Enhancement of Mechanical Properties of Zein-Based Nanofibers by Incorporation of Millet Gliadin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Millet Gliadin Isolation from Millet Grains
2.3. Characterization of Zein and Millet Gliadin
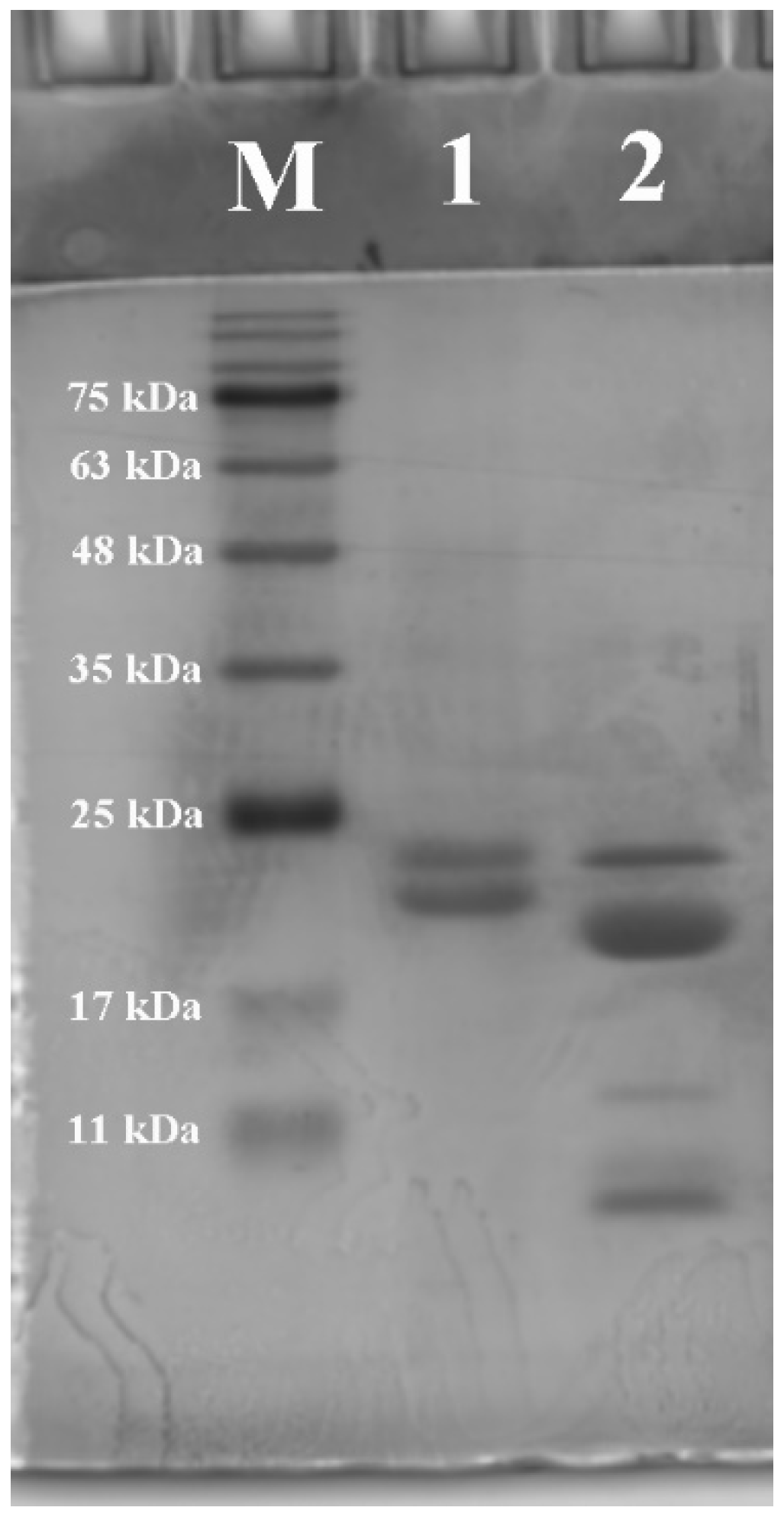
2.3.1. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.3.2. Gel Permeation Chromatography (GPC)
2.3.3. Amino Acid Composition
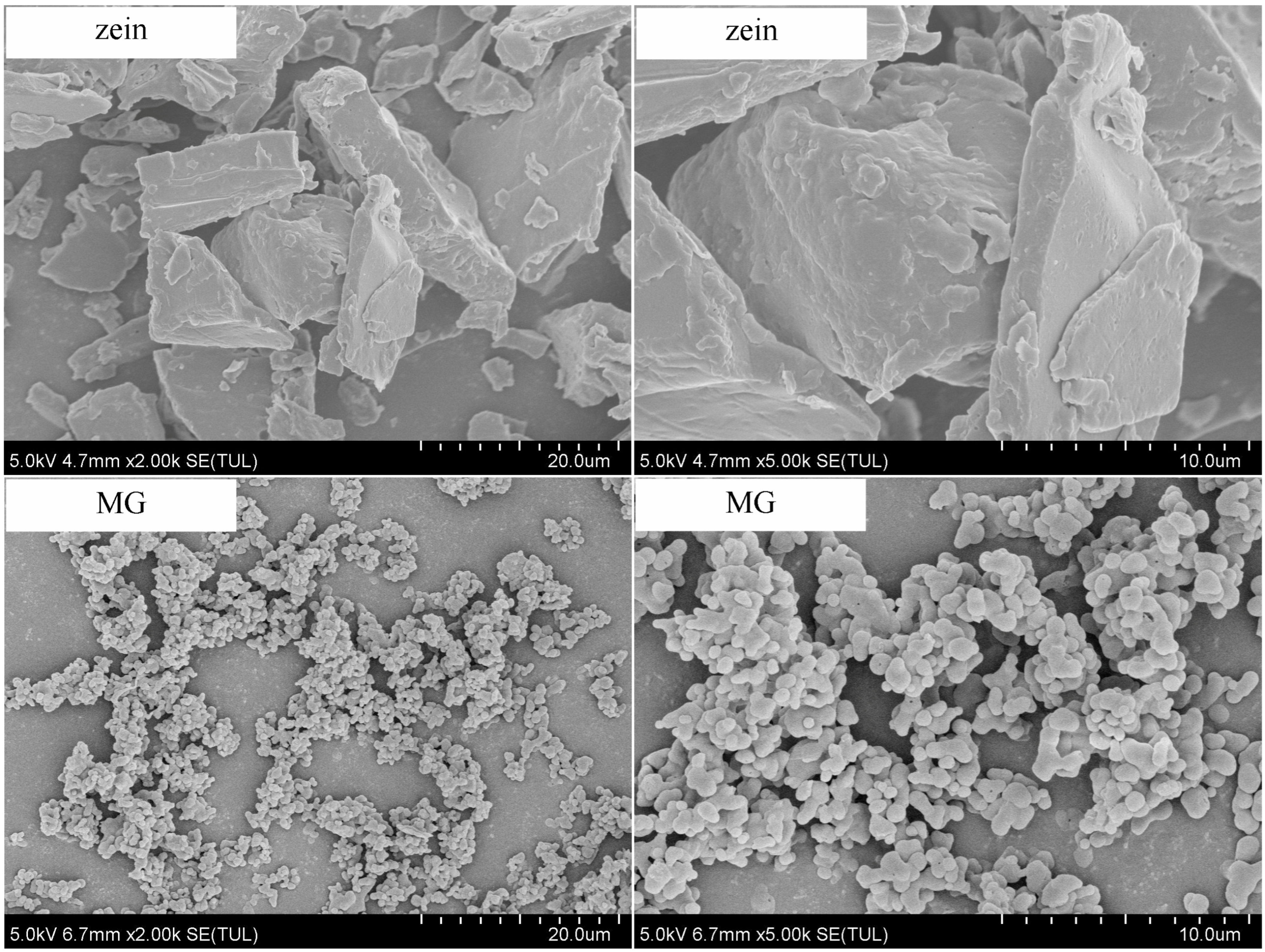
2.3.4. Micromorphology of Zein and Millet Gliadin
2.3.5. Intrinsic Fluorescence Spectroscopy
2.3.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.7. Viscosity of Zein and Millet Gliadin in Acetic Acid
2.4. Preparation of Electrospinning Nanofibers
2.5. Scanning Electron Microscopy (SEM)
2.6. Tensile Testing
2.7. Water Contact Angle (WCA)
2.8. Water Stability
2.9. Thermal Property
2.10. Attenuated Total Reflectance Infrared Spectroscopy (ATR-FTIR)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Composition and Structure of Zein and Millet Gliadin
3.1.1. Molecular Weight Profile and Amino Acid Composition
3.1.2. Structural Properties
3.1.3. Morphology
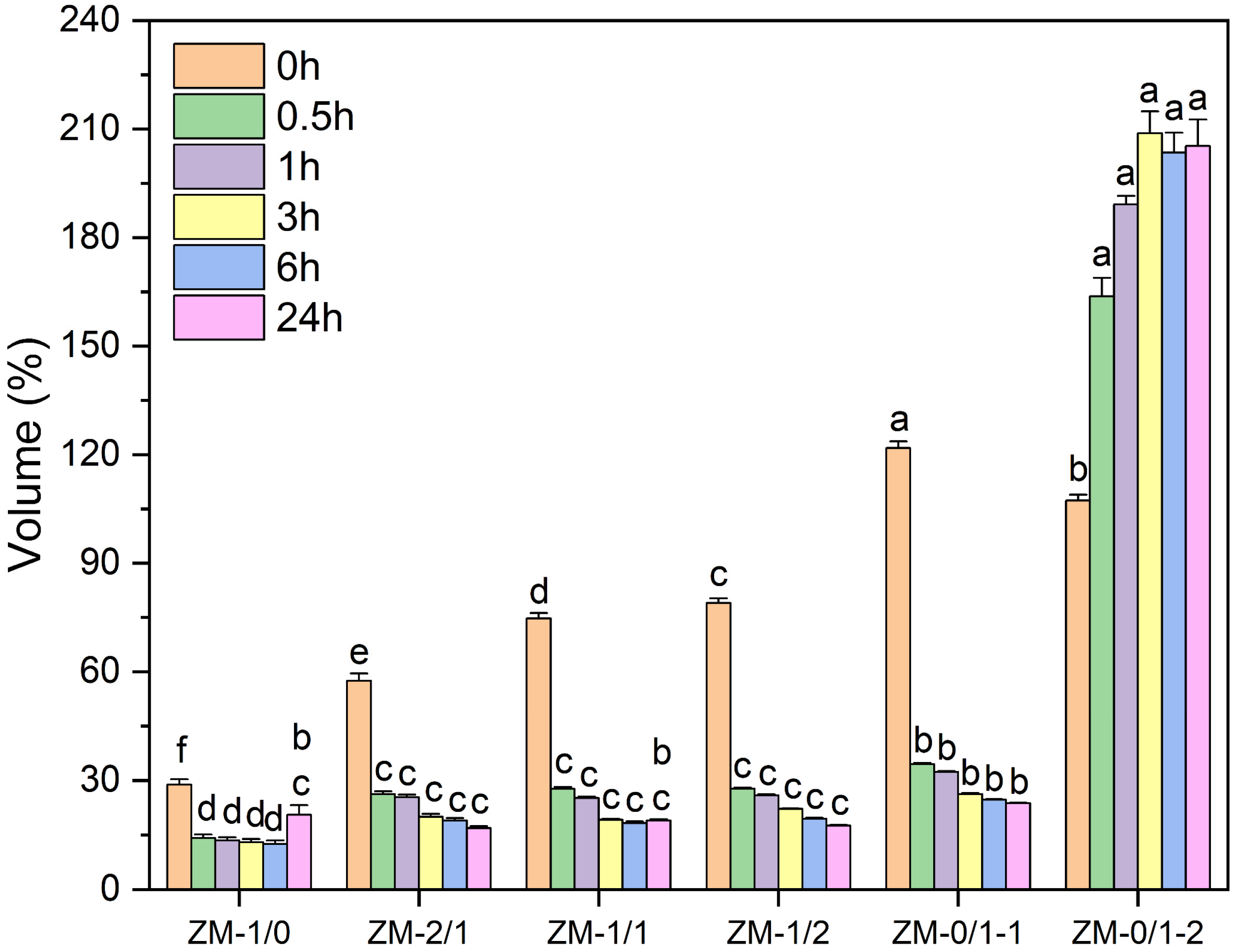
3.2. Solution Viscosity
3.3. Nanofiber Characterization
3.3.1. Morphology and Diameter Distribution of Nanofibers
3.3.2. Mechanical Properties
3.3.3. Surface Properties of Nanofibers
3.3.4. Water Stability of Nanofibers
3.3.5. Thermogravimetric Analysis (TGA)
3.3.6. Structures of Zein/Millet Gliadin Nanofibers
3.4. Composition Schematic Representation of Nanofibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karim, M.; Fathi, M.; Soleimanian-Zad, S.; Spigno, G. Development of sausage packaging with zein nanofibers containing tetradecane produced via needle-less electrospinning method. Food Packag. Shelf Life 2022, 33, 100911. [Google Scholar] [CrossRef]
- Palika, A.; Armanious, A.; Rahimi, A.; Medaglia, C.; Gasbarri, M.; Handschin, S.; Rossi, A.; Pohl, M.O.; Busnadiego, I.; Gubeli, C.; et al. An antiviral trap made of protein nanofibrils and iron oxyhydroxide nanoparticles. Nat. Nanotechnol. 