Analytical Investigation of Phthalates and Heavy Metals in Edible Ice from Vending Machines Connected to the Italian Water Supply
Abstract
1. Introduction
1.1. Phthalates (PAE) in Edible Ice from Vending Machines
1.2. Heavy Metals
2. Materials and Methods
2.1. Ice and Water Samples
2.2. Determination of PAEs Contamination in Ice Samples
2.2.1. Chemicals
2.2.2. SPME-GC/MS Experimental Conditions
2.3. Metals in Ice Samples
2.3.1. Chemicals and Standard Solution
2.3.2. Analysis in ICP-MS
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caggiano, G.; Marcotrigiano, V.; Trerotoli, P.; Diella, G.; Rutigliano, S.; Apollonio, F.; Marzella, A.; Triggiano, F.; Gramegna, M.; Lagravinese, D.; et al. Food hygiene surveillance in Italy: Is food ice a public health risk? Int. J. Environ. Res. Public Health 2020, 17, 2408. [Google Scholar] [CrossRef] [PubMed]
- Triggiano, F.; Apollonio, F.; Diella, G.; Marcotrigiano, V.; Caggiano, G. State of the art in hygienic quality of food Ice worldwide: A ten-year review. Microorganisms 2024, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Dzodzomenyo, M.; Dotse-Gborgbortsi, W.; Lapworth, D.; Wardrop, N.; Wright, J. Geographic distribution of registered packaged water production in Ghana: Implications for piped supplies, groundwater management and product transportation. Water 2017, 9, 142. [Google Scholar] [CrossRef]
- Kavaarpuo, G.; Wulifan, J.K. Extended producer responsibility—Potentials for managing plastic waste in Ghana. J. Sustain. Dev. Afr. 2013, 15, 29–39. [Google Scholar]
- Angnunavuri, P.N.; Attiogbe, F.; Mensah, B. Particulate plastics in drinking water and potential human health effects: Current knowledge for management of freshwater plastic materials in Africa. Environ. Pollut. 2023, 316, 120714. [Google Scholar] [CrossRef]
- Diduch, M.; Polkowska, Ż.; Namieśnik, J. Factors affecting the quality of bottled water. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 111–119. [Google Scholar] [CrossRef][Green Version]
- Martínez-Romo, A.; González-Mota, R.; Soto-Bernal, J.J.; Rosales-Candelas, I. Investigating the degradability of HDPE, LDPE, PE-BIO, and PE-OXO films under UV-B radiation. J. Spectrosc. 2015, 10, 586514. [Google Scholar] [CrossRef]
- Welle, F.; Franz, R. Microplastic in bottled natural mineral water—Literature review and considerations on exposure and risk assessment. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 2482–2492. [Google Scholar] [CrossRef]
- Dada, E.O.; Ikeh, R.K. Phthalate and metal concentrations in drinking water in Lagos, Nigeria. J. Health Pollut. 2018, 8, 180603. [Google Scholar] [CrossRef]
- Ibeto, C.; Ezeh, H.; Ugwu, I.; Aju, E. Impact of storage on levels of phthalates in sachet and bottled water brands in Enugu State, Nigeria. Int. J. Environ. Anal. Chem. 2022, 104, 1404–1416. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and mechanisms of phthalates’ action on neurological processes and neural health: A literature review. Pharmacol. Rep. 2021, 73, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Liu, B.; Bao, W. Phthalates and attributable mortality: A population-based longitudinal cohort study and cost analysis. Environ. Pollut. 2022, 292, 118021. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mat. 2017, 340, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Edjere, O.; Asibor, I.G.; Bassey, U. Distribution of phthalate esters in underground water from power transmission sitesin warri metropolis, Delta State, Nigeria. J. Appl. Sci. Environ. Manag. 2016, 20, 599–605. [Google Scholar]
- Wang, Y.; Qian, H. Phthalates and their impacts on human health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- North, M.L.; Takaro, T.K.; Diamond, M.L.; Ellis, A.K. Effects of phthalates on the development and expression of allergic disease and asthma. Ann. Allergy Asthma Immunol. 2014, 112, 496–502. [Google Scholar] [CrossRef]
- Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Comm. 2006, OJ L 396, 1–516.
- European Commission. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20140410 (accessed on 1 January 2023).
