E;  ), pickled mustard greens (F;
), pickled mustard greens (F;  ), pickled ginger (G;
), pickled ginger (G;  ), fermented tea leaves (H;
), fermented tea leaves (H;  ), pickled scallions (I;
), pickled scallions (I;  ), and pickled bud rieng (J;
), and pickled bud rieng (J;  ) samples. Important features are organized in descending order of variable importance in projection (VIP) scores (B). Squares in the VIP score panel express normalized chemical abundance with respect to the color range. The red color indicates a higher content of the corresponding chemical parameter. To better interpret the references to color in this figure, please see the statistical comparisons of the sample chemical properties in Table 2, Table 3 and Table 4.
) samples. Important features are organized in descending order of variable importance in projection (VIP) scores (B). Squares in the VIP score panel express normalized chemical abundance with respect to the color range. The red color indicates a higher content of the corresponding chemical parameter. To better interpret the references to color in this figure, please see the statistical comparisons of the sample chemical properties in Table 2, Table 3 and Table 4.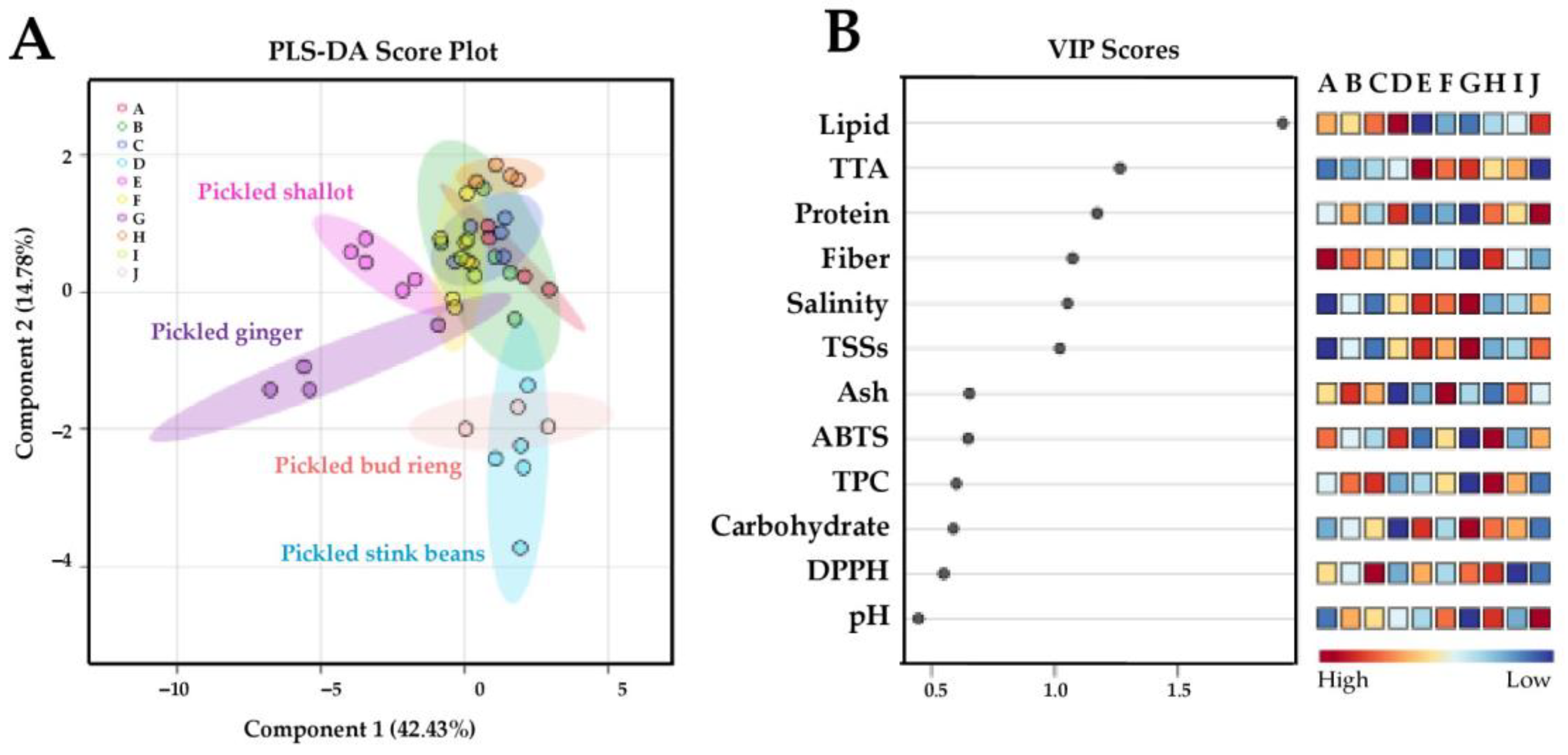
| Traditionally Fermented Thai Vegetables (Local Name) | N | Scientific Name | Province | Region |
|---|---|---|---|---|
| Pickled pak-kum | N = 4 | Crateva adansonii | Chiang Rai | Northern |
| (Pak-Kum Dong) | Tak | Northern | ||
| Chiang Rai | Northern | |||
| Trang | Southern | |||
| Pickled pak-sian | N = 5 | Cleome gynandra | Nakhon Si Thammarat | Southern |
| (Pak-Sian Dong) | Amnat Charoen | Northeastern | ||
| Ubon Ratchathani | Northeastern | |||
| Ubon Ratchathani | Northeastern | |||
| Trang | Southern | |||
| Pickled bamboo shoots (Nor-Mai Dong) | N = 5 | Bambusa vulgaris | Lampang | Northern |
| Prachuap Khiri Khan | Western | |||
| Bangkok | Central | |||
| Trang | Southern | |||
| Chiang Mai | Northern | |||
| Pickled stink beans | N = 5 | Parkia speciosa | Pattani | Southern |
| (Sator Dong) | Nakhon Si Thammarat | Southern | ||
| Nakhon Si Thammarat | Southern | |||
| Surat Thani | Southern | |||
| Songkhla | Southern | |||
| Pickled shallot | N = 5 | Allium oschaninii | Prachuap Khiri Khan | Western |