2021, 16, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.S.; Freag, M.S.; Gaber, S.M.; Al Oufy, A.; Abdallah, O.Y. Development of verapamil hydrochloride-loaded biopolymer-based composite electrospun nanofibrous mats: In vivo evaluation of enhanced burn wound healing without scar formation. Drug Des. Dev. Ther. 2023, 17, 1211–1231. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. Efficient removal of polycyclic aromatic hydrocarbons and heavy metals from water by electrospun nanofibrous polycyclodextrin membranes. ACS Omega 2019, 4, 7850–7860. [Google Scholar] [CrossRef] [PubMed]
- Ajalloueian, F.; Guerra, P.R.; Bahl, M.I.; Torp, A.M.; Hwu, E.T.; Licht, T.R.; Boisen, A. Multi-layer plga-pullulan-plga electrospun nanofibers for probiotic delivery. Food Hydrocoll. 2022, 123, 107112. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Cellulose nanowhiskers and fiber alignment greatly improve mechanical properties of electrospun prolamin protein fibers. ACS Appl. Mater. Interfaces 2014, 6, 1709–1718. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Fabrication and characterization of novel assembled prolamin protein nanofabrics with improved stability, mechanical property and release profiles. J. Mater. Chem. 2012, 22, 21592–21601. [Google Scholar] [CrossRef]
- Surendranath, M.; Ramesan, R.M.; Nair, P.; Parameswaran, R. Electrospun mucoadhesive zein/pvp fibroporous membrane for transepithelial delivery of propranolol hydrochloride. Mol. Pharm. 2023, 20, 508–523. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, K.; Qin, Z.; Zou, Y.; Zhong, H.; Zhang, H. Cross-linked gluten/zein nanofibers via maillard reaction with the loading of star anise essential oil/β-cyclodextrin inclusions for food-active packaging. Food Packag. Shelf Life 2022, 34, 100950. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Song, T. Electrospinning and crosslinking of zein nanofiber mats. J. Appl. Polym. Sci. 2007, 103, 380–385. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, P.; Li, J.; Zhang, H.; Weiss, J. Characterization of core-shell nanofibers electrospun from bilayer gelatin/gum arabic o/w emulsions crosslinked by genipin. Food Hydrocoll. 2021, 119, 106854. [Google Scholar] [CrossRef]
