Salicylic Acid Treatment Ameliorates Postharvest Quality Deterioration in ‘France’ Prune (Prunus domestica L. ‘Ximei’) Fruit by Modulating the Antioxidant System
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Material Postharvest Treatment
2.2. Determination of Physiological Quality Parameters
2.3. Determination of Bioactive Compounds
2.4. Determination of ROS Levels
2.5. Determination of Antioxidant Enzyme Activity
2.6. Analysis of Antioxidant Enzyme Gene Expression
2.7. Determination of Antioxidant Capacity
2.8. Statistical Analysis
3. Results
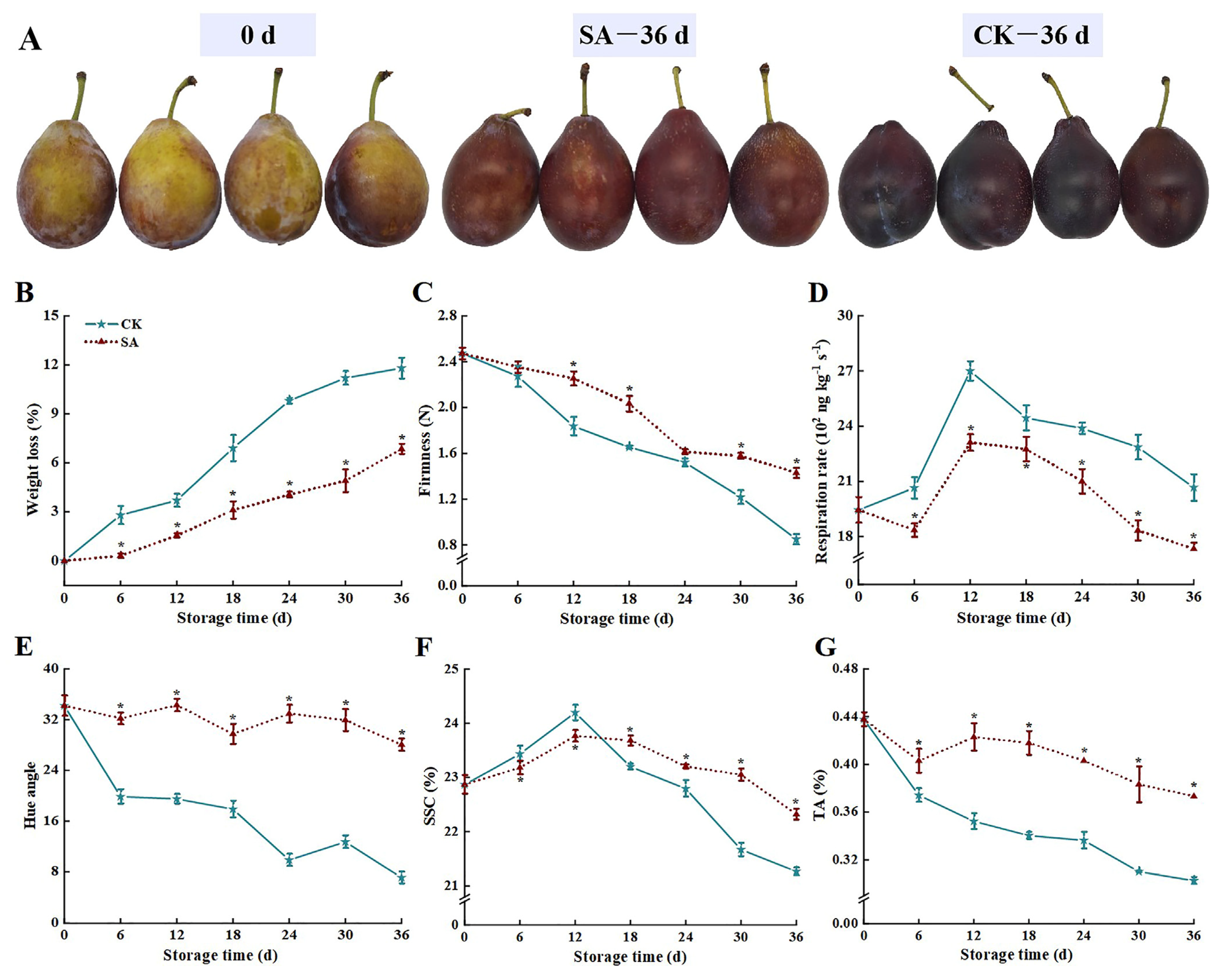
3.1. Physiological Quality Parameters
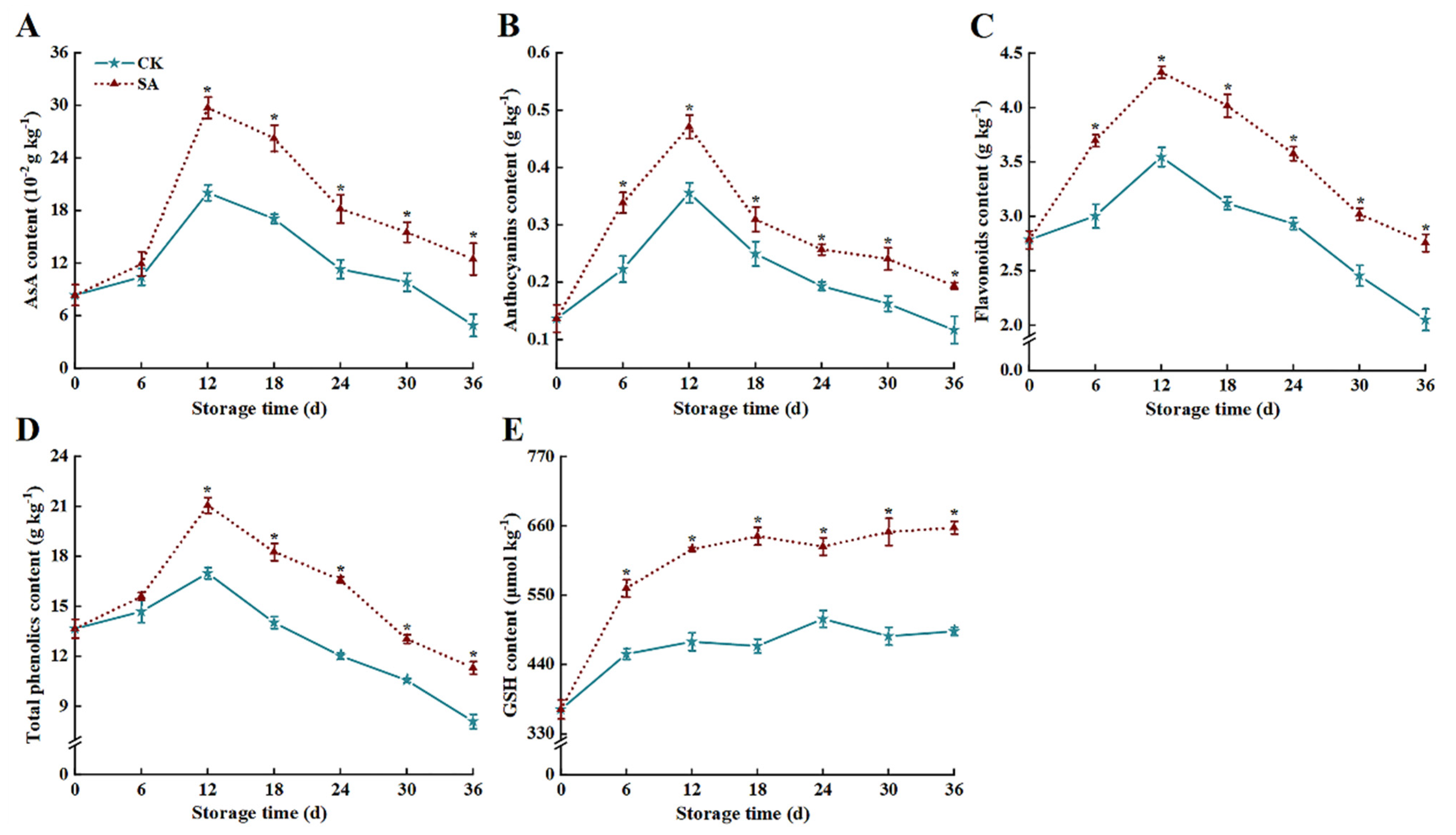
3.2. Bioactive Components
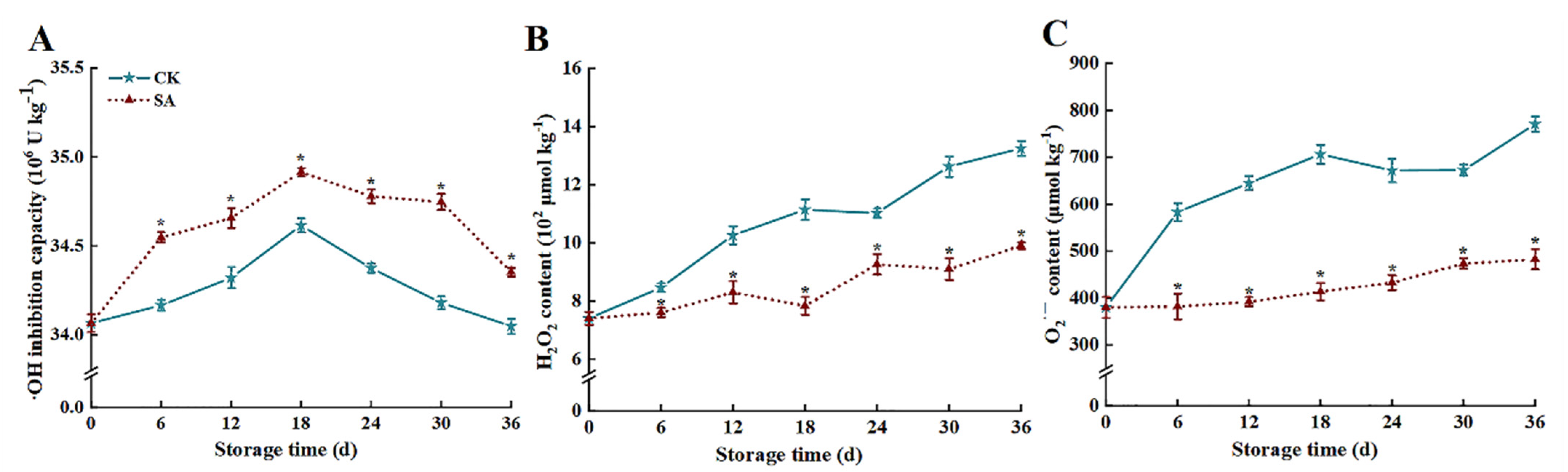
3.3. ROS Levels
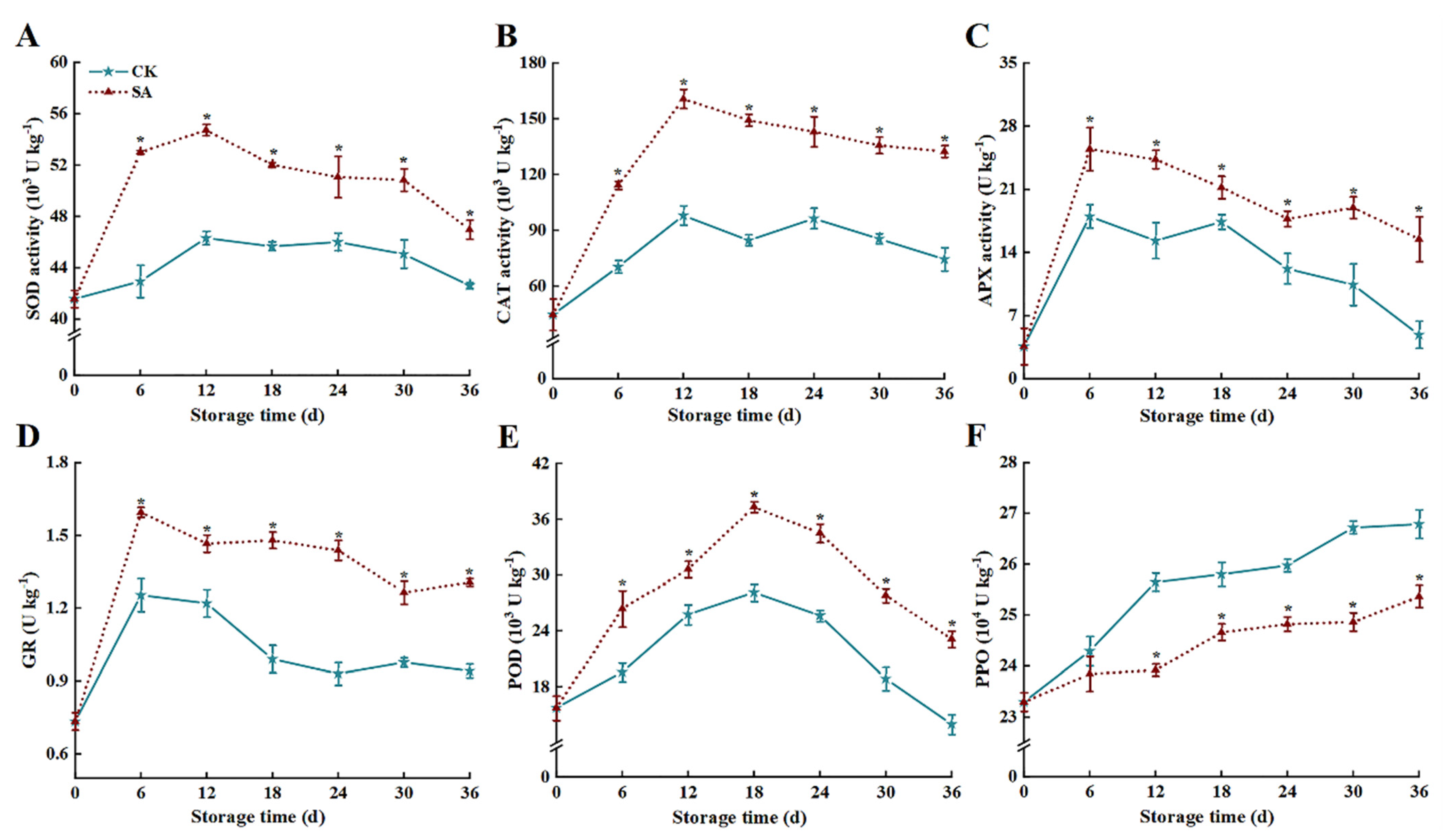
3.4. Antioxidant Enzyme Activity
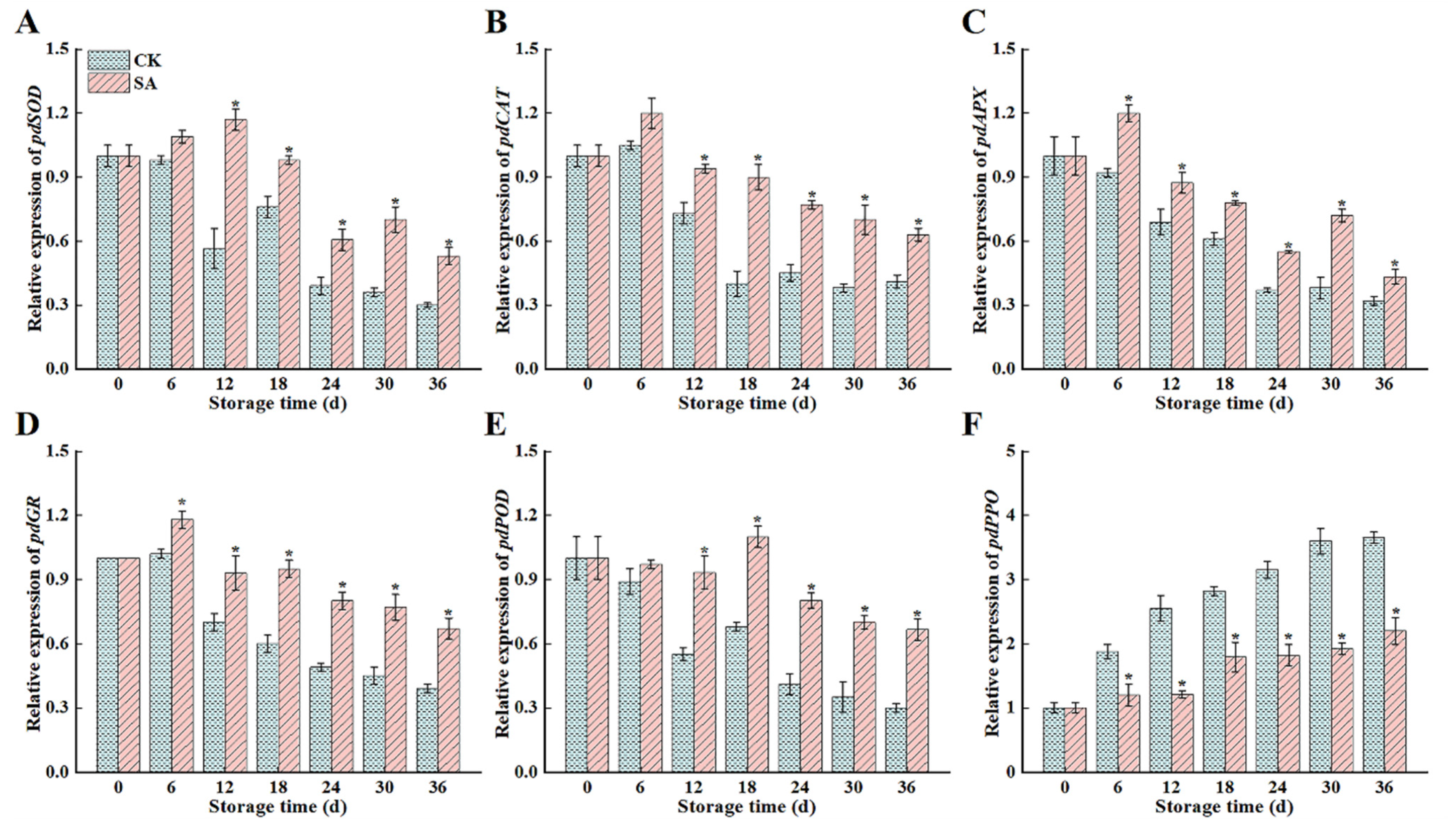
3.5. Antioxidant Enzyme Gene Expression
3.6. Antioxidant Capacity
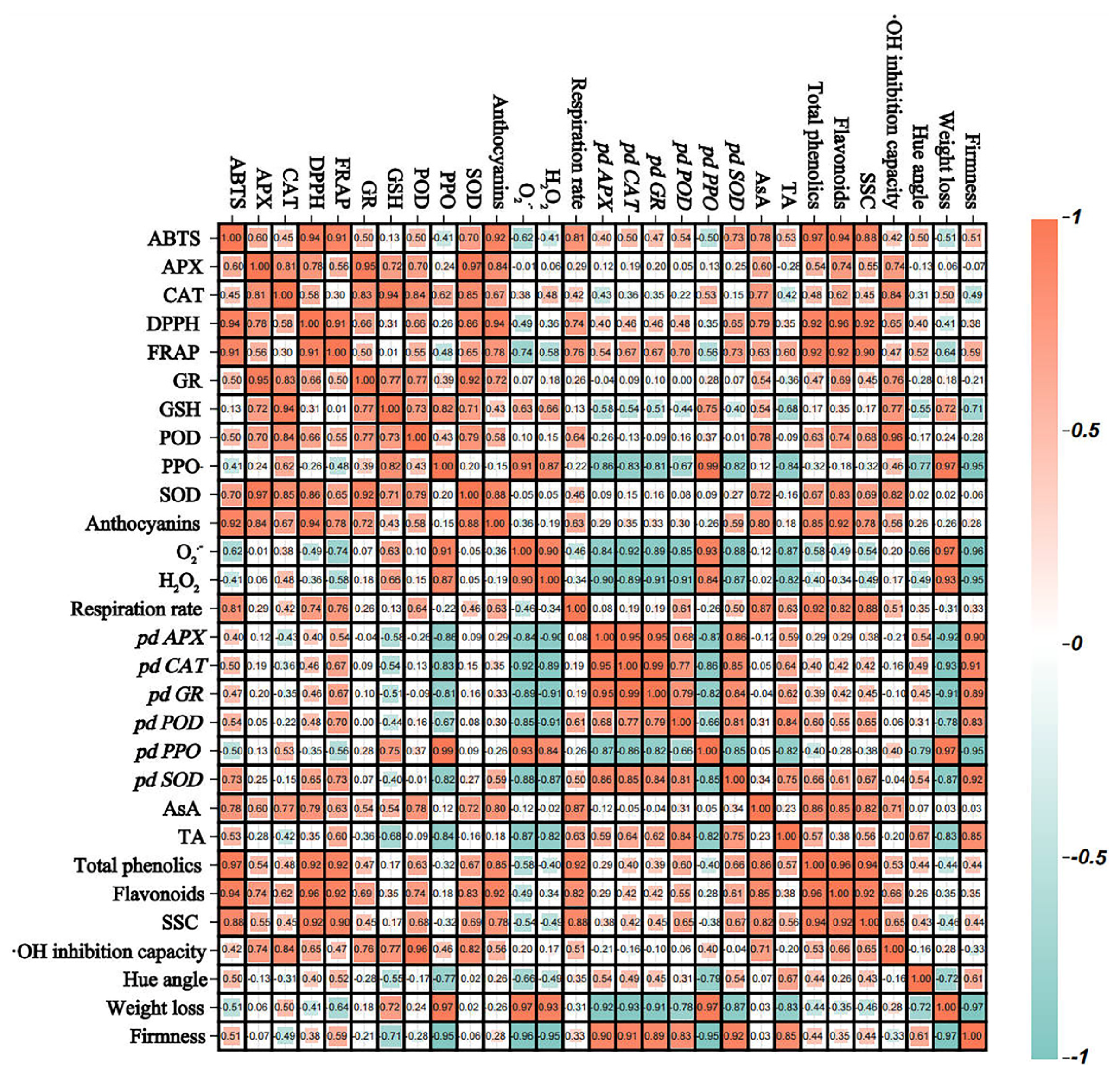
3.7. Correlation Analysis
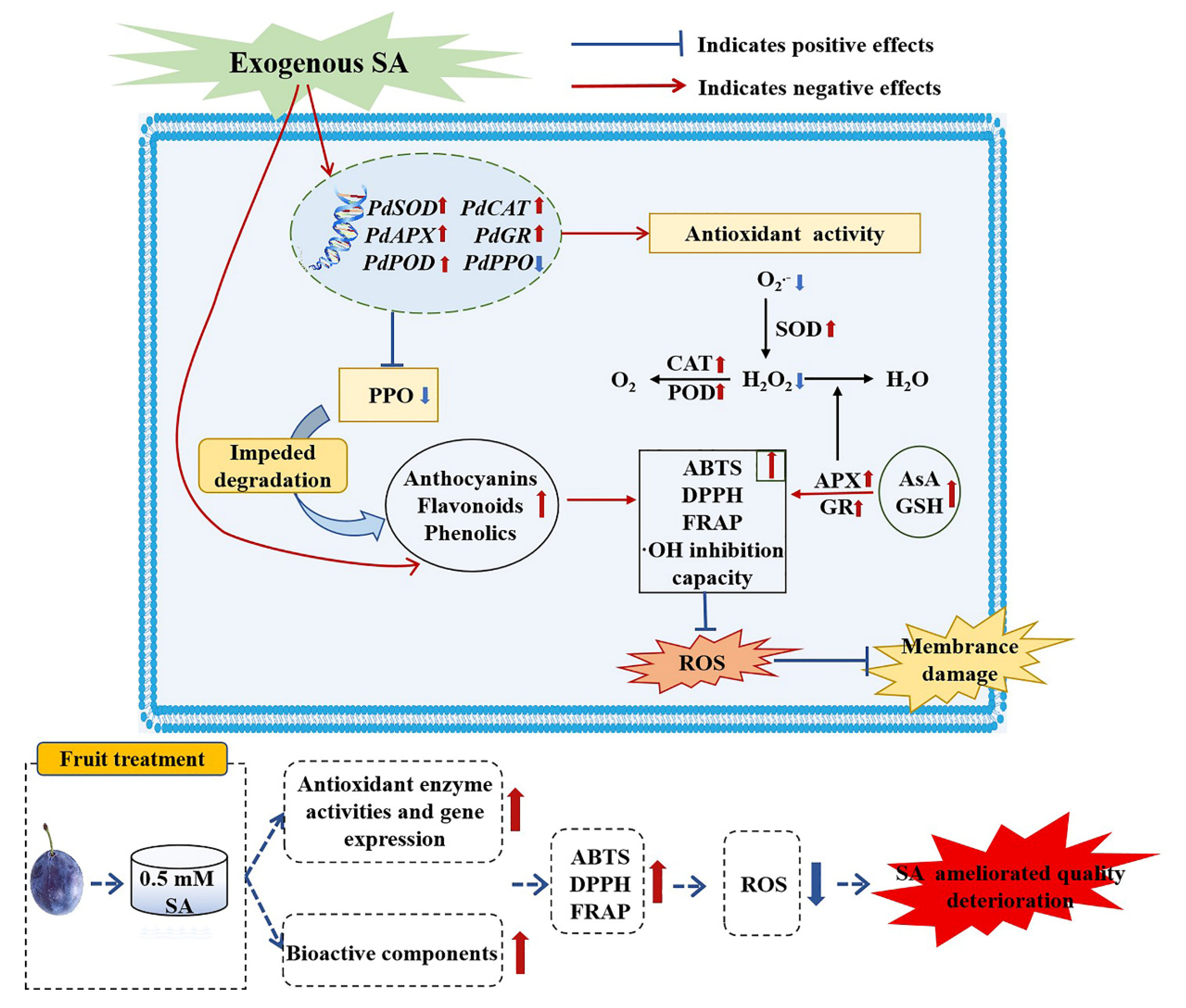
4. Discission
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Zhang, W.; Cheng, S.; Liu, Y.; Yang, W.; Wang, Y.; Guo, M.; Chen, G. Postharvest storage at near-freezing temperature maintained the quality and antioxidant properties of Prunus domestica L. cv. Ximei fruit. Sci. Hortic. 2022, 293, 110720. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Zhang, W.; Li, L.; Cheng, S.; Guo, M.; Chen, G. Transcriptome analysis reveals the mechanism of delayed softening of ‘France’ prune (Prunus domestica L.) during storage at near-freezing temperature. LWT-Food Sci. Technol. 2023, 189, 115446. [Google Scholar] [CrossRef]