| (Homdang Dong) | Bangkok | Central | ||
| Surat Thani | Southern | |||
| Si Sa Ket | Northeastern | |||
| Saraburi | Central | |||
| Pickled mustard greens (Pak-Kard Dong) | N = 7 | Brassica juncea | Nakhon Pathom | Central |
| Lampang | Northern | |||
| Bangkok | Central | |||
| Ratchaburi | Western | |||
| Kanchanaburi | Western | |||
| Chon Buri | Eastern | |||
| Chiang Mai | Northern | |||
| Pickled ginger | N = 4 | Zingiber officinale | Nakhon Pathom | Central |
| (King Dong) | Bangkok | Central | ||
| Bangkok | Central | |||
| Bangkok | Central | |||
| Fermented tea leaves | N = 4 | Camellia sinensis var. assamica | Phrae | Northern |
| (Miang) | Tak | Northern | ||
| Phayao | Northern | |||
| Chiang Mai | Northern | |||
| Pickled scallions | N = 3 | Allium fistulosum | Chiang Mai | Northern |
| (Ton-Hom Dong) | Amnat Charoen | Northeastern | ||
| Nakhon Ratchasima | Northeastern | |||
| Pickled bud rieng | N = 3 | Parkia timoriana | Phatthalung | Southern |
| (Nor-Rieng Dong) | Nakhon Si Thammarat | Southern | ||
| Nakhon Si Thammarat | Southern |
| Traditionally | Type | Part of Vegetables | Ingredients * | Fermentation Condition |
|---|---|---|---|---|
| Fermented Thai Vegetables | ||||
| (Local Name) | ||||
| Pickled pak-kum | A | Leaves | Salt | Room temperature, 3–7 days |
| (Pak-Kum Dong) | Rice-washed water | |||
| Cooked rice/Sticky rice | ||||
| Pickled pak-sian | B | Leaves | Salt | Room temperature, 3–7 days |
| (Pak-Sian Dong) | Rice-washed water | |||
| Pickled bamboo shoots | C | Tuber | Salt | Room temperature, 3–5 days |
| (Nor-Mai Dong) | Rice-washed water | |||
| Pickled stink beans | D | Seeds | Salt | Room temperature, 3–5 days |
| (Sator Dong) | Sugar | |||
| Water/Rice-washed water | ||||
| Pickled shallot | E | Tuber | Salt | Room temperature, 5–10 days |
| (Homdang Dong) | Sugar | |||
| Water/Rice-washed water | ||||
| Vinegar | ||||
| Pickled mustard greens | F | Leaves | Salt | Room temperature, 3–10 days |
| (Pak-Kard Dong) | Rice-washed water | |||
| Pickled ginger | G | Tuber | Salt | Room temperature, 5–7 days |
| (King Dong) | Sugar | |||
| Vinegar | ||||
| Water | ||||
| Fermented tea leaves | H | Leaves | Salt | Room temperature, 3–4 months |
| (Miang) | Water | |||