- Jiang, Q.; Reddy, N.; Zhang, S.; Roscioli, N.; Yang, Y. Water-stable electrospun collagen fibers from a non-toxic solvent and crosslinking system. J. Biomed. Mater. Res. Part A 2013, 101, 1237–1247. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Rangkupan, R.; Jeeratawatchai, H.; Kanokpanont, S.; Damrongsakkul, S. Influences of physical and chemical crosslinking techniques on electrospun type a and b gelatin fiber mats. Int. J. Biol. Macromol. 2010, 47, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Dippold, D.; Tallawi, M.; Tansaz, S.; Roether, J.A.; Boccaccini, A.R. Novel electrospun poly(glycerol sebacate)–zein fiber mats as candidate materials for cardiac tissue engineering. Eur. Polym. J. 2016, 75, 504–513. [Google Scholar] [CrossRef]
- Teng, D.; Wahid, A.; Zeng, Y. Zein/pvdf micro/nanofibers with improved mechanical property for oil adsorption. Polymer 2020, 188, 122118. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Self-crosslinked gliadin fibers with high strength and water stability for potential medical applications. J. Mater. Sci. Mater. Med. 2008, 19, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Orqueda, M.E.; Gómez-Mascaraque, L.G.; Isla, M.I.; López-Rubio, A. Crosslinked electrospun zein-based food packaging coatings containing bioactive chilto fruit extracts. Food Hydrocoll. 2019, 95, 496–505. [Google Scholar] [CrossRef]
- Ullah, S.; Hashmi, M.; Khan, M.Q.; Kharaghani, D.; Saito, Y.; Yamamoto, T.; Kim, I.S. Silver sulfadiazine loaded zein nanofiber mats as a novel wound dressing. RSC Adv. 2018, 9, 268–277. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Xu, X.; Liu, X.; Liu, F. Zein-based nano-delivery systems for encapsulation and protection of hydrophobic bioactives: A review. Front. Nutr. 2022, 9, 999373. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Chen, Z.; Wang, X. Effects of moderate-intensity pulsed electric field on the structure and physicochemical properties of foxtail millet (Setaria italica) prolamin. Cereal Chem. 2022, 100, 360–370. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, F.; Liu, Z.; Zhao, Q.; Xue, Y.; Shen, Q. Improvement of diabetes-induced metabolic syndrome by millet prolamin is associated with changes in serum metabolomics. Food Biosci. 2021, 44, 101434. [Google Scholar] [CrossRef]