- Kumari, P.; Barman, K.; Patel, V.B.; Siddiqui, M.W.; Kole, B. Reducing postharvest pericarp browning and preserving health promoting compounds of litchi fruit by combination treatment of salicylic acid and chitosan. Sci. Hortic. 2015, 197, 555–563. [Google Scholar] [CrossRef]
- Baswal, A.K.; Dhaliwal, H.S.; Singh, Z.; Mahajan, B.V.C.; Gill, K.S. Postharvest application of methyl jasmonate, 1-methylcyclopropene and salicylic acid extends the cold storage life and maintain the quality of ‘Kinnow’ mandarin (Citrus nobilis L. X C. deliciosa L.) fruit. Postharvest Biol. Technol. 2020, 161, 111064. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Zapata, P.J.; Guillén, F.; Valverde, J.M.; Serrano, M.; Valero, D. Preharvest application of methyl salicylate, acetyl salicylic acid and salicylic acid alleviated disease caused by Botrytis cinerea through stimulation of antioxidant system in table grapes. Int. J. Food Microbiol. 2020, 334, 108807. [Google Scholar] [CrossRef]
- Yang, W.; Guo, M.; Zhang, W.; Cheng, S.; Chen, G. Methyl salicylate and methyl jasmonate induce resistance to Alternaria tenuissima by regulating the phenylpropane metabolism pathway of winter jujube. Postharvest Biol. Technol. 2023, 204, 112440. [Google Scholar] [CrossRef]
- Yang, W.; Kang, J.; Liu, Y.; Guo, M.; Chen, G. Effect of salicylic acid treatment on antioxidant capacity and endogenous hormones in winter jujube during shelf life. Food Chem. 2022, 397, 133788. [Google Scholar] [CrossRef]
