Effect of Solid-State Fermentation of Hericium erinaceus on the Structure and Physicochemical Properties of Soluble Dietary Fiber from Corn Husk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Fermentation Strain Cultures
2.3. SSF of Corn Husks
2.4. Preparation of Corn Husk SDF
2.5. Structural Characteristics of Corn Bran SDF
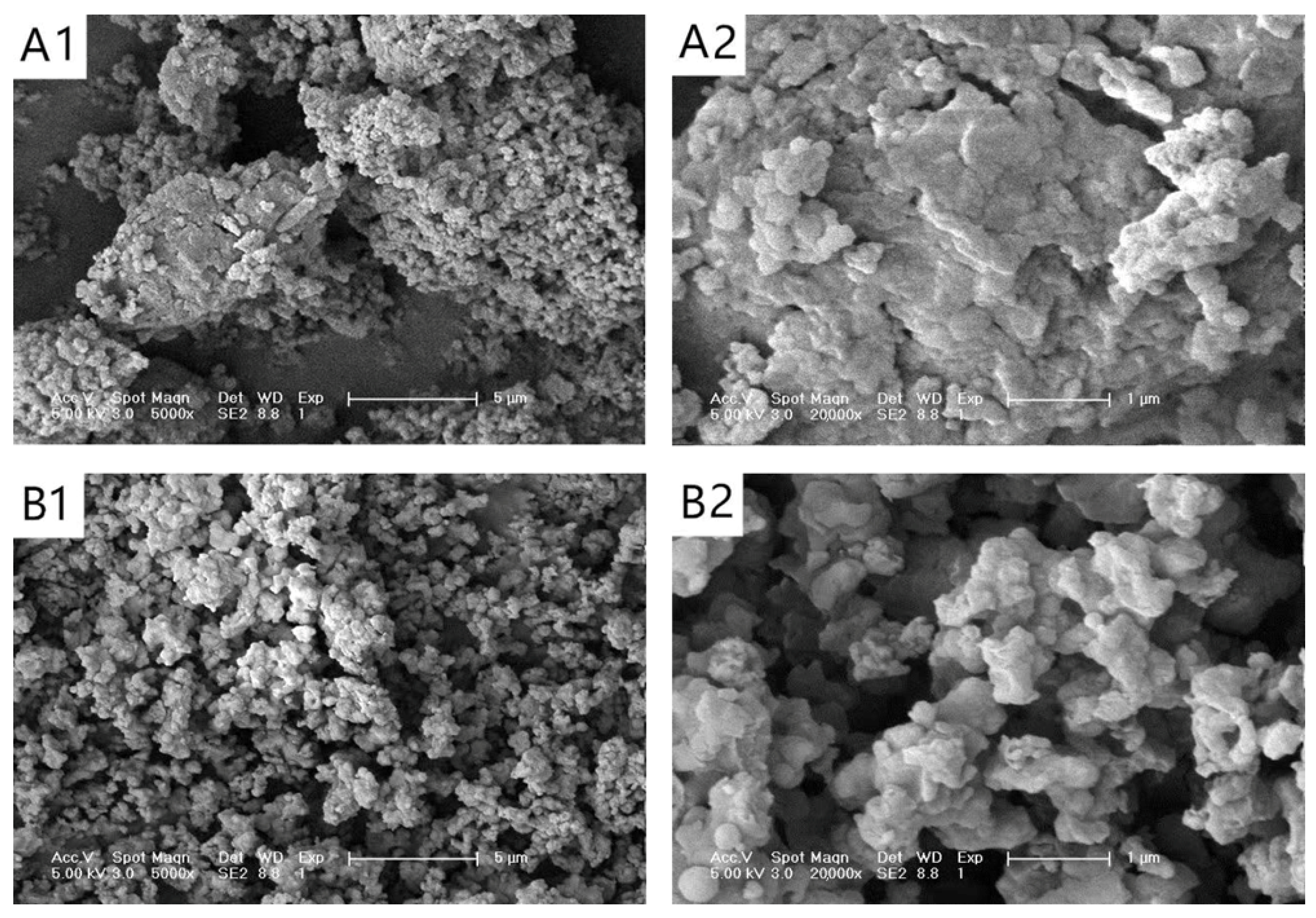
2.5.1. Scanning Electron Microscopy (SEM)
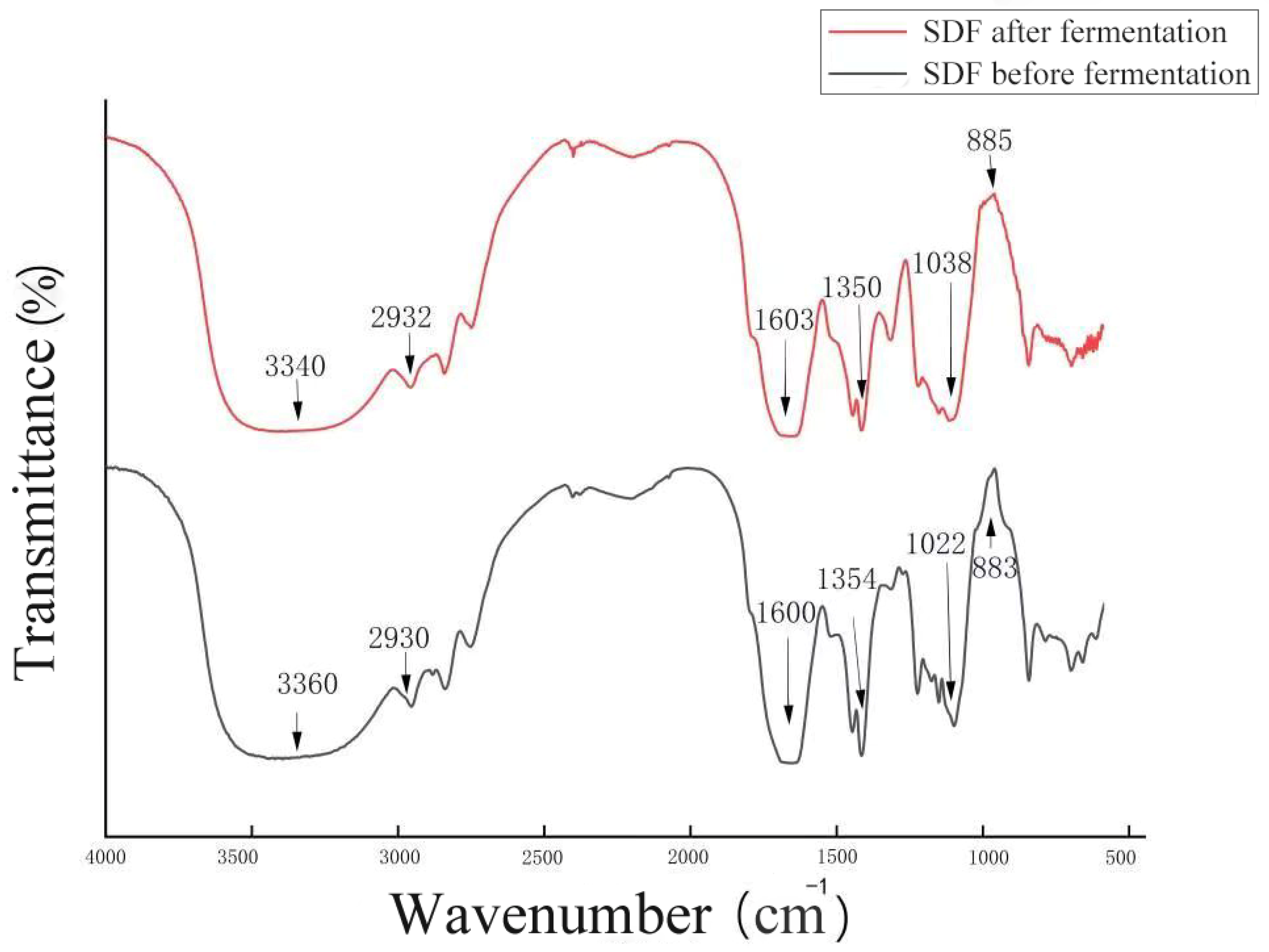
2.5.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.6. Corn Bran SDF Physicochemical Characteristics
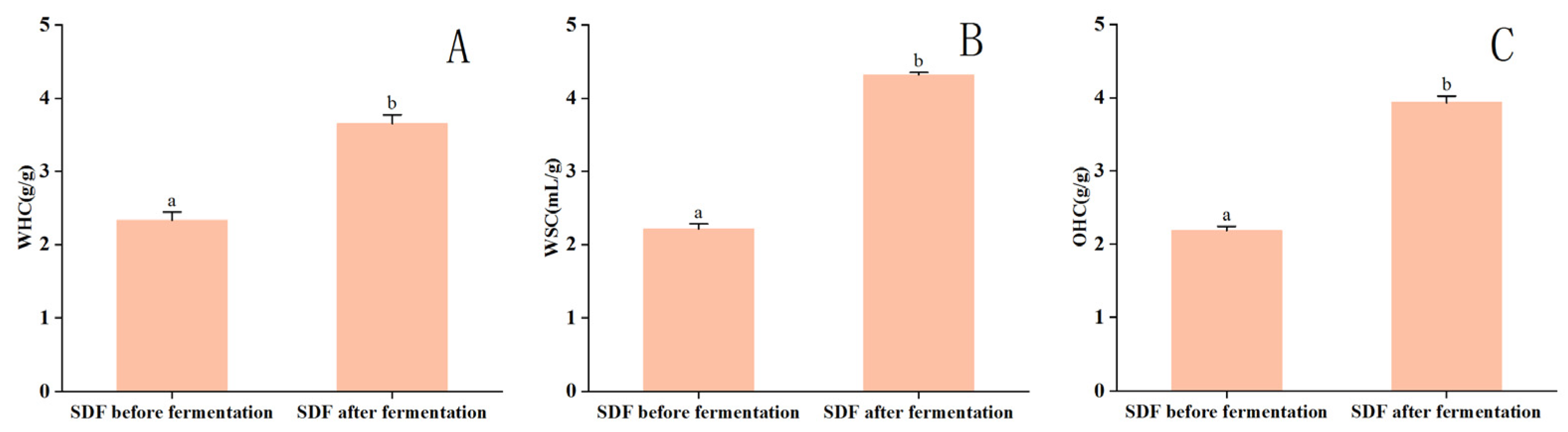
2.6.1. Water-Holding Capacity (WHC)
2.6.2. Water Soluble Capacity (WSC)
2.6.3. Oil-Holding Capacity (OHC)
2.7. Determining Nutrient Content
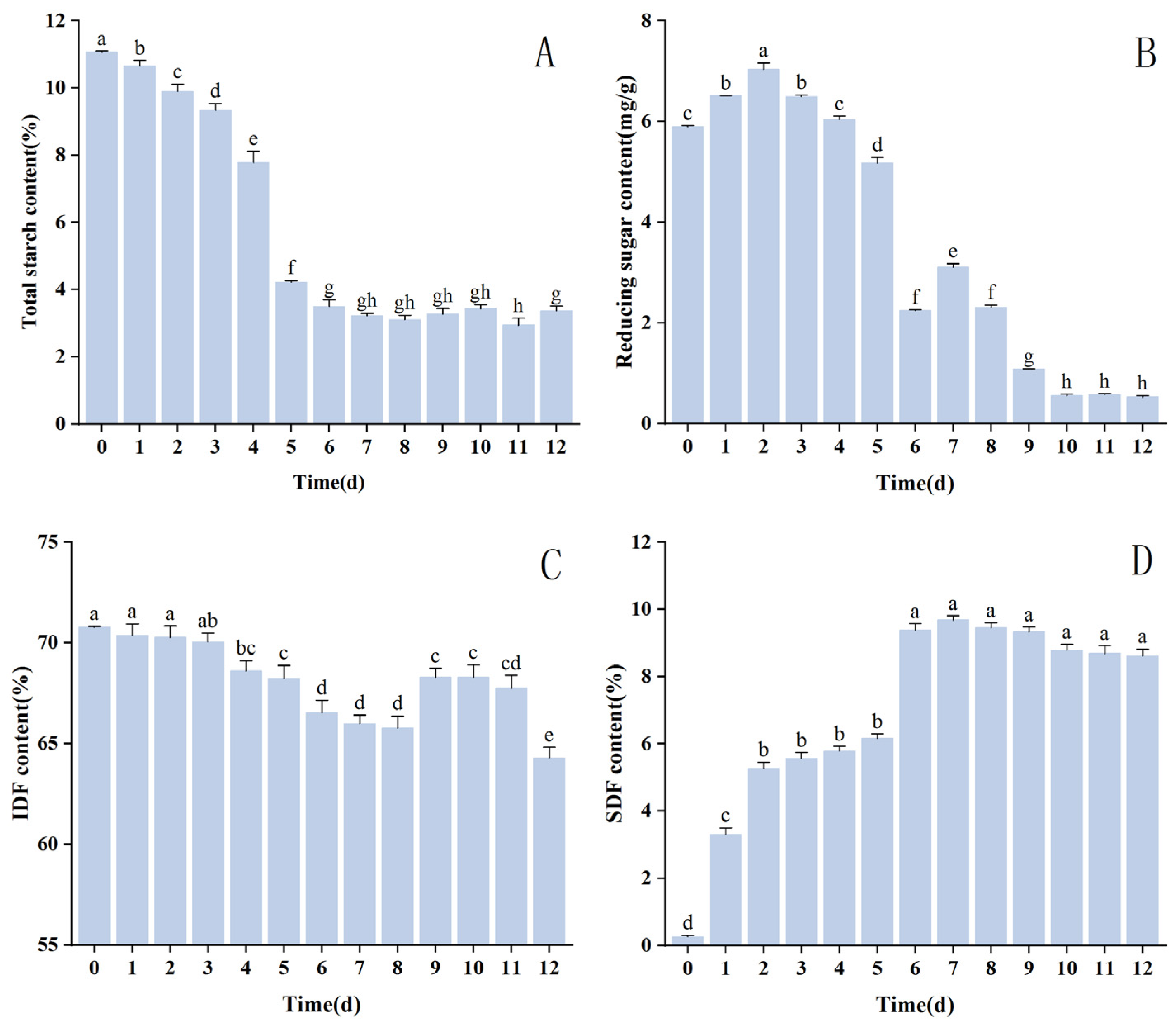
2.7.1. Total Starch
2.7.2. Reducing Sugar
2.7.3. DF
2.8. Determining Extracellular Enzyme Activity
2.8.1. Extraction and Preservation of Crude Enzyme Solution (CES)
2.8.2. Amylase
2.8.3. Carboxymethylcellulase (CMC) Enzyme
2.8.4. Hemicellulose (HC) Enzyme
2.9. Statistical Analysis
3. Results and Discussion
3.1. SEM Analysis
3.2. FT-IR Analysis
3.3. Physicochemical Properties of Corn Husk SDF Pre- and Post-Fermentation
3.4. Analysis of Changes in Nutrient Composition
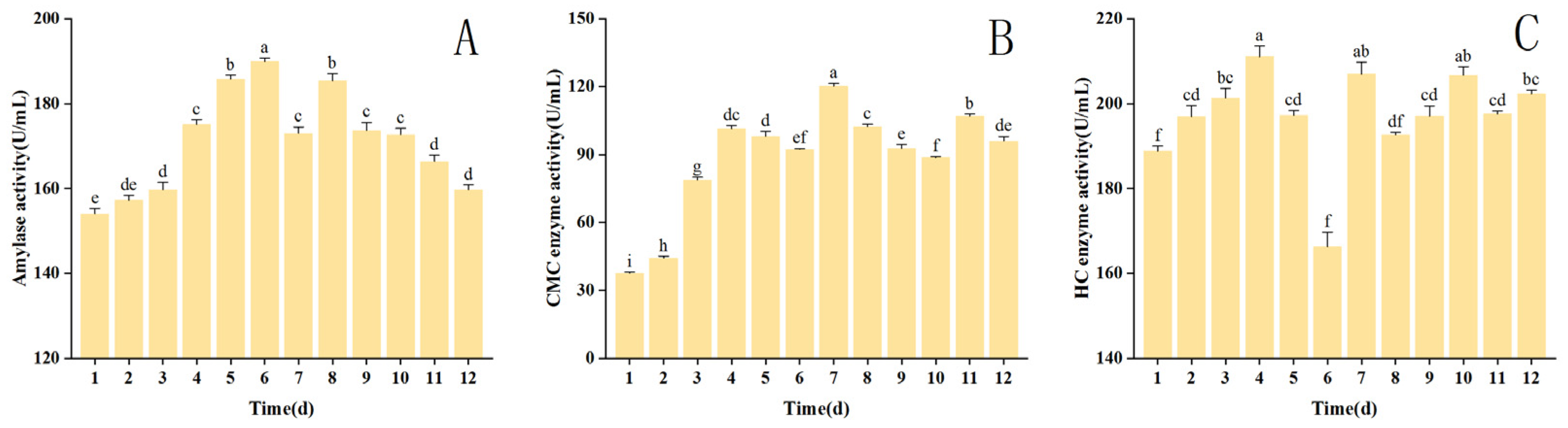
3.5. Changes in Extracellular Enzyme Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jiao, Y.; Chen, H.-D.; Han, H.; Chang, Y. Development and Utilization of Corn Processing by-Products: A Review. Foods 2022, 11, 3709. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Jensen, M.G. Dietary Fibres in the Regulation of Appetite and Food Intake. Importance of Viscosity. Appetite 2011, 56, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Baka, A.; Björck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of Diet Composition on Blood Glucose Regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef] [PubMed]