- Tumu, K.; Vorst, K.; Curtzwiler, G. Endocrine modulating chemicals in food packaging: A review of phthalates and bisphenols. Compr. Rev. Food Sci. Food. Saf. 2023, 2, 1337–1359. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Gómez-Regalado, M.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef]
- Celeiro, M.; Lamas, P.J.; Garcia-Jares, C.; Llompart, M. Pressurized liquid extraction-gas chromatography-mass spectrometry analysis of fragrance allergens, musks, phthalates and preservatives in baby wipes. J. Chrom. A 2015, 1384, 9–21. [Google Scholar] [CrossRef]
- European Commission Recomandation (EU) 2019/794: On a coordinated control plan with a view to establishing the prevalence of certain substances migrating from materials articles intended to come into contact with food. Off. J. Eur. Union. 2019, 129, 37–42.
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Koki, I.B.; Bayero, A.S.; Umar, A.; Yusuf, S. Health risk assessment of heavy metals in water, air, soil and fish. Afr. J. Pure Appl. Chem. 2015, 9, 204–210. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Colak, E.H.; Yomralioglu, T.; Nisanci, R.; Yildirim, V.; Duran, C. Geostatistical analysis of the relationship between heavy metals in drinking water and cancer incidence in residential areas in the Black Sea region of Turkey. J. Environ. Health 2015, 77, 86–93. [Google Scholar]
- Fan, Q.; He, J.; Xue, H.; Lü, C.; Sun, Y.; Shen, L.; Liang, Y.; Bai, S. Heavy metal pollution in the Baotou section of the Yellow River, China. Chem. Speciat. Bioavailab. 2008, 20, 65–76. [Google Scholar] [CrossRef]