- Gao, F.; Xie, W.; Zhang, H.; Li, S.; Li, T. Salicylic acid treatment regulates the tyrosine metabolism and antioxidant system to inhibit the browning of morels (Morchella sextelata). Postharvest Biol. Technol. 2022, 194, 112090. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.; Wang, J.; Wang, P.; Lu, D.; Deng, S.; Lei, H.; Gao, Y.; Tao, Y. Treatment with exogenous salicylic acid maintains quality, increases bioactive compounds, and enhances the antioxidant capacity of fresh goji (Lycium barbarum L.) fruit during storage. LWT-Food Sci. Technol. 2021, 140, 110837. [Google Scholar] [CrossRef]
- Gu, S.; Xu, D.; Zhou, F.; Feng, K.; Chen, C.; Jiang, A. Repairing ability and mechanism of methyl jasmonate and salicylic acid on mechanically damaged sweet cherries. Sci. Hortic. 2022, 292, 110567. [Google Scholar] [CrossRef]
- Niu, Y.; Ye, L.; Wang, Y.; Shi, Y.; Liu, Y.; Luo, A. Transcriptome analysis reveals salicylic acid treatment mitigates chilling injury in kiwifruit by enhancing phenolic synthesis and regulating phytohormone signaling pathways. Postharvest Biol. Technol. 2023, 205, 112483. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, J.; Xiao, X.; Zhang, M.; Yun, Z.; Zeng, Y.; Xu, J.; Cheng, Y.; Deng, X. Salicylic acid treatment reduces the rot of postharvest citrus fruit by inducing the accumulation of H2O2, primary metabolites and lipophilic polymethoxylated flavones. Food Chem. 2016, 207, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, F.; Wang, J.; Yang, Q.; Wang, P.; Zhao, H.; Wang, J.; Wang, C.; Xu, X. Salicylic acid inhibits the postharvest decay of goji berry (Lycium barbarum L.) by modulating the antioxidant system and phenylpropanoid metabolites. Postharvest Biol. Technol. 2021, 178, 111558. [Google Scholar] [CrossRef]
- Zhang, H.; Cun, Y.; Wang, J.; Wu, M.; Li, X.; Liang, Q.; Wang, C.; Zhao, L.; Deng, J. Acetylsalicylic acid and salicylic acid alleviate postharvest leaf senescence in Chinese flowering cabbage (Brassica rapa var. parachinensis). Postharvest Biol. Technol. 2022, 194, 112070. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Qu, H.; Yun, Z.; Wang, H.; Huang, Z.; Huang, H.; Jiang, Y. Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. 2015, 171, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kang, J.; Yang, W.; Guo, H.; Guo, M.; Chen, G. Incorporation of 1-methylcyclopropene and salicylic acid improves quality and shelf life of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) through regulating reactive oxygen species metabolism. Front. Nutr. 2022, 9, 940494. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, J.; Li, P.; Yan, Z.; Gao, L.; Wang, Q.; Jiang, A. Effect of methyl jasmonate on the quality of harvested broccoli after simulated transport. Food Chem. 2020, 319, 126561. [Google Scholar] [CrossRef]