- Solah, V.A.; Kerr, D.A.; Hunt, W.J.; Johnson, S.K.; Boushey, C.J.; Delp, E.J.; Meng, X.; Gahler, R.J.; James, A.P.; Mukhtar, A.S.; et al. Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals. Nutrients 2017, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.-L.; Cheng, Y.-K.; Jiang, Z.-Y.; Jin, Y.; Zhang, H.-S.; Liu, D.; Teng, L.-R.; Zhang, G.-R. Enzymo-Chemical Preparation, Physico-Chemical Characterization and Hypolipidemic Activity of Granular Corn Bran Dietary Fibre. J. Food Sci. Technol. 2015, 52, 1718–1723. [Google Scholar] [CrossRef]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterations in Physicochemical and Functional Properties of Buckwheat Straw Insoluble Dietary Fiber by Alkaline Hydrogen Peroxide Treatment. Food Chem. X 2019, 3, 100029. [Google Scholar] [CrossRef]
- Rose, D.J.; Inglett, G.E.; Liu, S.X. Utilisation of Corn (Zea mays) Bran and Corn Fiber in the Production of Food Components. J. Sci. Food Agric. 2010, 90, 915–924. [Google Scholar] [CrossRef]
- Li, R.; Wang, C.; Wang, Y.; Xie, X.; Sui, W.; Liu, R.; Wu, T.; Zhang, M. Extrusion Modification of Wheat Bran and Its Effects on Structural and Rheological Properties of Wheat Flour Dough. Foods 2023, 12, 1813. [Google Scholar] [CrossRef]