- Gupta, S.; Nayek, S.; Saha, R.N.; Satpati, S. Assessment of heavy metal accumulation in macrophyte, agricultural soil, and crop plants adjacent to discharge zone of sponge iron factory. Environ. Geol. 2008, 55, 731–739. [Google Scholar] [CrossRef]
- Wu, Q.; Tam, N.F.Y.; Leung, J.Y.S.; Zhou, X.; Fu, J.; Yao, B.; Huang, X.; Xia, L. Ecological risk and pollution history of heavy metals in Nansha mangrove, South China. Ecotox. Environ. Saf. 2014, 104, 143–151. [Google Scholar] [CrossRef]
- Amodio, M.; De Gennaro, G.; Di Gilio, A.; Tutino, M. Monitoring of the deposition of PAHs and metals produced by a steel plant in Taranto (Italy). Adv. Meteorol. 2014, 2014, 598301. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Rehman, K.; Fiza, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184 (accessed on 23 February 2023).
- Seo, C.; Lee, J.W.; Jeong, J.W.; Kim, T.S.; Lee, Y.; Gang, G.; Lee, S.G. Current technologies for heavy metal removal from food and environmental resources. Food Sci. Biotechnol. 2024, 33, 287–295. [Google Scholar] [CrossRef] [PubMed]
- De Vietro, N.; Aresta, A.M.; Gubitosa, J.; Rizzi, V.; Zambonin, C. Assessing the Conformity of Plasticizer-Free Polymers for Foodstuff Packaging Using Solid Phase Microextraction Coupled to Gas Chromatography/Mass Spectrometry. Separations 2024, 11, 25. [Google Scholar] [CrossRef]
- Aresta, A.M.; De Vietro, N.; Mancini, G.; Zambonin, C. Effect of a chitosan-based packaging material on the domestic storage of “ready-to-cook” meat products: Evaluation of biogenic amines production, phthalates migration, and in vitro antimicrobic activity’s impact on Aspergillus Niger. Separations 2024, 11, 159. [Google Scholar] [CrossRef]