- Kenny, O.; O’Beirne, D. Antioxidant phytochemicals in fresh-cut carrot disks as affected by peeling method. Postharvest Biol. Technol. 2010, 58, 247–253. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Jung, H.; Lee, S.-R.; Lee, H.-J.; Hwang, K.T.; Kim, T.-Y. Anti-oxidant, anti-proliferative and anti-inflammatory activities of the extracts from black raspberry fruits and wine. Food Chem. 2010, 123, 338–344. [Google Scholar] [CrossRef]
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Enzymatic antioxidants in response to methyl jasmonate and salicylic acid and their effect on chilling tolerance in lemon fruit [Citrus limon (L.) Burm. F.]. Sci. Hortic. 2017, 225, 659–667. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, W.; Liu, Y.; Sang, Y.; Yang, W.; Guo, M.; Cheng, S.; Chen, G. Effect of composite coating treatment and low-temperature storage on the quality and antioxidant capacity of Chinese jujube (Zizyphus jujuba cv. Junzao). Sci. Hortic. 2021, 288, 110372. [Google Scholar] [CrossRef]
- Rasouli, M.; Koushesh Saba, M.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Martinez-Romero, D.; Gimenez, M.J.; Serrano, M.; Garcia-Martinez, S.; Valero, D.; Valverde, J.M.; Zapata, P.J. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef]
- Fan, X.; Xi, Y.; Zhao, H.; Liu, B.; Cao, J.; Jiang, W. Improving fresh apricot (Prunus armeniaca L.) quality and antioxidant capacity by storage at near freezing temperature. Sci. Hortic. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Yang, C.; Duan, W.; Xie, K.; Ren, C.; Zhu, C.; Chen, K.; Zhang, B. Effect of salicylic acid treatment on sensory quality, flavor-related chemicals and gene expression in peach fruit after cold storage. Postharvest Biol. Technol. 2020, 161, 111089. [Google Scholar] [CrossRef]