- Ben-Romdhane, M.; Riahi, L.; Borji, M.; Riahi, J.; Chibani, F.; Ghorbel, A.; Zoghlami, N. Characterization of prolamin storage protein profiles in tunisian pearl millet landraces toward better utilization and improvement. Cereal Chem. 2023, 100, 1263–1272. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zhang, X.; Li, X.; Ma, J.; Jing, X.; Wang, X. Anti-inflammatory effect of a novel millet gliadin peptide on mice with colitis. J. Funct. Foods 2023, 111, 105912. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Liu, Z.; Shen, Q. Comparison of the characteristics of prolamins among foxtail millet varieties with different palatability: Structural, morphological, and physicochemical properties. Int. J. Biol. Macromol. 2021, 186, 194–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Chen, Z.; Wang, X. Purification and identification of antioxidant peptides from millet gliadin treated with high hydrostatic pressure. LWT-Food Sci. Technol. 2022, 164, 113654. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.Y.; Wu, Y.C.; Gong, P.X.; Li, H.J. Foxtail millet prolamin as an effective encapsulant deliver curcumin by fabricating caseinate stabilized composite nanoparticles. Food Chem. 2022, 367, 130764. [Google Scholar] [CrossRef]
- Zahabi, N.; Golmakani, M.T.; Fazaeli, M.; Ghiasi, F.; Khalesi, M. Electrospinning of glutelin-hordein incorporated with oliveria decumbens essential oil: Characterization of nanofibers. Colloids Surf. B 2021, 208, 112058. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Li, X.Y.; Liu, B.H.; Yin, Y.Q.; Zhang, H.L.; Zhang, Y.H. Comprehensive application possibility: Construction hydrophilic, amphiphilic and hydrophobic system of modified zein by enzymatic or cysteine modification. Food Hydrocoll. 2023, 135, 108159. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gomez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Biochemical properties of bioplastics made from wheat gliadins cross-linked with cinnamaldehyde. J. Agric. Food Chem. 2011, 59, 13212–13220. [Google Scholar] [CrossRef]