- Hu, L.; Du, H.; Liu, C.; Zhang, Y.; Yu, G.; Zhang, X.; Si, C.; Li, B.; Peng, H. Comparative Evaluation of the Efficient Conversion of Corn Husk Filament and Corn Husk Powder to Valuable Materials Via a Sustainable and Clean Biorefinery Process. ACS Sustain. Chem. Eng. 2018, 7, 1327–1336. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Modification of Deoiled Cumin Dietary Fiber with Laccase and Cellulase under High Hydrostatic Pressure. Carbohydr. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ratna, A.S.; Ghosh, A.; Mukhopadhyay, S. Advances and Prospects of Corn Husk as a Sustainable Material in Composites and Other Technical Applications. J. Clean. Prod. 2022, 371, 133563. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martinez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive Phenolic Compounds: Production and Extraction by Solid-State Fermentation. A Review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Wu, J.; Ren, L.; Zhao, N.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Solid-State Fermentation by Rhizopus Oryzae Improves Flavor of Wheat Bran for Application in Food. J. Cereal Sci. 2022, 107, 103536. [Google Scholar] [CrossRef]
- Jia, M.; Chen, J.; Liu, X.; Xie, M.; Nie, S.; Chen, Y.; Xie, J.; Yu, Q. Structural Characteristics and Functional Properties of Soluble Dietary Fiber from Defatted Rice Bran Obtained through Trichoderma Viride fermentation. Food Hydrocoll. 2019, 94, 468–474. [Google Scholar] [CrossRef]
- Sun, C.; Wu, X.; Chen, X.; Li, X.; Zheng, Z.; Jiang, S. Production and Characterization of Okara Dietary Fiber Produced by Fermentation with Monascus anka. Food Chem. 2020, 316, 126243. [Google Scholar] [CrossRef]