- Mustafa, M.A.; Ali, A.; Seymour, G.; Tucker, G. Treatment of dragonfruit (Hylocereus polyrhizus) with salicylic acid and methyl jasmonate improves postharvest physico-chemical properties and antioxidant activity during cold storage. Sci. Hortic. 2018, 231, 89–96. [Google Scholar] [CrossRef]
- Xiong, S.; Sun, X.; Tian, M.; Xu, D.; Jiang, A. 1-Methylcyclopropene treatment delays the softening of Actinidia arguta fruit by reducing cell wall degradation and modulating carbohydrate metabolism. Food Chem. 2023, 411, 135485. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Pu, Y.; Chen, L.; He, X.; Cao, J.; Jiang, W. Composite coating of guar gum with salicylic acid alleviates the quality deterioration of vibration damage in ‘Huangguan’ pear fruit through the regulation of antioxidant metabolism. Postharvest Biol. Technol. 2023, 205, 112476. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Luo, Y.; Sun, X.; Wang, J.; Luo, T.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 2020, 306, 125634. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, R.; Fang, X.; Tong, C.; Chen, H.; Gao, H. Effects of salicylic acid treatment on fruit quality and wax composition of blueberry (Vaccinium virgatum Ait). Food Chem. 2022, 368, 130757. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Xue, H.; Sun, Y.; Li, L.; Bi, Y.; Hussain, R.; Zhang, R.; Long, H.; Nan, M.; Pu, L. Acetylsalicylic acid (ASA) induced fusarium rot resistance and suppressed neosolaniol production by elevation of ROS metabolism in muskmelon fruit. Sci. Hortic. 