- Silva, P.M.; Prieto, C.; Andrade, C.C.P.; Lagaron, J.M.; Pastrana, L.M.; Coimbra, M.A.; Vicente, A.A.; Cerqueira, M.A. Hydroxypropyl methylcellulose-based micro- and nanostructures for encapsulation of melanoidins: Effect of electrohydrodynamic processing variables on morphological and physicochemical properties. Int. J. Biol. Macromol 2022, 202, 453–467. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Wang, P.; Zhang, M.; Liu, S.; Wang, R.; Li, Y.; Ren, F.; Fang, B. Improvement in the sustained-release performance of electrospun zein nanofibers via crosslinking using glutaraldehyde vapors. Foods 2024, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, Q.; Li, G.; Qiu, Y.; Wei, Q. Electrospun water-stable zein/ethyl cellulose composite nanofiber and its drug release properties. Mater. Sci. Eng. C 2017, 74, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liao, R.; Bu, N.; Zhang, D.; Pang, J.; Mu, R. Electrospun konjac glucomannan/polyvinyl alcohol long polymeric filaments incorporated with tea polyphenols for food preservations. Foods 2024, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Mickowska, B.; Socha, P.; Urminská, D.; Cieślik, E. Immunodetection, electrophoresis and amino acid composition of alcohol soluble proteins extracted from grains of selected varieties of pseudocereals, legumes, oat, maize and rice. Cereal Res. Commun. 2013, 41, 160–169. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Electrospinning of prolamin proteins in acetic acid: The effects of protein conformation and aggregation in solution. Macromol. Mater. Eng. 2012, 297, 902–913. [Google Scholar] [CrossRef]
- Hasan, S.; Isar, M.; Naeem, A. Macromolecular crowding stabilises native structure of alpha-chymotrypsinogen-a against hexafluoropropanol-induced aggregates. Int. J. Biol. Macromol. 2020, 164, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ma, H.; Wang, K.; Yagoub Ael, G.; Owusu, J.; Qu, W.; He, R.; Zhou, C.; Ye, X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015, 24, 55–64. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Xue, J.; Zhang, H.; Wang, F.; Liu, J. Mechanistic understanding of the effect of zein-chlorogenic acid interaction on the properties of electrospun nanofiber films. Food Chem. X 2022, 16, 100454. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; Ludescher, R.D.; McClements, D.J. Fluorescence quenching study of resveratrol binding to zein and gliadin: Towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem. 2015, 185, 261–267. [Google Scholar] [CrossRef]