- Si, J.; Yang, C.; Chen, Y.; Xie, J.; Tian, S.; Cheng, Y.; Hu, X.; Yu, Q. Structural Properties and Adsorption Capacities of Mesona Chinensis Benth Residues Dietary Fiber Prepared by Cellulase Treatment Assisted by Aspergillus niger or Trichoderma reesei. Food Chem. 2023, 407, 135149. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Li, F.; Liu, P. Active Polysaccharides from Lentinula Edodes and Pleurotus Ostreatus by Addition of Corn Straw and Xylosma Sawdust through Solid-State Fermentation. Int. J. Biol. Macromol. 2023, 228, 647–658. [Google Scholar] [CrossRef]
- Postemsky, P.; Bidegain, M.; González-Matute, R.; Figlas, N.; Cubitto, M. Pilot-Scale Bioconversion of Rice and Sunflower Agro-Residues into Medicinal Mushrooms and Laccase Enzymes through Solid-State Fermentation with Ganoderma lucidum. Bioresour. Technol. 2017, 231, 85–93. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, Y.; Yu, H.; Wang, Y.; Piao, C. Insoluble Dietary Fibre from Okara (Soybean Residue) Modified by Yeast Kluyveromyces marxianus. LWT 2020, 134, 110252. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium Erinaceus, an Amazing Medicinal Mushroom. Mycol. Prog. 2015, 14, 1–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Pan, H.; Pandey, A.; He, W.; Fan, L. Antioxidant and Hepatoprotective Potential of Endo-Polysaccharides from Hericium erinaceus Grown on Tofu Whey. Int. J. Biol. Macromol. 2012, 51, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, F.; Falletta, E.; Leri, M.; Angeloni, C.; Beghelli, D.; Giusti, L.; Milanesi, R.; Sampaio-Marques, B.; Ludovico, P.; Goppa, L.; et al. Anti-Aging and Neuroprotective Properties of Grifola frondosa and Hericium erinaceus Extracts. Nutrients 2022, 14, 4368. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Auletta, S.; Palladino, G.; Brandimarte, G.; D’onofrio, R.; Arboretto, G.; Imperio, G.; Ventura, A.; Cipullo, M.; et al. Hericium Erinaceus, a Medicinal Fungus with a Centuries-Old History: Evidence in Gastrointestinal Diseases. World J. Gastroenterol. 2023, 29, 3048. [Google Scholar] [CrossRef]