| Vinegar | ||||
| Pickled scallions | I | Leaves | Salt | Room temperature, 5–7 days |
| (Ton-Hom Dong) | Rice-washed water | |||
| Cooked rice/Sticky rice | ||||
| Pickled bud rieng | J | Seeds | Germinated seeds | Room temperature, 2–7 days |
| (Nor-Rieng Dong) | Salt | |||
| Sugar | ||||
| Water/Rice-washed water |
| Traditionally Fermented Thai Vegetables | TSSs (°Brix) | pH | TTA (%) | Salinity (%) |
|---|---|---|---|---|
| Pickled pak-kum | 2.92 ± 0.60 c | 3.51 ± 0.33 b | 0.37 ± 0.11 d | 2.40 ± 0.49 c |
| Pickled pak-sian | 4.15 ± 0.56 c | 3.72 ± 0.53 b | 0.37 ± 0.05 d | 3.47 ± 0.45 c |
| Pickled bamboo shoots | 5.21 ± 1.40 c | 3.62 ± 0.28 b | 0.53 ± 0.12 d | 4.33 ± 1.18 c |
| Pickled stink beans | 5.41 ± 1.61 c | 3.61 ± 0.20 b | 0.56 ± 0.15 cd | 4.83 ± 0.78 c |
| Pickled shallot | 12.61 ± 3.72 b | 3.60 ± 0.20 b | 1.35 ± 0.29 a | 13.85 ± 3.19 b |
| Pickled mustard greens | 5.62 ± 1.14 c | 3.63 ± 0.21 b | 1.07 ± 0.30 ab | 4.70 ± 0.97 c |
| Pickled ginger | 21.90 ± 2.86 a | 3.22 ± 0.14 b | 1.35 ± 0.37 a | 20.91 ± 0.07 a |
| Fermented tea leaves | 4.44 ± 1.13 c | 4.20 ± 0.28 a | 0.85 ± 0.05 bc | 3.68 ± 0.96 c |
| Pickled scallions | 4.78 ± 1.31 c | 3.54 ± 0.14 b | 1.01 ± 0.06 b | 4.02 ± 1.09 c |
| Pickled bud rieng | 4.33 ± 0.72 c | 4.28 ± 0.80 a | 0.45 ± 0.03 d | 3.47 ± 0.58 c |
| Traditionally Fermented Thai Vegetables | Crude Lipid | Crude Protein | Crude Fiber | Ash | Carbohydrate |
|---|---|---|---|---|---|
| Pickled pak-kum | 4.79 ± 0.86 cd | 4.77 ± 0.65 b | 24.44 ± 3.73 a | 20.48 ± 2.70 bc | 43.71 ± 4.81 bc |
| Pickled pak-sian | 5.90 ± 0.71 c | 5.65 ± 1.23 b | 16.53 ± 3.68 b | 27.23 ± 5.43 b | 41.37 ± 8.09 bc |
| Pickled bamboo shoots | 4.27 ± 1.27 cde | 3.37 ± 0.65 c | 15.23 ± 3.71 bc | 37.36 ± 8.23 a | 42.76 ± 12.69 bc |
| Pickled stink beans | 33.90 ± 1.29 a | 6.75 ± 0.24 a | 14.11 ± 3.10 bc | 9.96 ± 0.96 d | 10.73 ± 0.05 d |
| Pickled shallot | 0.62 ± 0.05 f | 2.38 ± 0.44 d | 6.26 ± 3.22 d | 13.44 ± 5.01 cd | 72.90 ± 5.84 a |
| Pickled mustard greens | 2.57 ± 0.44 ef | 3.76 ± 0.54 c | 12.20 ± 1.72 bc | 41.64 ± 6.03 a | 37.60 ± 5.14 c |
| Pickled ginger | 1.03 ± 0.10 f | 0.29 ± 0.13 e | 3.77 ± 0.43 d | 9.31 ± 3.02 d | 75.25 ± 2.35 a |
| Fermented tea leaves | 2.65 ± 0.28 def | 5.59 ± 0.81 b | 22.19 ± 4.29 d | 8.86 ± 4.16 d | 55.55 ± 6.19 b |