- Yu, X.; Liu, J.; Liu, Y.; Fang, G. Critical role of glutelin in ultrasound-assisted isolation of corn starch. Int. J. Food Sci. Technol. 2021, 57, 489–498. [Google Scholar] [CrossRef]
- Mattice, K.D.; Marangoni, A.G. Functionalizing zein through antisolvent precipitation from ethanol or aetic acid. Food Chem. 2020, 313, 126127. [Google Scholar] [CrossRef] [PubMed]
- Wongsasulak, S.; Tongsin, P.; Intasanta, N.; Yoovidhya, T. Effect of glycerol on solution properties governing morphology, glass transition temperature, and tensile properties of electrospun zein film. J. Appl. Polym. Sci. 2010, 118, 910–919. [Google Scholar] [CrossRef]
- Miri, M.A.; Habibi Najafi, M.B.; Movaffagh, J.; Ghorani, B. Encapsulation of ascorbyl palmitate in zein by electrospinning technique. J. Polym. Environ. 2020, 29, 1089–1098. [Google Scholar] [CrossRef]
- Yao, Z.C.; Chang, M.W.; Ahmad, Z.; Li, J.S. Encapsulation of rose hip seed oil into fibrous zein films for ambient and on demand food preservation via coaxial electrospinning. J. Food Eng. 2016, 191, 115–123. [Google Scholar] [CrossRef]
- Selling, G.W.; Woods, K.K.; Sessa, D.; Biswas, A. Electrospun zein fibers using glutaraldehyde as the crosslinking reagent: Effect of time and temperature. Macromol. Chem. Phys. 2008, 209, 1003–1011. [Google Scholar] [CrossRef]
- Wang, L.; Mu, R.J.; Li, Y.; Lin, L.; Lin, Z.; Pang, J. Characterization and antibacterial activity evaluation of curcumin loaded konjac glucomannan and zein nanofibril films. LWT-Food Sci. Technol. 2019, 113, 108293. [Google Scholar] [CrossRef]
- Shao, P.; Liu, Y.; Ritzoulis, C.; Niu, B. Preparation of zein nanofibers with cinnamaldehyde encapsulated in surfactants at critical micelle concentration for active food packaging. Food Packag. Shelf Life 2019, 22, 100385. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhao, M.; Barker, S.A.; Belton, P.S.; Craig, D.Q.M. A spectroscopic and thermal investigation into the relationship between composition, secondary structure and physical characteristics of electrospun zein nanofibers. Mater. Sci. Eng. C 2019, 98, 409–418. [Google Scholar] [CrossRef]
