Encapsulation of Phenolic Compounds Extracted from Beet By-Products: Analysis of Physical and Chemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Materials and Chemicals
2.3. Preparation of Beetroot By-Products Extracts
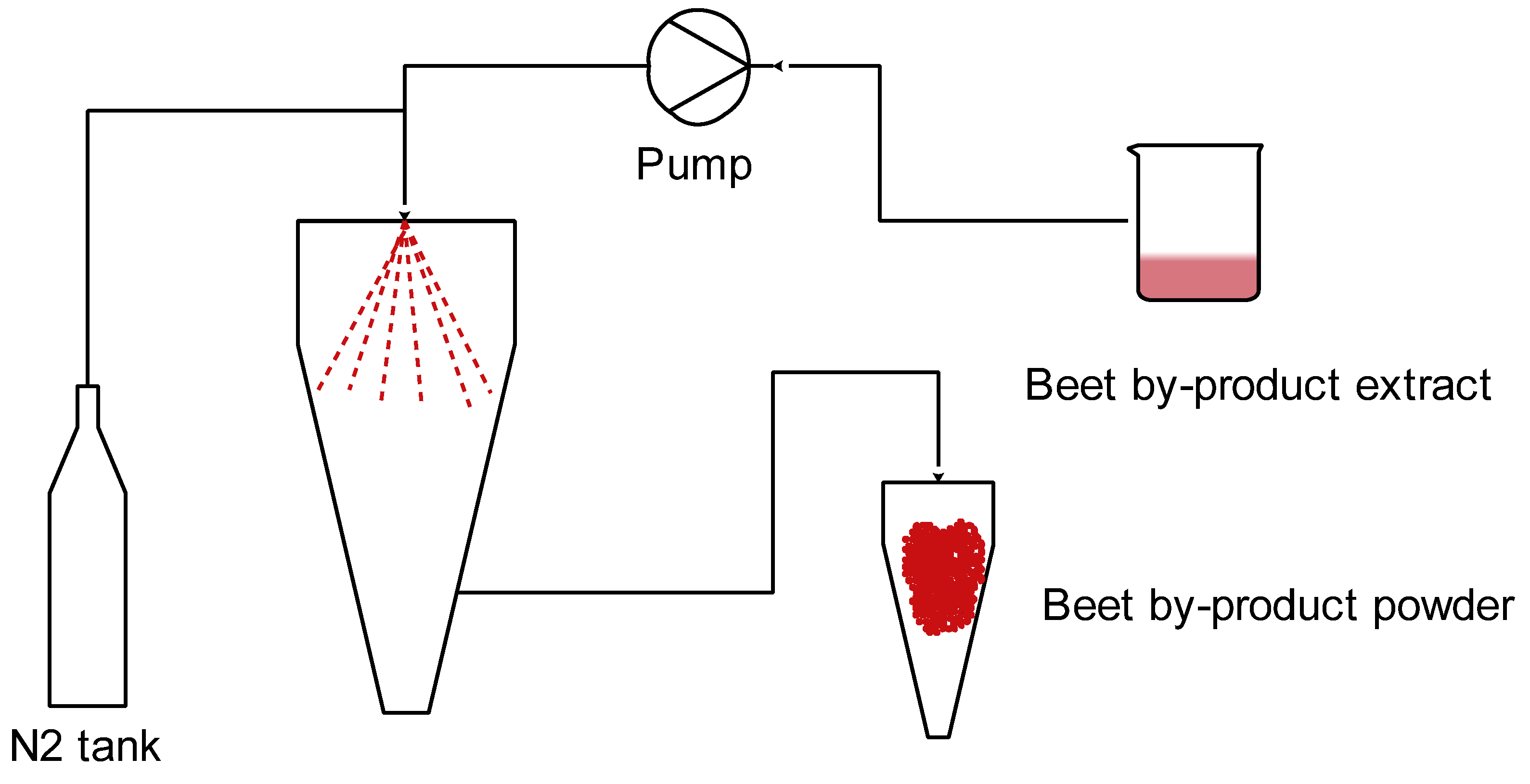
2.4. Spray Drying to Encapsulate Antioxidants from Beetroot By-Products
2.4.1. Preparation of Sample
2.4.2. Spray-Drying Processing
2.4.3. Determination of the Spray-Drying Yield
2.5. Physical Properties Analysis
2.5.1. Moisture Determination
2.5.2. Water Activity (aw)
2.5.3. Color
2.5.4. Density
2.5.5. Hygroscopicity
2.5.6. Degree of Caking
2.5.7. Dispersibility
2.5.8. Morphology of Encapsulated Product
2.6. Chemicals Analysis
2.6.1. Determination of Total Phenols
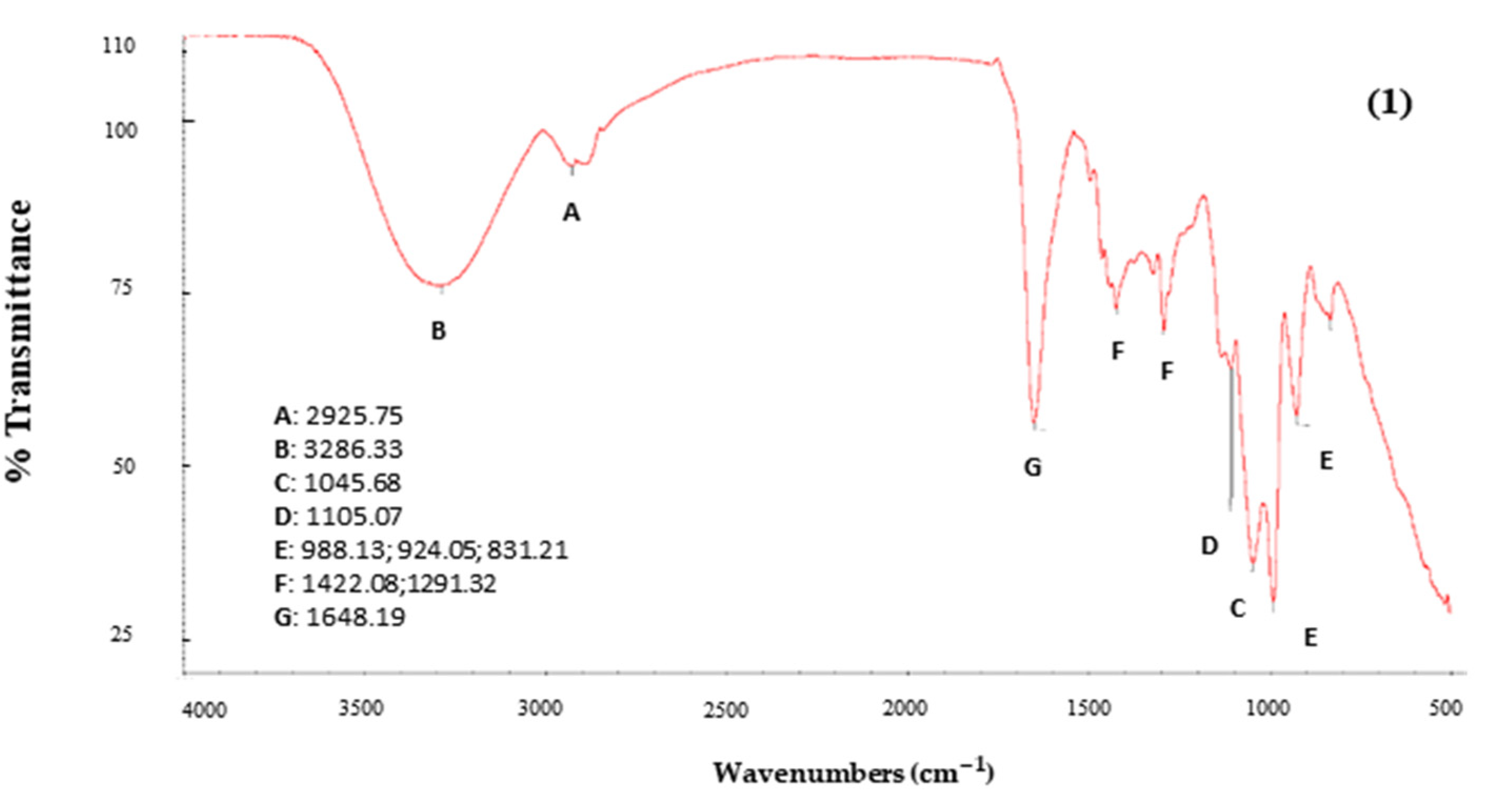
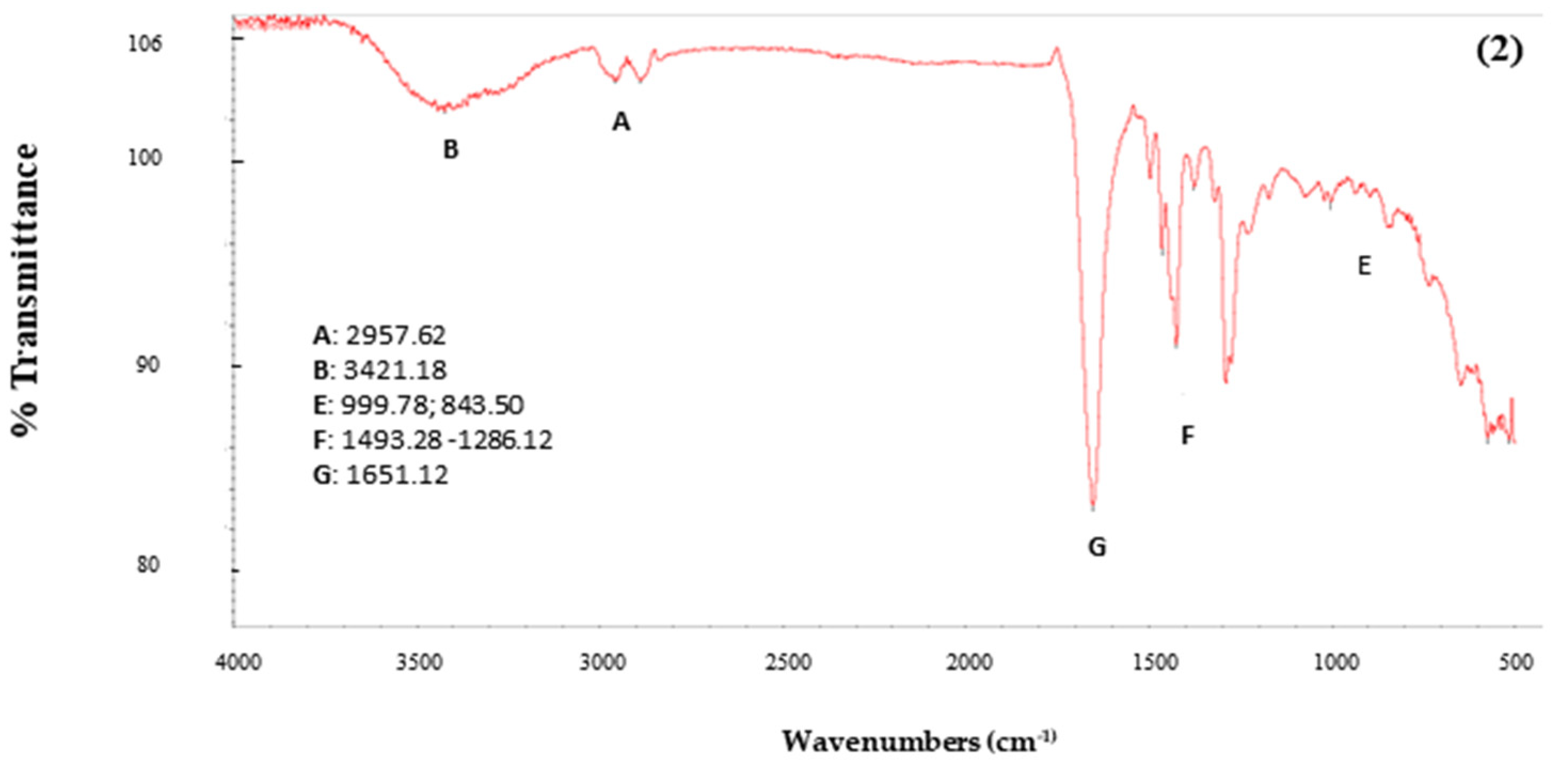
2.6.2. Infrared Spectroscopy (FTIR)
2.6.3. X-ray Fluorescence (XRF)
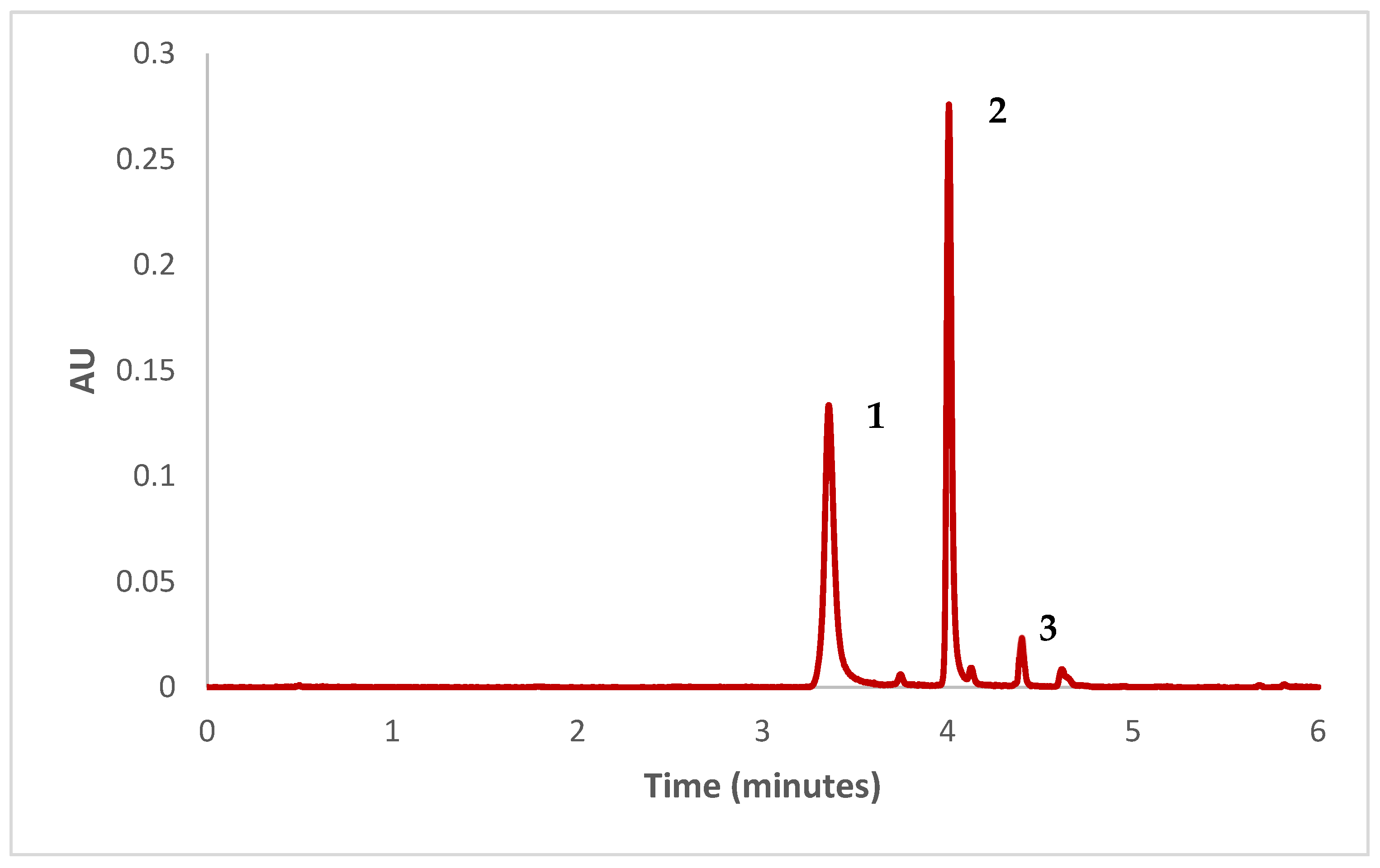
2.7. Betalains Determination
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Characterization of Beetroot By-Product