- Chianese, E.; Tirimberio, G.; Appolloni, L.; Dinoi, A.; Contini, D.; Di Gilio, A.; Palmisani, J.; Cotugno, P.; MIniero, D.V.; Cammino, G.; et al. Chemical characterization of PM10 from ship emissions: A study on samples from hydrofoil exhaust stacks. Environ. Scie. Pollut. Res. 2022, 29, 17723–17736. [Google Scholar] [CrossRef]
- Reyes, J.M.; Price, P.S. An analysis of cumulative risks based on biomonitoring data for six phthalates using the Maximum Cumulative Ratio. Environ. Int. 2018, 112, 77–84. [Google Scholar] [CrossRef]
- Abtahi, M.; Dobaradaran, S.; Torabbeigi, M.; Jorfi, S.; Gholamnia, R.; Koolivand, A.; Darabi, H.; Kavousi, A.; Saeedi, R. Health risk of phthalates in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in Tehran. Iran. Environ. Res. 2019, 173, 469–479. [Google Scholar] [CrossRef]
- Angnunavuri, P.N.; Attiogbe, F.; Mensah, B. Effect of storage on the levels of phthalates in high-density polyethylene (HDPE) film-packaged drinkng water. Sci. Total Environ. 2022, 845, 157347. [Google Scholar] [CrossRef]
- Alhaddad, F.A.; Abu-Dieyeh, M.; Da’ana, D.; Helaleh, M.; Al-Ghouti, M.A. Occurrence and removal characteristics of phthalate esters from bottled drinking water using silver modified roasted date pits. J. Environ. Health Sci. Eng. 2021, 19, 733–751. [Google Scholar] [CrossRef]
- Radfard, M.; Hashemi, H.; Baghapour, M.; Azhdarpoor, A. Prediction of human health risk and disability-adjusted life years induced by heavy metals exposure through drinking water in Fars Province, Iran. Sci. Rep. 2023, 13, 19080. [Google Scholar] [CrossRef]