- Turasan, H.; Cakmak, M.; Kokini, J. Fabrication of zein-based electrospun nanofiber decorated with gold nanoparticles as a SERS platform. J. Mater. Sci. 2019, 54, 8872–8891. [Google Scholar] [CrossRef]








| Samples | Zein (g) | Millet Gliadin (g) | Eugenol (g) | Acetic Acid (mL) |
|---|---|---|---|---|
| ZM-1/0 | 30 | 0 | 0 | 100 |
| ZM-2/1 | 20 | 8.33 | 0 | 100 |
| ZM-1/1 | 15 | 12.50 | 0 | 100 |
| ZM-1/2 | 10 | 16.67 | 0 | 100 |
| ZM-0/1-1 | 0 | 25 | 0 | 100 |
| ZM-0/1-2 | 0 | 30 | 0 | 100 |
| Zein | Millet Gliadin | |
|---|---|---|
| Amino acids (g/100 g protein) | ||
| Glycine (Gly) | 1.12 | 0.90 |
| Alanine (Ala) | 9.12 | 9.41 |
| Proline (Pro) | 6.51 | 5.28 |
| Valine (Val) | 1.63 | 2.17 |
| Methionine (Met) | 0.53 | 1.52 |
| Isoleucine (Ile) | 1.15 | 1.72 |
| Leucine (Leu) | 13.04 | 10.29 |
| Phenylalanine (Phe) | 4.62 | 3.53 |
| Cysteine (Cys) | 0.15 | 0.53 |
| Tyrosine (Tyr) | 3.38 | 2.13 |
| Histidine (His) | 0.78 | 0.86 |
| Arginine (Arg) | 0.92 | 0.87 |
| Threonine (Thr) | 1.10 | 1.64 |
| Aspartic (Asp) | 4.93 | 5.57 |
| Glutamic acid (Glu) | 19.95 | 18.22 |
| Serine (Ser) | 5.04 | 4.65 |
| Lysine (Lys) | 0.08 | 0.05 |
| Hydrophobic amino acids | 36.59 | 33.92 |
| Hydrophilic amino acids | 9.66 | 8.95 |
| Molecular weight | ||
| Mn | 5681 | 3401 |
| Mw | 13,330 | 7623 |
| Samples | Secondary Structure Composition (%) | |||
|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | |
| Zein | 25.40 ± 0.45 b | 32.63 ± 0.63 a | 18.56 ± 0.39 d | 23.40 ± 0.21 c |
| Millet gliadin | 25.24 ± 0.05 b | 33.51 ± 0.11 a | 19.24 ± 0.03 d | 22.01 ± 0.09 c |
| Samples | Fiber Diameter (nm) | Fiber Diameter Distribution | Span |
|---|---|---|---|
| ZM-1/0 | 208.22 ± 47.04 a | 0.05 | 0.64 |
| ZM-2/1 | 265.99 ± 54.87 a | 0.04 | 0.56 |
| ZM-1/1 | 281.33 ± 49.78 a | 0.03 | 0.49 |
| ZM-1/2 | 298.72 ± 55.39 a | 0.03 | 0.49 |
| ZM-0/1-1 | 260.99 ± 49.75 a | 0.04 | 0.51 |
| ZM-0/1-2 | 634.79 ± 152.73 b | 0.06 | 0.73 |