- Sokół, S.; Golak-Siwulska, I.; Sobieralski, K.; Siwulski, M.; Górka, K. Biology, Cultivation, and Medicinal Functions of the Mushroom Hericium Erinaceum. Acta Mycol. 2015, 50, 2. [Google Scholar] [CrossRef]
- Fen, L.; Xuwei, Z.; Nanyi, L.; Puyu, Z.; Shuang, Z.; Xue, Z.; Pengju, L.; Qichao, Z.; Haiping, L. Screening of Lignocellulose-Degrading Superior Mushroom Strains and Determination of Their Cmcase and Laccase Activity. Sci. World J. 2014, 2014, 763108. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Liu, Q.N.; Sun, X.Z.; Ji, W.T.; Xu, D.; Zhao, Y.N.; Cai, D. Process Optimization of Hericium Erinaceus Fermentation for Modifying Dietary Fiber in Corn Husk; Tianjin Food Research Institute Co., Ltd.: Tianjin, China, 2022. [Google Scholar]
- Singh, J.; Singh, N.; Chugh, R.K.; Yadav, N.; Chauhan, R. Efficacy of Different Culture Media on Growth and Characteristics of Mycelium of Monkey Head Mushroom (Hericium erinaceus). Plant Dis. Res. 2021, 36, 27–31. [Google Scholar] [CrossRef]
- Chen, L.; Yao, J.-N.; Chen, H.-P.; Zhao, Z.-Z.; Li, Z.-H.; Feng, T.; Liu, J.-K. Hericinoids A–C, Cyathane Diterpenoids from Culture of Mushroom Hericium Erinaceus. Phytochem. Lett. 2018, 27, 94–100. [Google Scholar] [CrossRef]
- Yu, X.H.; Gu, Z.X.; Tu, K.; Shao, R.; Jin, X.J. Optimization of Solid State Fermentation Media for Soluble Dietary Fiber Production by Hericium Erinaceus. Adv. Mater. Res. 2012, 421, 81–89. [Google Scholar] [CrossRef]