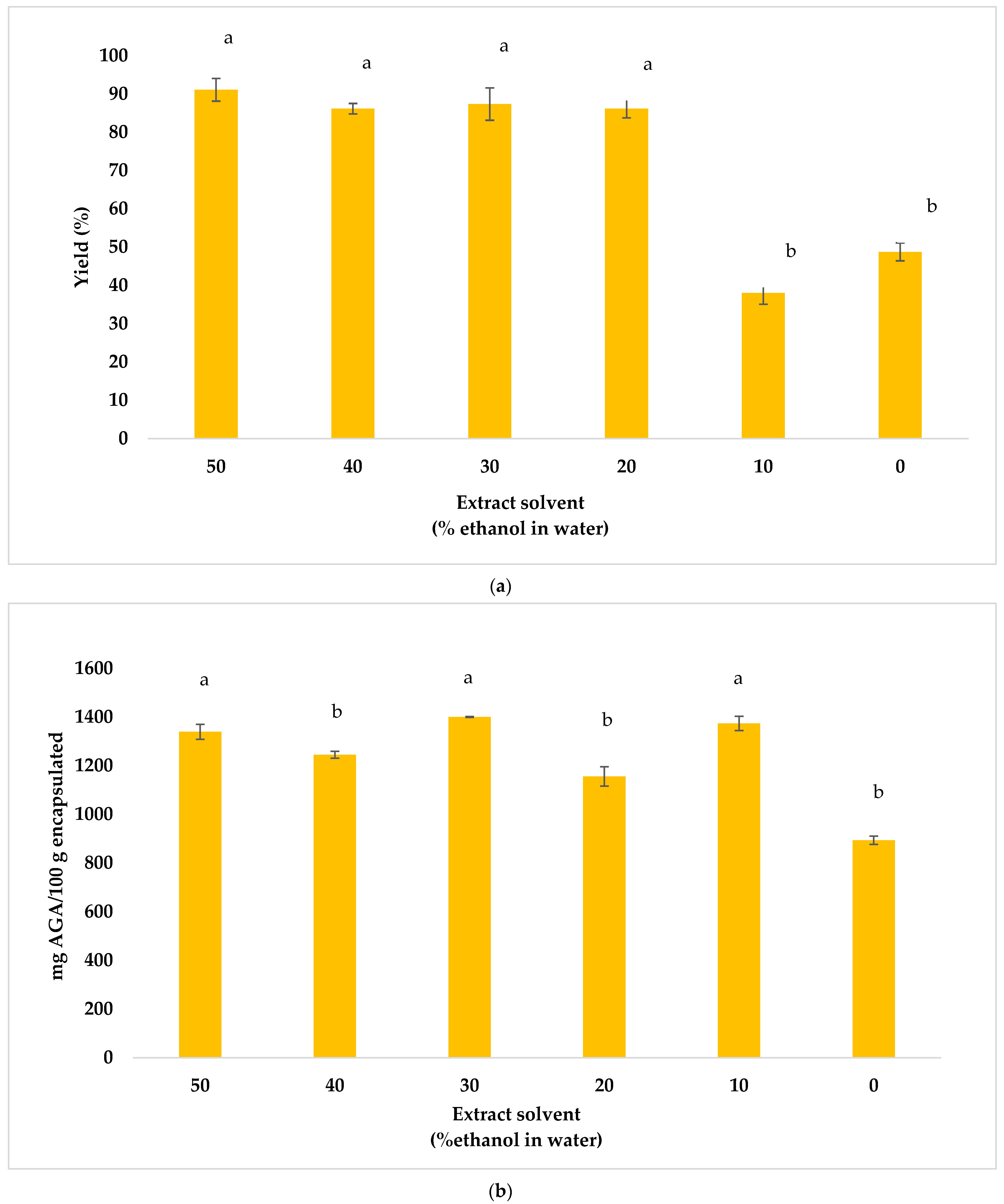
3.2. Encapsulation of Antioxidants from Beetroot By-Products
3.3. Optimization of the Influence of the Factors
3.4. Physical Properties of Encapsulated Product
3.4.1. Color
3.4.2. Moisture and Water Activity (WA)
3.4.3. Density, Solubility and Dispersibility
3.4.4. Hygroscopicity and Degree of Caking
3.4.5. Morphology of Encapsulated Beetroot By-Product Antioxidants
3.5. Chemical Properties of Atomized Beet By-Product Powder
3.5.1. Infrared Spectroscopy (FTIR)
3.5.2. X-ray Fluorescence (XRF)
3.6. Betalains
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otegbayo, B.O.; Akwa, I.M.; Tanimola, A.R. Physico-chemical properties of beetroot (Beta vulgaris L.) wine produced at varying fermentation days. Sci. Afr. 2020, 8, e00420. [Google Scholar] [CrossRef]
- Olumese, F.E.; Oboh, H.A. Antioxidant and Antioxidant capacity of raw and processed Nigerian Beetroot (Beta vulgaris). Niger. J. Basic Appl. Sci. 2017, 24, 35–40. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional Characteristics of Commercial Beetroot Products and Beetroot Juice Prepared from Seven Beetroot Varieties Grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as Substrates for Tyrosinase. An Approach to the Role of Tyrosinase in the Biosynthetic Pathway of Betalains. Plant Physiol. 2005, 138, 421–432. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.d.L.; De Carvalho, M.V.; Melo, L. Health promoting and sensory properties of phenolic compounds in food. Rev. Ceres 2014, 61, 764–779. [Google Scholar] [CrossRef]
- Milenkovic, D.; Deval, C.; Dubray, C.; Mazur, A.; Morand, C. Hesperidin Displays Relevant Role in the Nutrigenomic Effect of Orange Juice on Blood Leukocytes in Human Volunteers: A Randomized Controlled Cross-Over Study. PLoS ONE 2011, 6, e26669. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Eurostat. Agriculture, Forestry and Fishery Statistics, 2017th ed.; Imprimerie Centrale: Luxembourg, 2017. [Google Scholar]
- Singh, B.; Hathan, B.S. Process optimization of spray drying of beetroot Juice. J. Food Sci. Technol. 2017, 54, 2241–2250. [Google Scholar] [CrossRef]
- do Carmo, E.L.; Teodoro, R.A.R.; Félix, P.H.C.; Fernandes, R.V.d.B.; de Oliveira, É.R.; Veiga, T.R.L.A.; Borges, S.V.; Botrel, D.A. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2018, 249, 51–59. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Martínez-Monzó, J. Beetroot Microencapsulation with Pea Protein Using Spray Drying: Physicochemical, Structural and Functional Properties. Appl. Sci. 2021, 11, 6658. [Google Scholar] [CrossRef]
- Gawałek, J. Effect of spray dryer scale size on the properties of dried beetroot juice. Molecules 2021, 26, 6700. [Google Scholar] [CrossRef] [PubMed]
- Bazaria, B.; Kumar, P. Effect of whey protein concentrate as drying aid and drying parameters on physicochemical and functional properties of spray dried beetroot juice concentrate. Food Biosci. 2016, 14, 21–27. [Google Scholar] [CrossRef]
- Bazaria, B.; Kumar, P. Optimization of spray drying parameters for beetroot juice powder using response surface methodology (RSM). J. Saudi Soc. Agric. Sci. 2018, 17, 408–415. [Google Scholar] [CrossRef]
- Janiszewska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- AOAC INTERNATIONAL. Moisture in Dried Fruits. In Official Methods Analysis, 21st ed.; Latimer, D.G., Ed.; AOAC Int.: Rockville, MD, USA, 2019. [Google Scholar]
- Shenoy, P.; Viau, M.; Tammel, K.; Innings, F.; Fitzpatrick, J.; Ahrné, L. Effect of powder densities, particle size and shape on mixture quality of binary food powder mixtures. Powder Technol. 2015, 272, 165–172. [Google Scholar] [CrossRef]
- Oliveira Menezes, D.; Clemente, E.; Correia da Costa, J.M. Hygroscopic behavior and degree of caking of grugru palm (Acrocomia aculeata) powder. J. Food Sci. Technol. 2012, 51, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- GEA Niro Research Laboratory Analytical Methods of Dry Milk Products. 2005. Available online: https://www.gea.com/en/products/dryers-particle-processing/spray-dryers/ (accessed on 31 July 2024).