| Samples | Weight Loss at 25–100 °C (%) | Weight Loss at 100–225 °C (%) | Weight Loss at 225–600 °C (%) | Residue at 600 °C (%) |
|---|---|---|---|---|
| ZM-1/0 | 2.85 ± 0.11 b | 4.45 ± 0.08 e | 72.59 ± 0.21 c | 19.88 ± 0.17 a |
| ZM-2/1 | 2.68 ± 0.06 b | 5.05 ± 0.10 d | 74.41 ± 0.19 b | 17.58 ± 0.16 c |
| ZM-1/1 | 3.25 ± 0.08 a | 5.37 ± 0.14 c | 74.15 ± 0.20 b | 16.83 ± 0.18 d |
| ZM-1/2 | 2.36 ± 0.12 c | 6.17 ± 0.19 b | 74.41 ± 0.24 b | 16.84 ± 0.19 d |
| ZM-0/1-1 | 2.13 ± 0.10 d | 6.40 ± 0.17 b | 74.46 ± 0.18 b | 16.79 ± 0.21 d |
| ZM-0/1-2 | 0.87 ± 0.13 e | 7.70 ± 0.15 a | 81.96 ± 0.26 a | 18.04 ± 0.19 b |
| Samples | Secondary Structures (%) | ||
|---|---|---|---|
| β-Sheet | α-Helix | β-Turn | |
| ZM-1/0 | 42.87 ± 0.15 c | 33.55 ± 0.35 d | 23.58 ± 0.45 a |
| ZM-2/1 | 43.84 ± 0.15 b | 33.87 ± 0.41 d | 22.29 ± 0.46 b |
| ZM-1/1 | 41.32 ± 0.29 d | 34.93 ± 0.52 c | 21.49 ± 0.32 c |
| ZM-1/2 | 41.29 ± 0.47 d | 35.37 ± 0.46 c | 21.58 ± 0.23 c |
| ZM-0/1-1 | 48.48 ± 0.39 a | 37.14 ± 0.40 b | 11.15 ± 0.37 e |
| ZM-0/1-2 | 40.07 ± 0.38 e | 39.83 ± 0.35 a | 17.38 ± 0.41 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, P.; Liu, S.; Wang, R.; Li, Y.; Wang, X.; Ren, F.; Luo, J.; Fang, B. Enhancement of Mechanical Properties of Zein-Based Nanofibers by Incorporation of Millet Gliadin. Foods 2024, 13, 2900. https://doi.org/10.3390/foods13182900
Wang S, Wang P, Liu S, Wang R, Li Y, Wang X, Ren F, Luo J, Fang B. Enhancement of Mechanical Properties of Zein-Based Nanofibers by Incorporation of Millet Gliadin. Foods. 2024; 13(18):2900. https://doi.org/10.3390/foods13182900
Chicago/Turabian StyleWang, Shumin, Pengjie Wang, Siyuan Liu, Ran Wang, Yixuan Li, Xiaoyu Wang, Fazheng Ren, Jie Luo, and Bing Fang. 2024. "Enhancement of Mechanical Properties of Zein-Based Nanofibers by Incorporation of Millet Gliadin" Foods 13, no. 18: 2900. https://doi.org/10.3390/foods13182900
APA StyleWang, S., Wang, P., Liu, S., Wang, R., Li, Y., Wang, X., Ren, F., Luo, J., & Fang, B. (2024). Enhancement of Mechanical Properties of Zein-Based Nanofibers by Incorporation of Millet Gliadin. Foods, 13(18), 2900. https://doi.org/10.3390/foods13182900