- Liu, W.; Lun, Y. Determination of Sub-Ppb Level of Phthalates in Water by auto-SPME and GC–MS. Application 5989-7726EN; Agilent Technologies: Santa Clara, CA, USA, 2008. [Google Scholar]
- European Union Council. Decision No 2455/2001/EC establishing the list of priority substances in the field of water policy amending Directive 2000/60/EC Off. J. Eur. Commun. 2001, L 331, 1–5.
- Jeddi, M.; Rastkari, N.; Ahmadkhaniha, R.; Yunesian, M. Concentrations of phthalates in bottled water under common storage conditions: Do they pose a health risk to children? Int. J. Food Sci. 2015, 69, 256–265. [Google Scholar] [CrossRef]
- Sulentic, R.; Dumitrascu, R.; Deziel, N.; Gurzau, A. Phthalate exposure from drinking water in romanian adolescents. Int. J. Environ. Res. Public Health 2018, 15, 2109. [Google Scholar] [CrossRef] [PubMed]
- DECRETO LEGISLATIVO 23 febbraio 2023, n. 18. Attuazione della direttiva (UE) 2020/2184 del Parlamento europeo e del Consiglio, del 16 dicembre 2020, concernente la qualità delle acque destinate al consumo umano (23G00025). Available online: https://www.gazzettaufficiale.it/eli/id/2023/03/06/23G00025/sg (accessed on 23 February 2023).
- Abdo, N.; Alhamid, A.; Abu-Dalo, M.; Graboski-Bauer, A.; Al Harahsheh, M. Potential health risk assessment of mixtures of heavy metals in drinking water. Groundw. Sustain. Dev. 2024, 25, 101147. [Google Scholar] [CrossRef]
- Russo, G.; Laneri, S.; Di Lorenzo, R.; Neri, I.; Dini, I.; Ciampaglia, R.; Grumetto, L. Monitoring of pollutants content in bottled and tap drinking water in Italy. Molecules 2022, 27, 3990. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Misra, A.K.; Ranjan, R.K.; Wanjari, N.; Rajak, R.; Yadav, S.K.; Rai, R.; Khan, A. Assessment of heavy metal and E. coli contamination in water sources of the east and south districts, Sikkim Himalaya, India. Water Conserv. Sci. Eng. 2024, 9, 22. [Google Scholar] [CrossRef]