| Pickled scallions | 3.70 ± 0.30 de | 4.96 ± 0.11 b | 13.83 ± 2.09 b | 26.45 ± 5.95 b | 47.63 ± 3.59 bc |
| Pickled bud rieng | 21.96 ± 5.10 b | 7.58 ± 1.17 a | 11.48 ± 2.97 d | 11.57 ± 2.60 d | 22.32 ± 1.36 d |
| Traditionally Fermented Thai Vegetables | TPC (µg GAE/g) | DPPH (% Inhibition) | ABTS (% Decolorization) |
|---|---|---|---|
| Pickled pak-kum | 434.19 ± 70.52 cd | 67.40 ± 9.67 bc | 77.43 ± 3.00 b |
| Pickled pak-sian | 504.66 ± 33.77 bc | 76.62 ± 1.35 abc | 72.96 ± 6.08 b |
| Pickled bamboo shoots | 599.81 ± 70.21 b | 81.41 ± 3.85 ab | 94.37 ± 0.11 a |
| Pickled stink beans | 347.71 ± 82.79 de | 51.34 ± 8.06 d | 79.74 ± 9.68 b |
| Pickled shallot | 496.94 ± 55.65 bc | 66.71 ± 6.55 c | 49.68 ± 5.88 c |
| Pickled mustard greens | 490.42 ± 64.55 bc | 83.77 ± 2.86 a | 94.26 ± 0.12 a |
| Pickled ginger | 199.54 ± 50.68 f | 72.30 ± 3.31 abc | 29.95 ± 8.12 d |
| Fermented tea leaves | 871.50 ± 80.25 a | 71.99 ± 8.96 abc | 94.57 ± 0.26 a |
| Pickled scallions | 449.19 ± 80.60 cd | 78.92 ± 4.66 abc | 38.06 ± 0.92 d |
| Pickled bud rieng | 264.59 ± 40.20 ef | 65.34 ± 9.16 c | 70.31 ± 6.58 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan-utai, W.; Settachaimongkon, S.; La-ongkham, O.; Pornpukdeewattana, S.; Hamwane, M.; Lorpeunge, C.; Adame, M.; Yodbumprenge, C. Physicochemical, Nutritional, and Antioxidant Properties of Traditionally Fermented Thai Vegetables: A Promising Functional Plant-Based Food. Foods 2024, 13, 2848. https://doi.org/10.3390/foods13172848
Pan-utai W, Settachaimongkon S, La-ongkham O, Pornpukdeewattana S, Hamwane M, Lorpeunge C, Adame M, Yodbumprenge C. Physicochemical, Nutritional, and Antioxidant Properties of Traditionally Fermented Thai Vegetables: A Promising Functional Plant-Based Food. Foods. 2024; 13(17):2848. https://doi.org/10.3390/foods13172848
Chicago/Turabian StylePan-utai, Wanida, Sarn Settachaimongkon, Orawan La-ongkham, Soisuda Pornpukdeewattana, Marisa Hamwane, Chalantorn Lorpeunge, Masnavee Adame, and Charisa Yodbumprenge. 2024. "Physicochemical, Nutritional, and Antioxidant Properties of Traditionally Fermented Thai Vegetables: A Promising Functional Plant-Based Food" Foods 13, no. 17: 2848. https://doi.org/10.3390/foods13172848