- Du, X.; Wang, L.; Huang, X.; Jing, H.; Ye, X.; Gao, W.; Bai, X.; Wang, H. Effects of Different Extraction Methods on Structure and Properties of Soluble Dietary Fiber from Defatted Coconut Flour. LWT 2021, 143, 111031. [Google Scholar] [CrossRef]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave Assisted Extraction with Three Modifications on Structural and Functional Properties of Soluble Dietary Fibers from Grapefruit Peel. Food Hydrocoll. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Zeng, W.; Huang, Y.; Yang, X. Properties of Dietary Fiber from Citrus Obtained through Alkaline Hydrogen Peroxide Treatment and Homogenization Treatment. Food Chem. 2020, 311, 125873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion Process Improves the Functionality of Soluble Dietary Fiber in Oat Bran. J. Cereal Sci. 2011, 54, 98–103. [Google Scholar] [CrossRef]
- Caprez, A.; Arrigoni, E.; Amadò, R.; Neukom, H. Influence of Different Types of Thermal Treatment on the Chemical Composition and Physical Properties of Wheat Bran. J. Cereal Sci. 1986, 4, 233–239. [Google Scholar] [CrossRef]
- GB/T 5009.9-2016; Determination of Starch Content in Food. Standards Press of China: Beijing, China, 2016.
- Johnson, N.A.N.; Ekumah, J.-N.; Adade, S.Y.-S.S.; Li, Y.; Betchem, G.; Issaka, E.; Ma, Y. Phytochemical and Structural Changes of Chickpea Beverage Prepared Using Ultrasound-Assisted Fermentation with Optimized Ultrasound Parameters Modelled by Response Surface Methodology. Beverages 2023, 9, 62. [Google Scholar] [CrossRef]
- GB/T 5009.88-2014; Determination of Dietary Fiber in Foods. Standards Press of China: Beijing, China, 2014.