- Izah, S.C.; Chakrabarty, N.; Srivastav, A.L. A review on heavy metal concentration in potable water sources in Nigeria: Human health effects and mitigating measures. Expo. Health 2016, 8, 285–304. [Google Scholar] [CrossRef]
- Khanniri, E.; Esmaeili, S.; Akbari, M.E.; Molaee-aghaee, E.; Sohrabvandi, S.; Akbari, N.; Sadighara, P.; Aslani, R. Determination of heavy metals in municipal water network of Tehran, Iran: A health risk assessment with a focus on carcinogenicity. Int. J. Cancer Manag. 2023, 16, e137240. [Google Scholar] [CrossRef]
- Tracy, J.W.; Guo, A.; Liang, K.; Bartram, J.; Fisher, M. Sources of and solutions to toxic metal and metalloid contamination in small rural drinking water systems: A rapid review. Int. J. Environ. Res. Public Health 2020, 17, 7076. [Google Scholar] [CrossRef]
- Gonzalez, S.; Lopez-Roldan, R.; Cortina, J.-L. Presence of metals in drinking water distribution networks due to pipe material leaching: A review. Toxicol. Environ. Chem. 2013, 95, 870–889. [Google Scholar] [CrossRef]
- Stern, B.R.; Lagos, G. Are there health risks from the migration of chemical substances from plastic pipes into drinking water? A review. Hum. Ecol. Risk Assess. 2008, 14, 753–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.P. Leaching of lead from new unplasticized polyvinyl chloride (uPVC) pipes into drinking water. Environ. Sci. Pollut. Res. 2015, 22, 8405–8411. [Google Scholar] [CrossRef] [PubMed]