2020, 265, 109264. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, Y.; Wu, X.; Liu, Z.; Zou, J.; Chen, Y.; Su, N.; Cui, J. Increased antioxidative capacity and decreased cadmium uptake contribute to hemin-induced alleviation of cadmium toxicity in Chinese cabbage seedlings. Ecotoxicol. Environ. Saf. 2019, 177, 47–57. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, J.; Xie, J.; Deng, L.; Yao, S.; Zeng, K. Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid, Pichia membranaefaciens and oligochitosan. Postharvest Biol. Technol. 2018, 142, 81–92. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Zuo, J.; Gao, L.; Lv, J.; Wang, Q. Methyl jasmonate delays postharvest ripening and senescence in the non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2016, 120, 76–83. [Google Scholar] [CrossRef]
- Sinha, A.; Gill, P.P.S.; Jawandha, S.K.; Kaur, P.; Grewal, S.K. Chitosan-enriched salicylic acid coatings preserves antioxidant properties and alleviates internal browning of pear fruit under cold storage and supermarket conditions. Postharvest Biol. Technol. 2021, 182, 111721. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef]
- Fan, X.; Du, Z.; Cui, X.; Ji, W.; Ma, J.; Li, X.; Wang, X.; Zhao, H.; Liu, B.; Guo, F.; et al. Preharvest methyl salicylate treatment enhance the chilling tolerance and improve the postharvest quality of apricot during low temperature storage. Postharvest Biol. Technol. 2021, 177, 111535. [Google Scholar] [CrossRef]