- Daou, C.; Zhang, H. Functional and Physiological Properties of Total, Soluble, and Insoluble Dietary Fibres Derived from Defatted Rice Bran. J. Food Sci. Technol. 2014, 51, 3878–3885. [Google Scholar] [CrossRef]
- Hussien, S.A.; Doosh, K. Extraction, Purification and Characterization of Β-Galactosidase from Tomato (Lycopersicom esculentum). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Sarangthem, I.; Rajkumari, L.; Ngashangva, N.; Nandeibam, J.; Yendrembam, R.B.S.; Mukherjee, P.K. Isolation and Characterization of Bacteria from Natural Hot Spring and Insights into the Thermophilic Cellulase Production. Curr. Microbiol. 2023, 80, 64. [Google Scholar] [CrossRef]
- Ottenheim, C.; Verdejo, C.; Zimmermann, W.; Wu, J.C. Hemicellulase Production by Aspergillus Niger Dsm 26641 in Hydrothermal Palm Oil Empty Fruit Bunch Hydrolysate and Transcriptome Analysis. J. Biosci. Bioeng. 2014, 118, 696–701. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Chen, Y.; Xie, J.; Song, Y.; Chang, X.; Liu, S.; Wang, Z.; Hu, X.; Yu, Q. Effects of Fermentation on the Structural Characteristics and in Vitro Binding Capacity of Soluble Dietary Fiber from Tea Residues. LWT 2020, 131, 109818. [Google Scholar] [CrossRef]
- Si, J.; Yang, C.; Ma, W.; Chen, Y.; Xie, J.; Qin, X.; Hu, X.; Yu, Q. Screen of High Efficiency Cellulose Degrading Strains and Effects on Tea Residues Dietary Fiber Modification: Structural Properties and Adsorption Capacities. Int. J. Biol. Macromol. 2022, 220, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Liao, J.-S.; Qi, J.-R.; Jiang, W.-X.; Yang, X.-Q. Structural and Physicochemical Properties of Pectin-Rich Dietary Fiber Prepared from Citrus Peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Effects of Extraction Methods and Particle Size Distribution on the Structural, Physicochemical, and Functional Properties of Dietary Fiber from Deoiled Cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, B.; Yadav, M.P.; Bhinder, S.; Singh, N. Isolation of Arabinoxylan and Cellulose-Rich Arabinoxylan from Wheat Bran of Different Varieties and Their Functionalities. Food Hydrocoll. 2021, 112, 106287. [Google Scholar] [CrossRef]
- Ullah, I.; Yin, T.; Xiong, S.; Zhang, J.; Din, Z.-U.; Zhang, M. Structural Characteristics and Physicochemical Properties of Okara (Soybean Residue) Insoluble Dietary Fiber Modified by High-Energy Wet Media Milling. LWT-Food Sci. Technol. 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y. Physicochemical and Functional Properties of Coconut (Cocos nucifera L.) Cake Dietary Fibres: Effects of Cellulase Hydrolysis, Acid Treatment and Particle Size Distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved Physicochemical and Functional Properties of Dietary Fiber from Millet Bran Fermented by Bacillus Natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical Profile, Functional and Antioxidant Properties of Tomato Peel Fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Chantaro, P.; Devahastin, S.; Chiewchan, N. Production of Antioxidant High Dietary Fiber Powder from Carrot Peels. LWT-Food Sci. Technol. 2008, 41, 1987–1994. [Google Scholar] [CrossRef]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health Benefits of Finger Millet (Eleusine coracana L.) Polyphenols and Dietary Fiber: A Review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, L.; Xu, A.; Huang, J.; Ma, S. Impact of Fermented Wheat Bran Dietary Fiber Addition on Dough Rheological Properties and Noodle Quality. Front. Nutr. 2022, 9, 952525. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lei, H.; Zhen, X.; Liu, J.; Xie, W.; Tang, Q.; Gou, D.; Zhao, J. Advancements in Modifying Insoluble Dietary Fiber: Exploring the Microstructure, Physicochemical Properties, Biological Activity, and Applications in Food Industry—A Review. Food Chem. 2024, 458, 140154. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yu, O.K.; Byun, M.S.; Cha, Y.S. Okara, a Soybean by-Product, Prevents High Fat Diet-Induced Obesity and Improves Serum Lipid Profiles in C57bl/6j Mice. Food Sci. Biotechnol. 2016, 25, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, M.; Li, Y.; Zhuang, W.; Guo, Z. Effect of Steam Explosion Modified Soluble Dietary Fiber from Tremella Fuciformis Stem on the Quality and Digestibility of Biscuits. Int. J. Biol. Macromol. 2024, 265, 130905. [Google Scholar] [CrossRef]