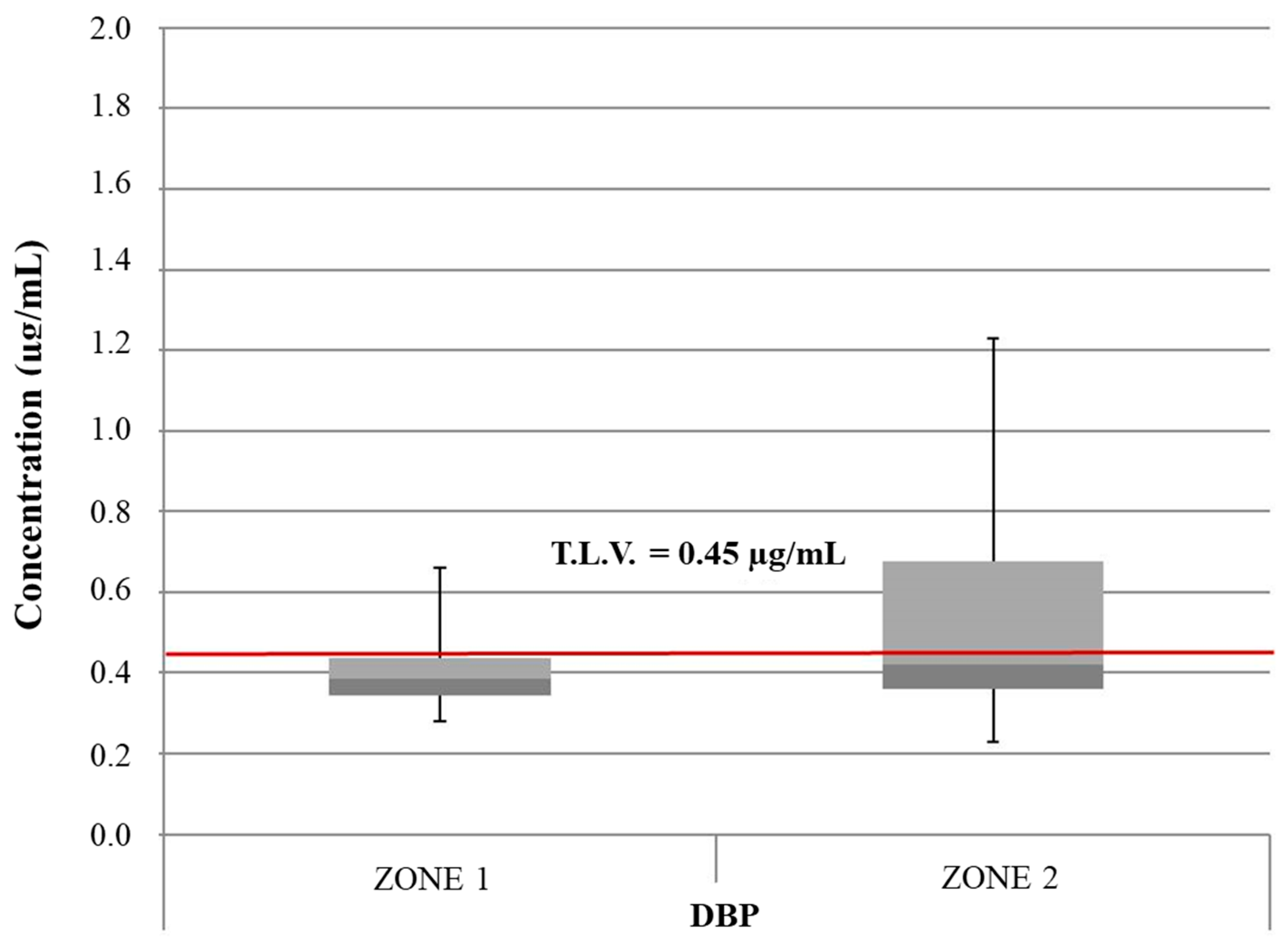
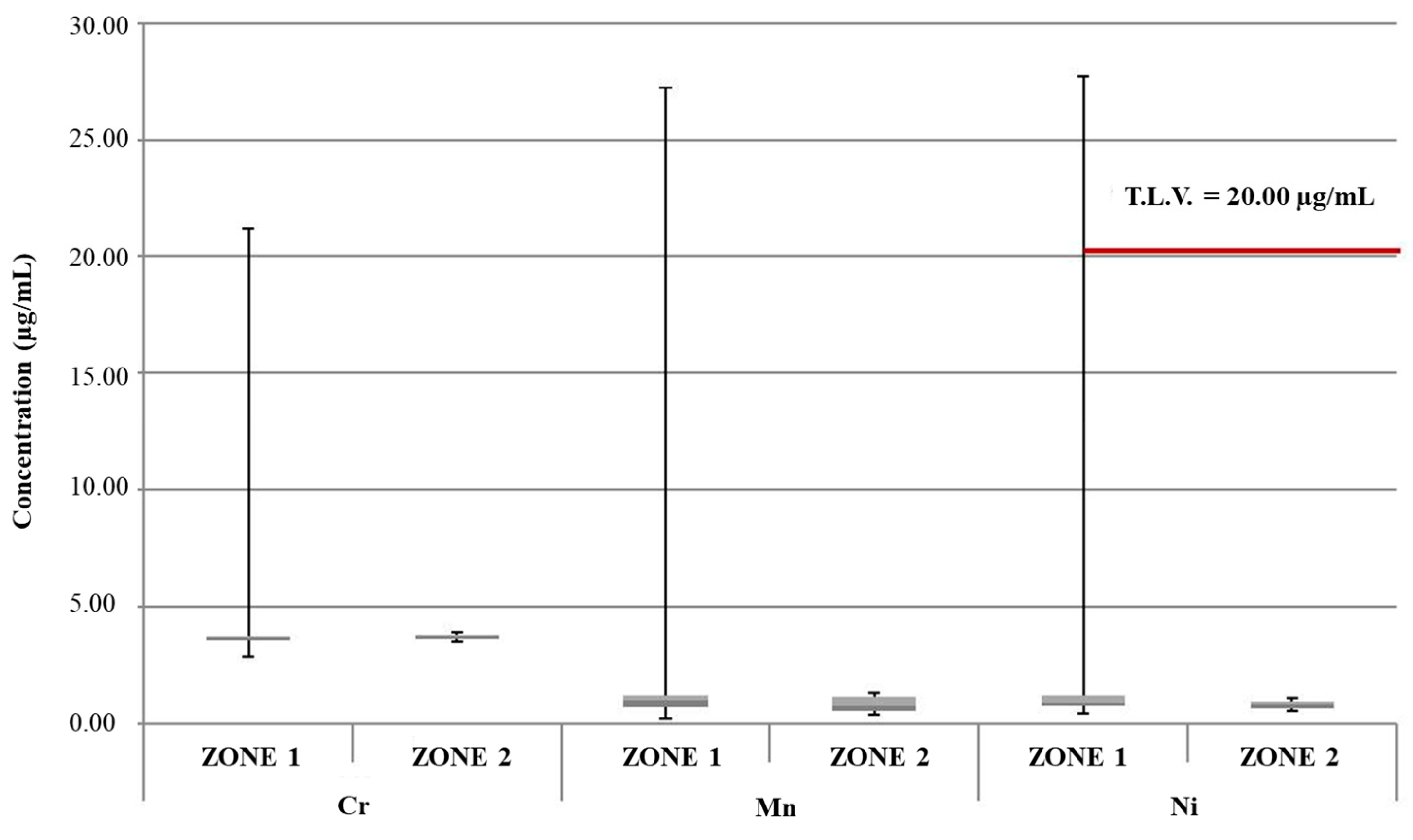
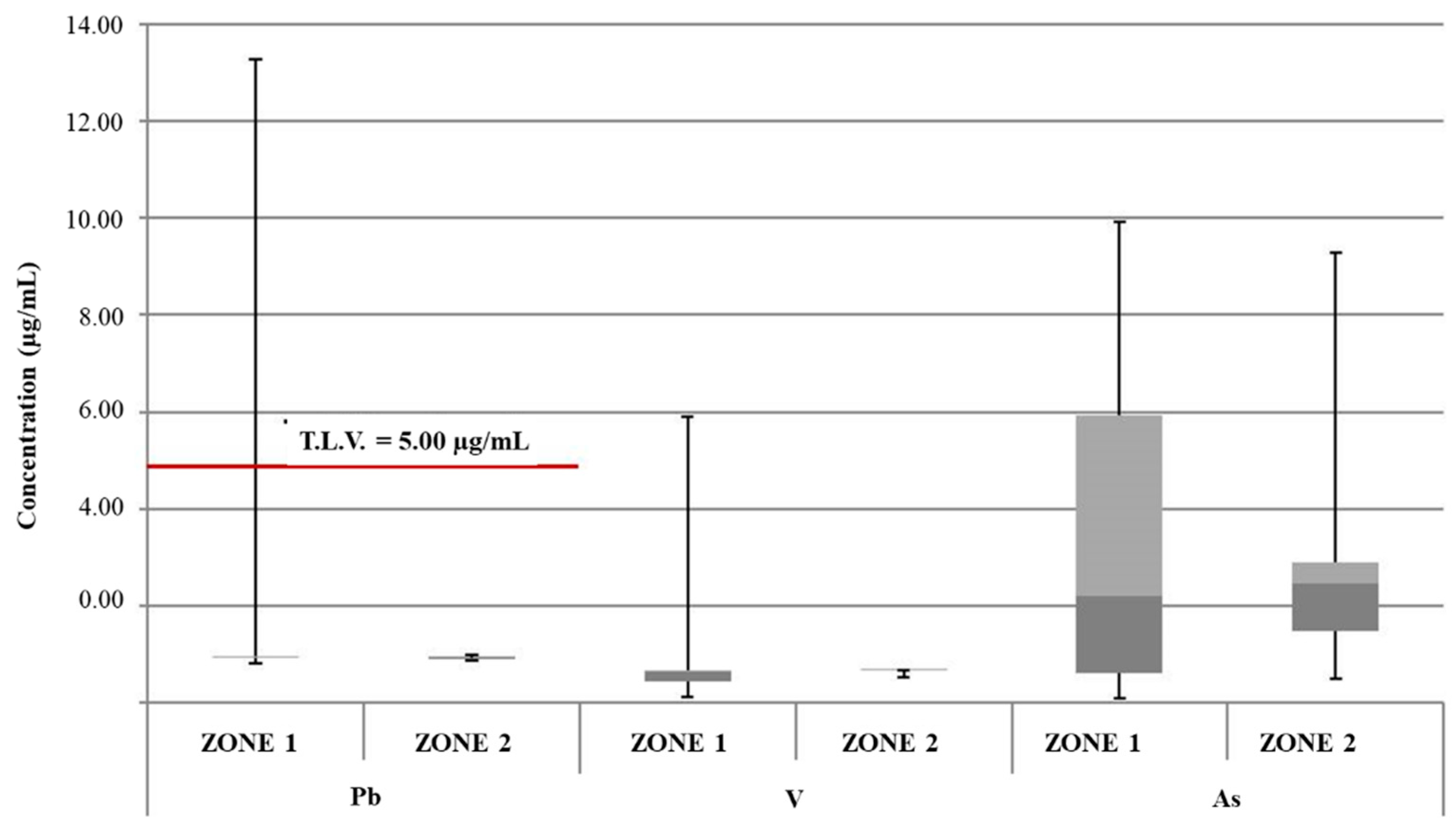

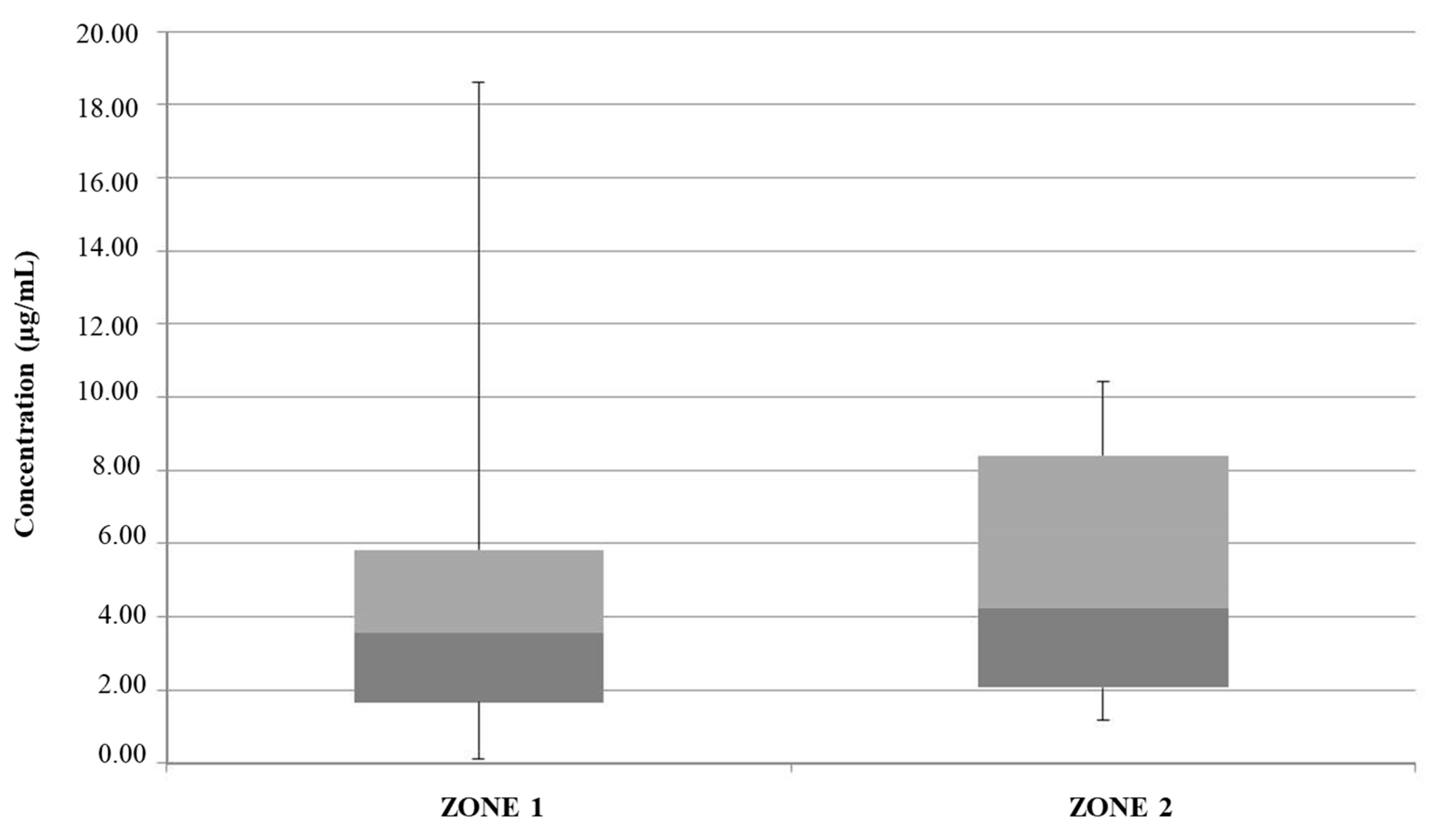

| Mean Value Range (μg/L) | [49] | [47] | [50] | [48] | [46] | Study Zone 1 | Study Zone 2 |
|---|---|---|---|---|---|---|---|
| Al | / | / | / | 44.99 (11.40–90.79) | / | 4.31 (0.12–18.60) | 5.13 (1.19–10.43) |
| V | / | / | / | / | / | 0.92 (0.12–5.90) | 0.66 (0.53–0.68) |
| Cr | (0.65–0.85) | 0.34 (0.15–0.55) | 25.0 (ND–78.0) | 0.63 (0.06–2.45) | 2.9 (0.1–19.8) | 4.11 (2.86–21.15) | 3.69 (3.50–3.87) |
| Mn | (0.51–0.80) | / | / | 23.77 (0.79–217.46) | 2.8 (ND–29.3) | 2.90 (0.21–27.23) | 0.86 (0.39–1.33) |
| Fe | (0.42–0.51) | / | 28.0 (3.0–5.06) | 58.74 (5.08–133.45) | 156.2 (ND–4072) | 25.67 (21.21–128.16) | 22.47 (21.76–23.59) |
| Co | (0.34–0.44) | / | / | / | 0.1 (ND–1.2) | 0.48 (0.03–0.85) | 0.53 (0.51–0.53) |
| Ni | (0.29–0.46) | 1.02 (0.19–2.44) | 0.2 (ND–1.9) | 1.07 (0.11–2.73) | 16.8 (0.2–517.3) | 4.05 (0.45–27.70) | 0.81 (0.52–1.11) |
| Cu | (0.23–0.26) | / | 0.1 (ND–1.7) | 5.48 (0.71–25.85) | 43.5 (0.4–584) | 8.49 (0.59–73.01) | 1.75 (1.56–2.00) |
| Zn | (0.19–0.24) | / | 13.0 (0.4–70.2) | 241.22 (34.01–988.25) | 244.8 (3.7–1849) | 30.18 (2.44–291.89) | 6.13 (5.25–7.55) |
| As | / | 1.05 (0.54–1.58) | 0.8 (0.4–1.1) | 0.68 (ND–5.86) | 0.5 (ND–3.3) | 3.37 (0.11–9.93) | 2.74 (0.49–9.28) |
| Se | / | / | / | / | / | 0.35 (0.18–1.19) | 0.38 (0.36–0.44) |
| Cd | / | 0.92 (0.26–1.58) | 1.0 (0.8–4.8) | / | 0.1 (ND–0.7) | 0.55 (0.49–0.94) | 0.50 (0.48–0.50) |