- Abdalla, A.A.; Mohammed, M.A.; Mudawi, H.A. Production and Quality Assessment of Instant Baobab (Adansonia digitata L.). Adv. J. Food Sci. Technol. 2010, 2, 125–133. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Cai, Y.; Corke, H. Amaranthus betacyanin pigments applied in model food systems. J. Food Sci. 1999, 64, 869–873. [Google Scholar] [CrossRef]
- Schmidt, S. Water Mobility in Foods. In Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 61–102. [Google Scholar]
- Tuberoso, G.; Jerkovic, I.; Sarais, G.; Congiu, F.; Marijanovic, Z.; Kus, P.M. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L* C*ab h°ab chromaticity coordinates. Food Chem. 2014, 145, 284–291. [Google Scholar] [CrossRef]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Boesch, C. Application of an Ultrasound-Assisted Extraction Method to Recover Betalains and Polyphenols from Red Beetroot Waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Jung, H.; Lee, Y.J.; Yoon, W.B. Effect of Moisture Content on the Grinding Process and Powder Properties in Food: A Review. Processes 2018, 6, 69. [Google Scholar] [CrossRef]
- Bhusari, S.N.; Muzaffar, K.; Kumar, P. Effect of carrier agents on physical and microstructural properties of spray dried tamarind pulp powder. Powder Technol. 2014, 266, 354–364. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-djomeh, Z.; Ashtari, A.K.; Omid, M. Food and Bioproducts Processing Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Pereira Bicudo, M.; Jó, J.; De Oliveira, G.A.; Chaimsohn, F.; Sierakowski, M.R.; De Freitas, R.A.; Hoffmann, R. Microencapsulation of Juçara (Euterpe edulis M.) Pulp by Spray Drying Using Different Carriers and Drying Temperatures. Dry. Technol. Int. J. 2015, 33, 37–41. [Google Scholar] [CrossRef]
- Colina Irezabal, M.L. Deshidratación de Alimentos; Trillas: Mexico City, Mexico, 2010. [Google Scholar]
- Kapoor, R.; Feng, H. Characterization of physicochemical, packing and microstructural properties of beet, blueberry, carrot and cranberry powders: The effect of drying methods. Powder Technol. 2022, 395, 290–300. [Google Scholar] [CrossRef]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Rehydration process of milk protein concentrate powder monitored by static light scattering. Food Hydrocoll. 2009, 23, 1958–1965. [Google Scholar] [CrossRef]