- Ghorai, S.; Banik, S.P.; Verma, D.; Chowdhury, S.; Mukherjee, S.; Khowala, S. Fungal Biotechnology in Food and Feed Processing. Food Res. Int. 2009, 42, 577–587. [Google Scholar] [CrossRef]
- Kitamoto, Y.; Gruen, H.E. Distribution of Cellular Carbohydrates During Development of the Mycelium and Fruitbodies of Flammulina velutipes. Plant Physiol. 1976, 58, 485–491. [Google Scholar] [CrossRef]
- Yoon, J.-J.; Munir, E.; Miyasou, H.; Hattori, T.; Shimada, M.; Terashita, T. A Possible Role of the Key Enzymes of the Glyoxylate and Gluconeogenesis Pathways for Fruit-Body Formation of the Wood-Rotting Basidiomycete Flammulina velutipes. Mycoscience 2002, 43, 327–332. [Google Scholar] [CrossRef]
- Lin, D.; Long, X.; Huang, Y.; Yang, Y.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Effects of Microbial Fermentation and Microwave Treatment on the Composition, Structural Characteristics, and Functional Properties of Modified Okara Dietary Fiber. LWT 2020, 123, 109059. [Google Scholar] [CrossRef]
- Abd-Aziz, S.; Shahrim, Z.; Sabaratnam, V.; Rahman, N.; Hassan, M.; Karim, M. Production of Reducing Sugars by Trichoderma Sp. Kupm0001 during Solid Substrate Fermentation of Sago Starch Processing Waste Hampas. Res. J. Microbiol. 2008, 3, 569–579. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of Modification Methods on Physicochemical and Structural Properties of Soluble Dietary Fiber from Corn Bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shao, S.; Liu, N.; Liu, Q.; Jacquemyn, H.; Xing, X. Extracellular Enzyme Activities and Carbon/Nitrogen Utilization in Mycorrhizal Fungi Isolated from Epiphytic and Terrestrial Orchids. Front. Microbiol. 2021, 12, 787820. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Awe, F.A.; Akinyanju, J.A. Amylase Synthesis in Aspergillus Flavus and Aspergillus Niger Grown on Cassava Peel. J. Ind. Microbiol. 1992, 10, 55–59. [Google Scholar] [CrossRef]
- Uguru, G.; Akinyanju, J.; Sani, A. The Use of Yam Peel for Growth of Locally Isolated Aspergillus Niger and Amylase Production. Enzym. Microb. Technol. 1997, 21, 48–51. [Google Scholar] [CrossRef]
- Kapoor, S.; Khanna, P.K.; Katyal, P. Effect of Supplementation of Wheat Straw on Growth and Lignocellulolytic Enzyme Potential of Lentinus Edodes. World J. Agric. Sci. 2009, 5, 328–331. [Google Scholar]
- Li, P.; Liang, H.; Lin, W.-T.; Feng, F.; Luo, L. Microbiota Dynamics Associated with Environmental Conditions and Potential Roles of Cellulolytic Communities in Traditional Chinese Cereal Starter Solid-State Fermentation. Appl. Environ. Microbiol. 2015, 81, 5144–5156. [Google Scholar] [CrossRef]
- Tokimoto, K. Activities of Enzymes in Bed Logs of Lentinus Edodes during Fruitbody Development. Rep. Tottori Mycol. Inst. 1987, 25, 24–35. [Google Scholar]
- Terashita, T.; Murao, R.; Yoshikawa, K.; Shishiyama, J. Changes in Carbohydrase Activities during Vegetative Growth and Development of Fruit-Bodies of Hypsizygus marmoreus Grown in Sawdust-Based Culture. J. Wood Sci. 1998, 44, 234–236. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Ma, F.; Tang, C.; Tang, Q.; Zhang, X. Investigation of Lignocellulolytic enzymes during Different Growth Phases of Ganoderma Lucidum Strain G0119 Using Genomic, Transcriptomic and Secretomic Analyses. PLoS ONE 2018, 13, e0198404. [Google Scholar] [CrossRef]
- Yang, S.S.; Wang, J.Y. Protease and Amylase Production of Streptomyces Rimosus in Submerged and Solid State Cultivations. Bot. Bull. Acad. Sin. 1999, 40, 59–265. [Google Scholar]
- Lechner, B.E.; Papinutti, V.L. Production of Lignocellulosic Enzymes During Growth and Fruiting of the Edible Fungus Lentinus tigrinus on Wheat Straw. Process Biochem. 2006, 41, 594–598. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, H.; Liu, Q.; Xiu, L.; Cai, D.; Liu, J. Effect of Solid-State Fermentation of Hericium erinaceus on the Structure and Physicochemical Properties of Soluble Dietary Fiber from Corn Husk. Foods 2024, 13, 2895. https://doi.org/10.3390/foods13182895
Ban H, Liu Q, Xiu L, Cai D, Liu J. Effect of Solid-State Fermentation of Hericium erinaceus on the Structure and Physicochemical Properties of Soluble Dietary Fiber from Corn Husk. Foods. 2024; 13(18):2895. https://doi.org/10.3390/foods13182895
Chicago/Turabian StyleBan, He, Qiannan Liu, Lin Xiu, Dan Cai, and Jingsheng Liu. 2024. "Effect of Solid-State Fermentation of Hericium erinaceus on the Structure and Physicochemical Properties of Soluble Dietary Fiber from Corn Husk" Foods 13, no. 18: 2895. https://doi.org/10.3390/foods13182895