| Pb | (0.31–0.39) | 0.48 (0.26–0.58) | 2.4 (0.9–9.4) | / | 4.8 (ND–73.5) | 1.26 (0.82–13.26) | 0.93 (0.90–0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vietro, N.; Triggiano, F.; Cotugno, P.; Palmisani, J.; Di Gilio, A.; Zambonin, C.; de Gennaro, G.; Mancini, G.; Aresta, A.M.; Diella, G.; et al. Analytical Investigation of Phthalates and Heavy Metals in Edible Ice from Vending Machines Connected to the Italian Water Supply. Foods 2024, 13, 2910. https://doi.org/10.3390/foods13182910
De Vietro N, Triggiano F, Cotugno P, Palmisani J, Di Gilio A, Zambonin C, de Gennaro G, Mancini G, Aresta AM, Diella G, et al. Analytical Investigation of Phthalates and Heavy Metals in Edible Ice from Vending Machines Connected to the Italian Water Supply. Foods. 2024; 13(18):2910. https://doi.org/10.3390/foods13182910
Chicago/Turabian StyleDe Vietro, Nicoletta, Francesco Triggiano, Pietro Cotugno, Jolanda Palmisani, Alessia Di Gilio, Carlo Zambonin, Gianluigi de Gennaro, Giovanna Mancini, Antonella Maria Aresta, Giusy Diella, and et al. 2024. "Analytical Investigation of Phthalates and Heavy Metals in Edible Ice from Vending Machines Connected to the Italian Water Supply" Foods 13, no. 18: 2910. https://doi.org/10.3390/foods13182910
APA StyleDe Vietro, N., Triggiano, F., Cotugno, P., Palmisani, J., Di Gilio, A., Zambonin, C., de Gennaro, G., Mancini, G., Aresta, A. M., Diella, G., Marcotrigiano, V., Sorrenti, G. T., Marzocca, P., Lampedecchia, M., Sorrenti, D. P., D’Aniello, E., Gramegna, M., Nencha, A., Caputo, A., ... Caggiano, G. (2024). Analytical Investigation of Phthalates and Heavy Metals in Edible Ice from Vending Machines Connected to the Italian Water Supply. Foods, 13(18), 2910. https://doi.org/10.3390/foods13182910