- Jelena, Č.; Vanja, Š.; Vesna, T.Š.; Gordana, Ć.; Jasna, Č.B.; Senka, P.; Milica, H.K.; Ljiljana, P. Encapsulation of beetroot juice: A study on the apllication of pumpkin oil cake protein as new carrier agent. J. Microencapsul. 2019, 3, 121–133. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Shittu, T.A.; Lawal, M.O. Factors affecting instant properties of powdered cocoa beverages. Food Chem. 2007, 100, 91–98. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine “Carabao” var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Resistant maltodextrin’s effect on the physicochemical and structure properties of spray dried orange juice powders. Eur. Food Res. Technol. 2021, 247, 1125–1132. [Google Scholar] [CrossRef]
- Dhiman, A.; Suhag, R.; Singh, D.; Thakur, D.; Chhikara, S.; Prabhakar, P.K. Status of beetroot processing and processed products: Thermal and emerging technologies intervention. Trends Food Sci. Technol. 2021, 114, 443–458. [Google Scholar] [CrossRef]
- Biswas, P.; Sen, D.; Mazumder, S.; Basak, C.B.; Doshi, P. Temperature Mediated Morphological Transition during Drying of Spray Colloidal Droplets. Langmuir 2016, 32, 2464–2473. [Google Scholar] [CrossRef]
- Trojanowska, A.; Nogalska, A.; Valls, R.G.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. Phys. Sci. Rev. 2017, 2, 20170020. [Google Scholar] [CrossRef]
- Mohsenin, N. Physical Properties of Plant and Animal Materials; Taylor & Francis: London, UK; New York, NY, USA, 2019. [Google Scholar]
- Mondragón Cortez, P. Principios y Aplicaciones de la Espectroscoia de Infrarrojo en el Análisis de Alimentos y Bebidas; Primera: Guadalajara, Mexico, 2020; ISBN 9781626239777. [Google Scholar]
- Janiszewska, E.; Wáodarczyk, J. Influence of Spray Drying Conditions on Beetroot Pigments Retention after Microencapsulation Process. Acta Agrophys. 2013, 20, 343–356. [Google Scholar]

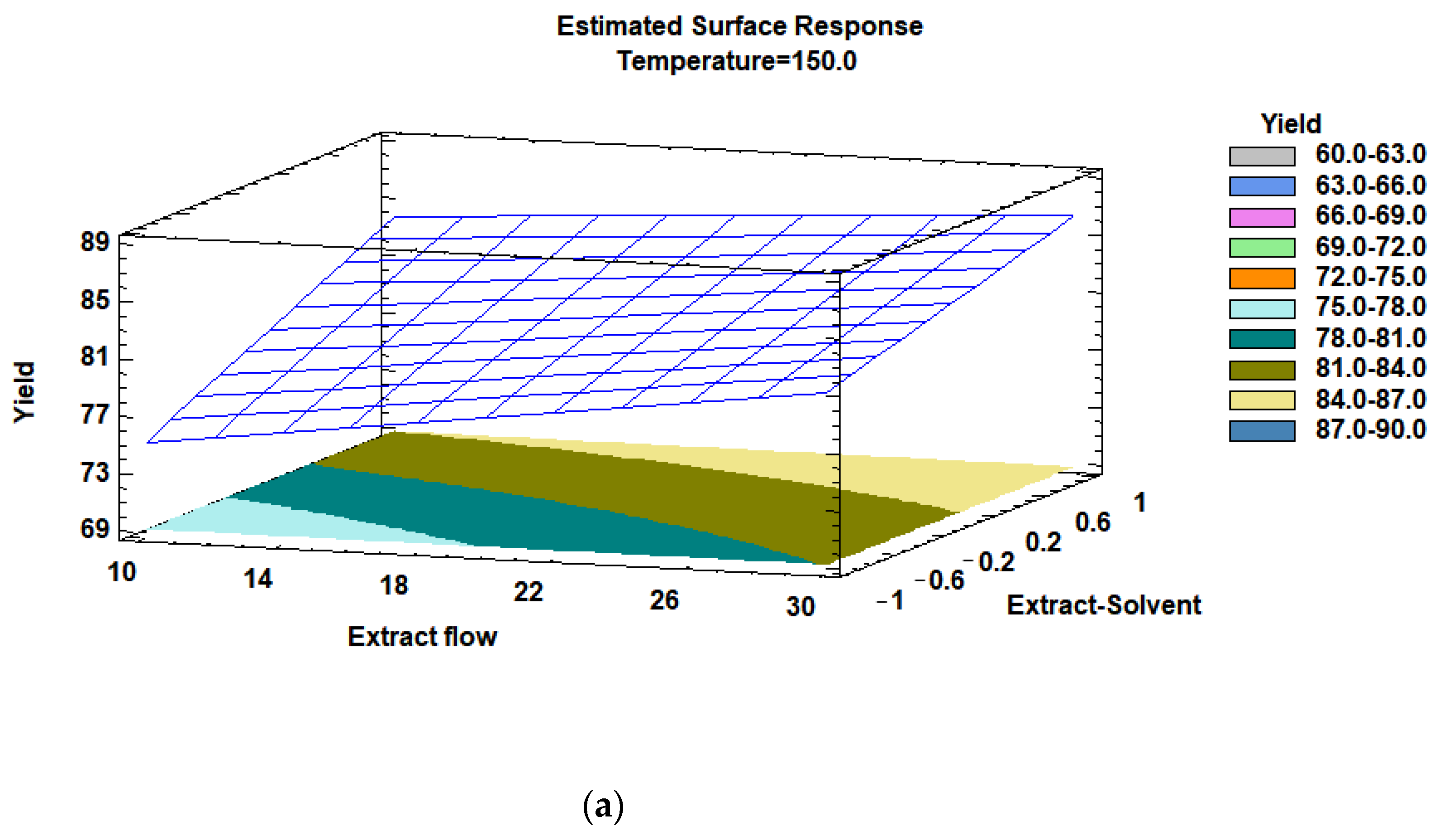
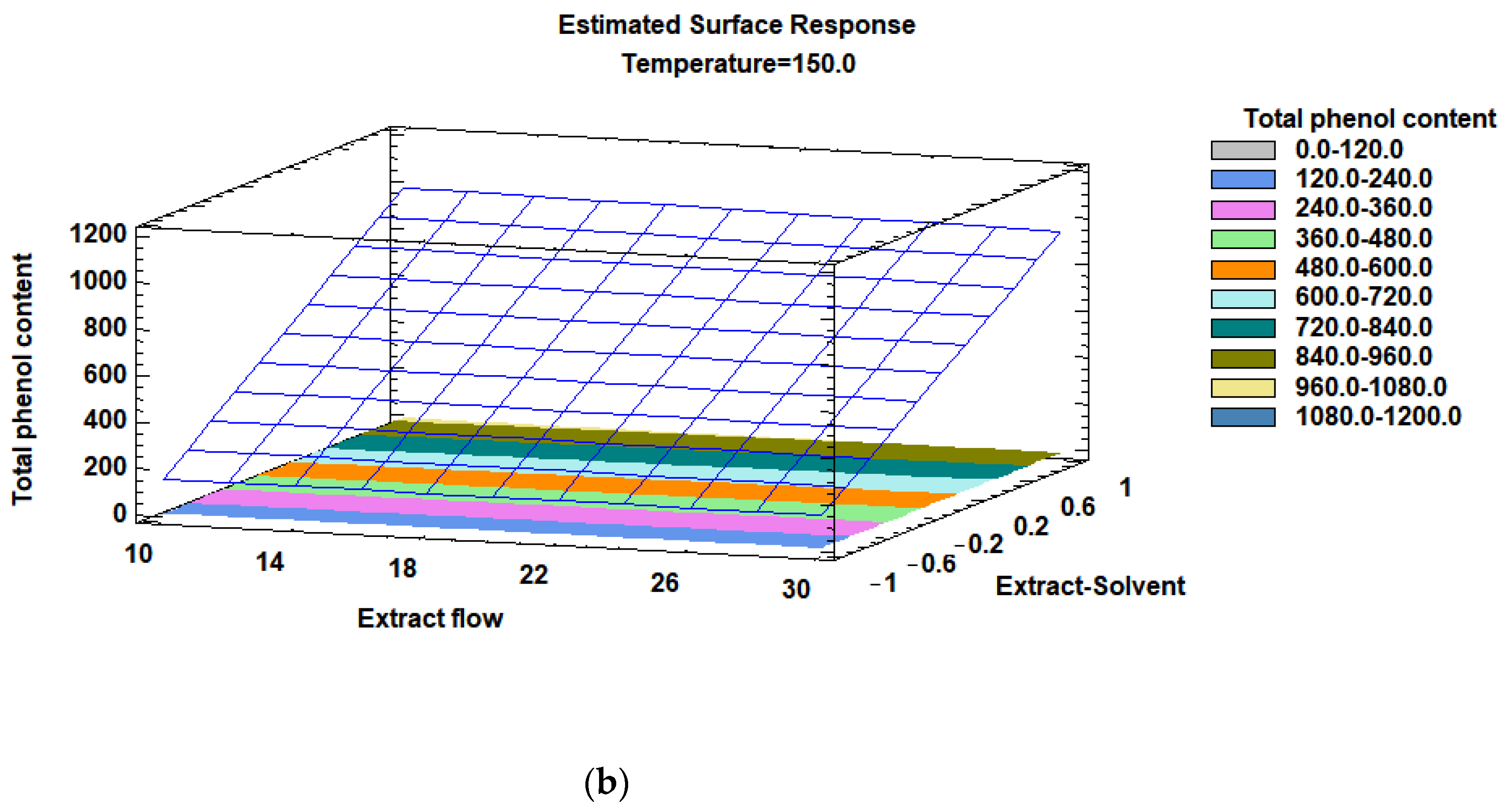

| Spray Dyer Factors | Levels | Unit | |