| Gene | Gene ID | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| Actin | 103339527 | TCAACCCTAAGGCAAAC | GTGGCTGACACCATCTC |
| PdSOD | 103339036 | TTCAGATAAGGAGCGTCAC | CATTCCCAGTAGTCTTGCT |
| PdPPO | 103324677 | CCGCACAGGCATAAGCAC | GGCACCAAAGTCACCACC |
| PdGR | 103324330 | GGCTGTCGGTGATGTTA | TTTGGCTTGTTCTATTGC |
| PdCAT | 103336676 | CAGGGAAAGCACAGTAT | TCAAGTGGGTCAAAGTC |
| PdPOD | 103342243 | TCCTCCCACTTCTACCC | ACATTTGCTTTGCCTGA |
| PdAPX | 103340411 | GGGTTGTTGCTGTGGAG | CCTTGTTTGGCATCTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Zhang, W.; Yang, W.; An, S.; Guo, M.; Chen, G. Salicylic Acid Treatment Ameliorates Postharvest Quality Deterioration in ‘France’ Prune (Prunus domestica L. ‘Ximei’) Fruit by Modulating the Antioxidant System. Foods 2024, 13, 2871. https://doi.org/10.3390/foods13182871
Zhang X, Liu Y, Zhang W, Yang W, An S, Guo M, Chen G. Salicylic Acid Treatment Ameliorates Postharvest Quality Deterioration in ‘France’ Prune (Prunus domestica L. ‘Ximei’) Fruit by Modulating the Antioxidant System. Foods. 2024; 13(18):2871. https://doi.org/10.3390/foods13182871
Chicago/Turabian StyleZhang, Xinling, Yuxing Liu, Weida Zhang, Wanting Yang, Shuaibing An, Minrui Guo, and Guogang Chen. 2024. "Salicylic Acid Treatment Ameliorates Postharvest Quality Deterioration in ‘France’ Prune (Prunus domestica L. ‘Ximei’) Fruit by Modulating the Antioxidant System" Foods 13, no. 18: 2871. https://doi.org/10.3390/foods13182871