|---|---|---|---|
| −1 | +1 | ||
| x1, Temperature | 140 | 160 | °C |
| x2, Extract flow | 10 | 30 | % pump capacity |
| x3, Extract-Solvent | 50 | 100 | % ethanol in water |
| Physical Property | Dehydrated By-Product | Encapsulated Product |
|---|---|---|
| Color | ||
| L | 34.3 ± 0.4 | 51.02 ± 0.03 |
| a* | 20.3 ± 0.1 | 35.36 ± 2.21 |
| b* | 3.5 ± 0.5 | 6.67 ± 0.63 |
| h* | 80.68 ± 0.58 | 10.69 ± 0.34 |
| C* | 20.38 ± 0.32 | 35.99 ± 2.29 |
| Water activity | 0.30 ± 0.00 | 0.19 ± 0.01 |
| Moisture (%) | 6.21 ± 0.08 | 6.01 ± 0.28 |
| Density (g/mL) | - | 0.42 ± 0.03 |
| Solubility (%) | - | 84.47 ± 4.36 |
| Dispersibility (DO) | - | 0.95 ± 0.02 |
| Hygroscopicity (%) | - | 15.14 ± 0.94 |
| Degree of caking (%) | - | 100 ± 0.00 |
| Spray Dryer Factor | Conditions | |
|---|---|---|
| Yield | Total Phenols Content | |
| Temperature, °C | 160 | 160 |
| Extract Flow, % | 30% | 10% |
| Extract–Solvent, % ethanol in water | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guamán-Balcázar, M.d.C.; Montero, M.; Celi, A.; Montes, A.; Carrera, C.; Pereyra, C.; Meneses, M.Á. Encapsulation of Phenolic Compounds Extracted from Beet By-Products: Analysis of Physical and Chemical Properties. Foods 2024, 13, 2859. https://doi.org/10.3390/foods13182859
Guamán-Balcázar MdC, Montero M, Celi A, Montes A, Carrera C, Pereyra C, Meneses MÁ. Encapsulation of Phenolic Compounds Extracted from Beet By-Products: Analysis of Physical and Chemical Properties. Foods. 2024; 13(18):2859. https://doi.org/10.3390/foods13182859
Chicago/Turabian StyleGuamán-Balcázar, María del Cisne, Magdalena Montero, Alejandro Celi, Antonio Montes, Ceferino Carrera, Clara Pereyra, and Miguel Ángel Meneses. 2024. "Encapsulation of Phenolic Compounds Extracted from Beet By-Products: Analysis of Physical and Chemical Properties" Foods 13, no. 18: 2859. https://doi.org/10.3390/foods13182859
APA StyleGuamán-Balcázar, M. d. C., Montero, M., Celi, A., Montes, A., Carrera, C., Pereyra, C., & Meneses, M. Á. (2024). Encapsulation of Phenolic Compounds Extracted from Beet By-Products: Analysis of Physical and Chemical Properties. Foods, 13(18), 2859. https://doi.org/10.3390/foods13182859








