Cookies Fortified with Clitoria ternatea Butterfly Pea Flower Petals: Antioxidant Capacity, Nutritional Composition, and Sensory Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Preparation of Cookies
2.3. Chemical Composition
2.4. Antioxidative Potential Analysis
2.5. Lipid Peroxide Value Analysis
2.6. Microbiological Identification
2.7. Sensory Evaluations
2.8. Colour Measurement
2.9. Statistical Analysis
3. Results and Discussion
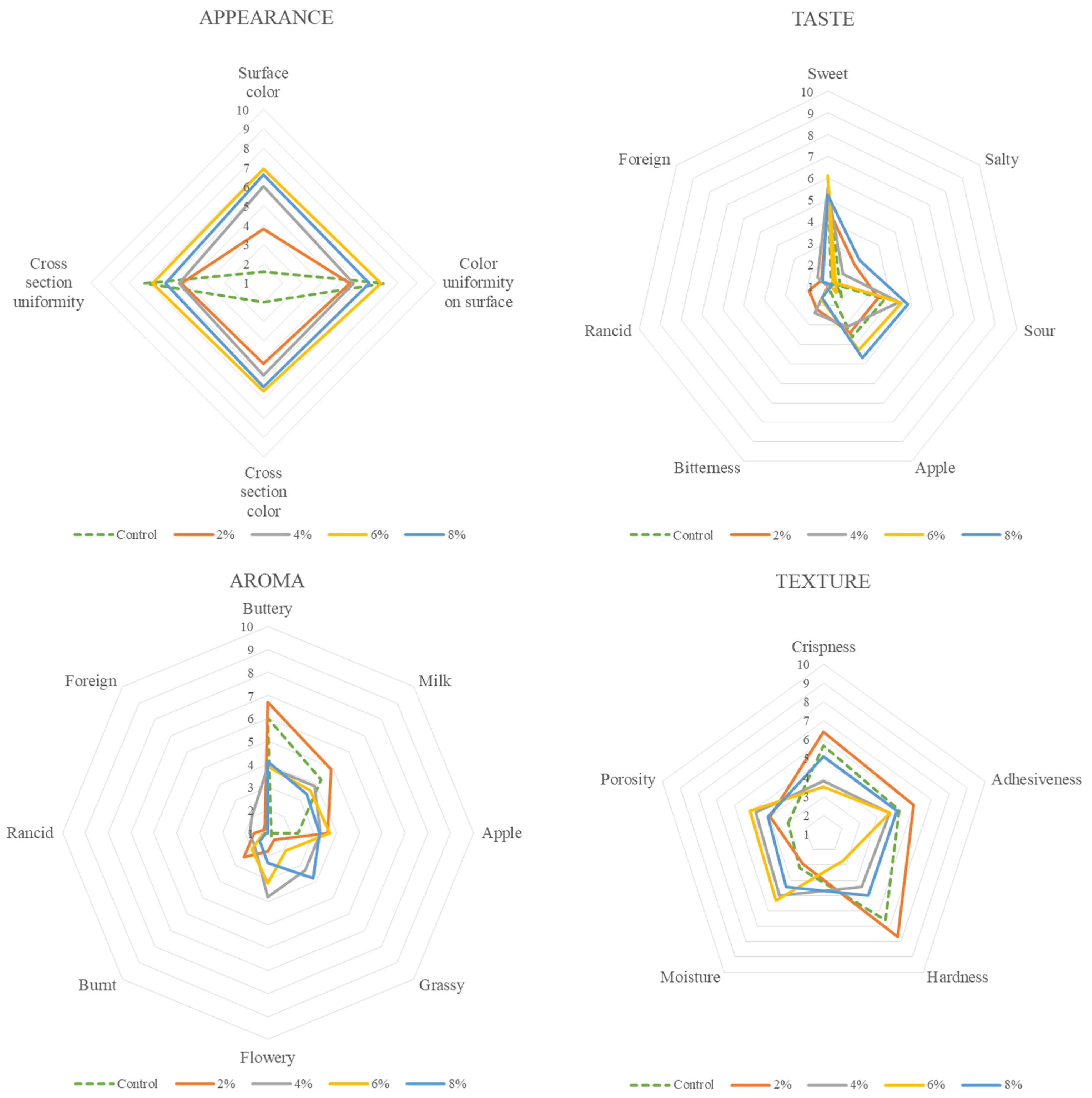
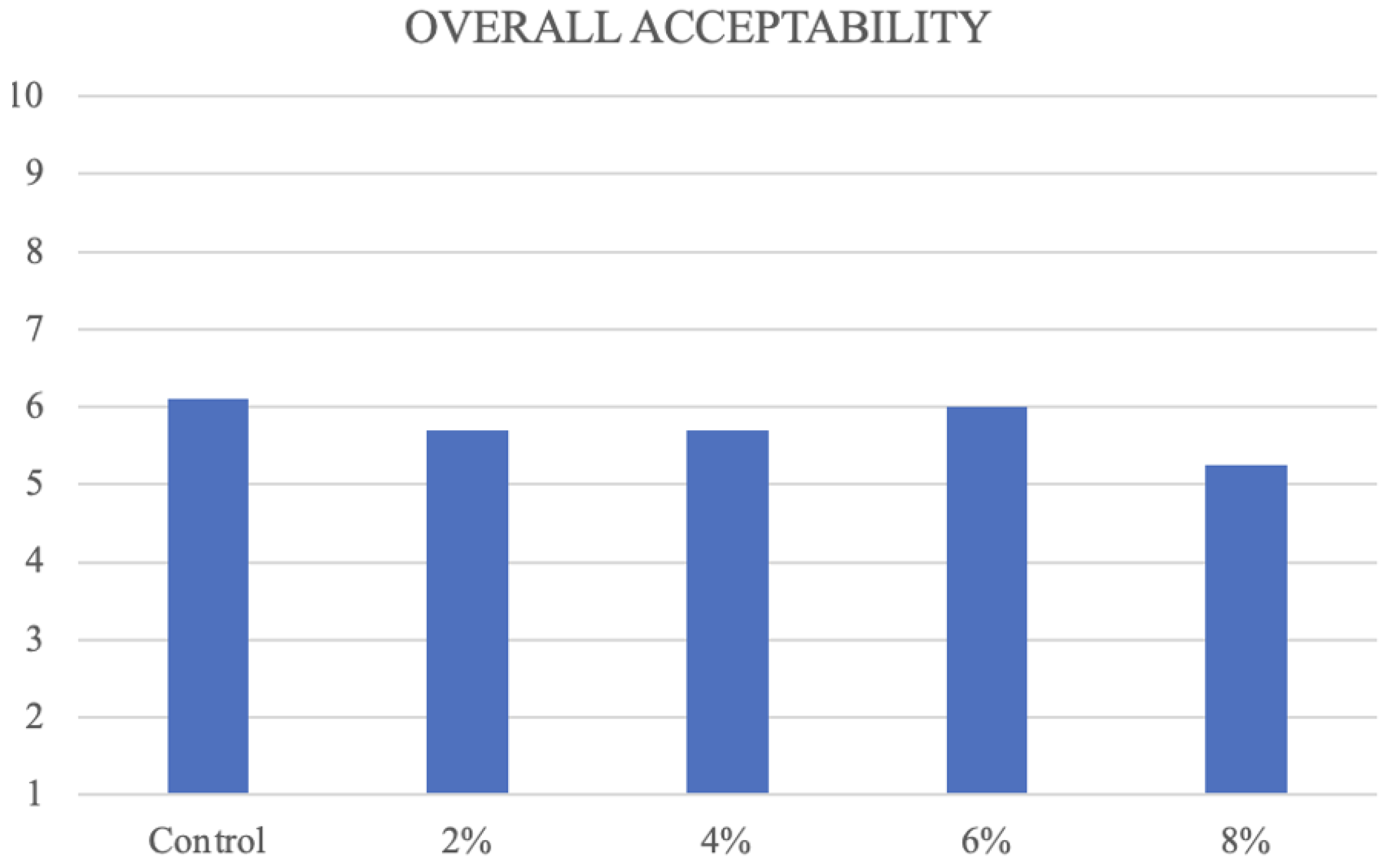
3.1. Sensory Profiling of Fresh Samples
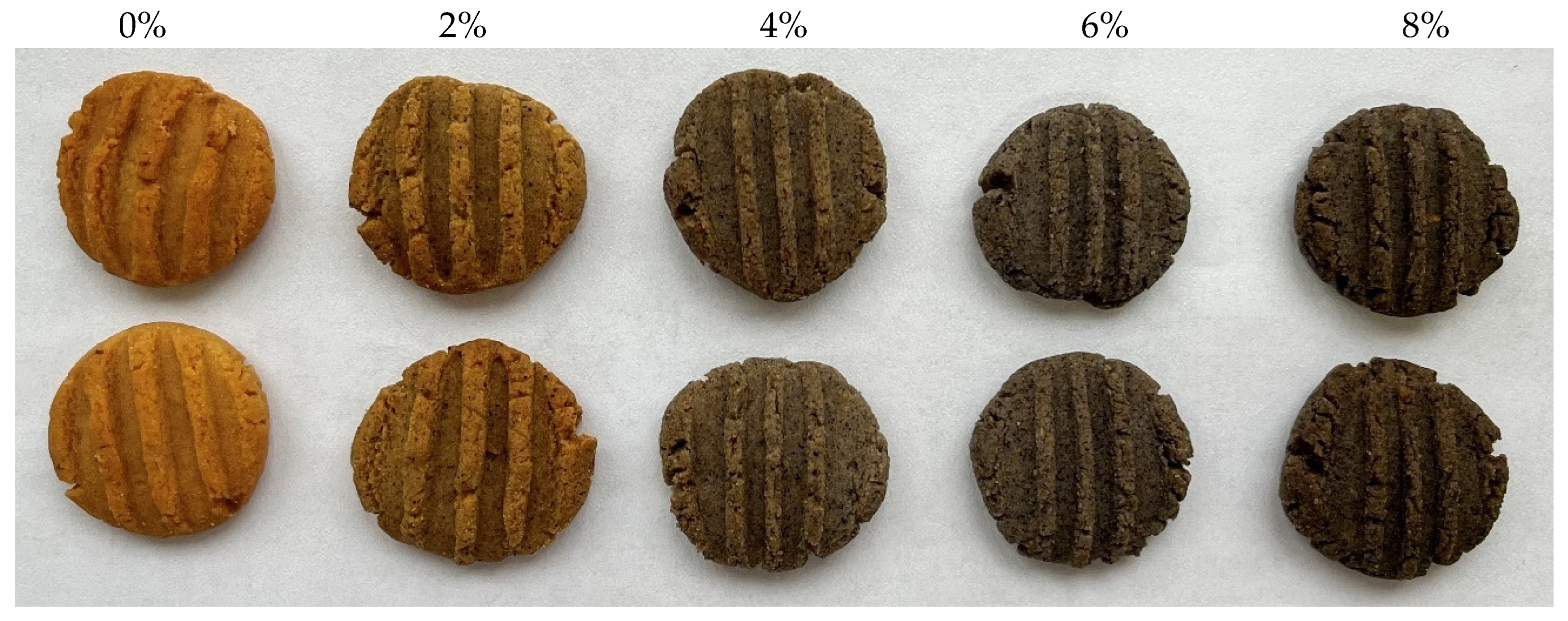
3.2. Colour Measurement
3.3. Chemical Composition
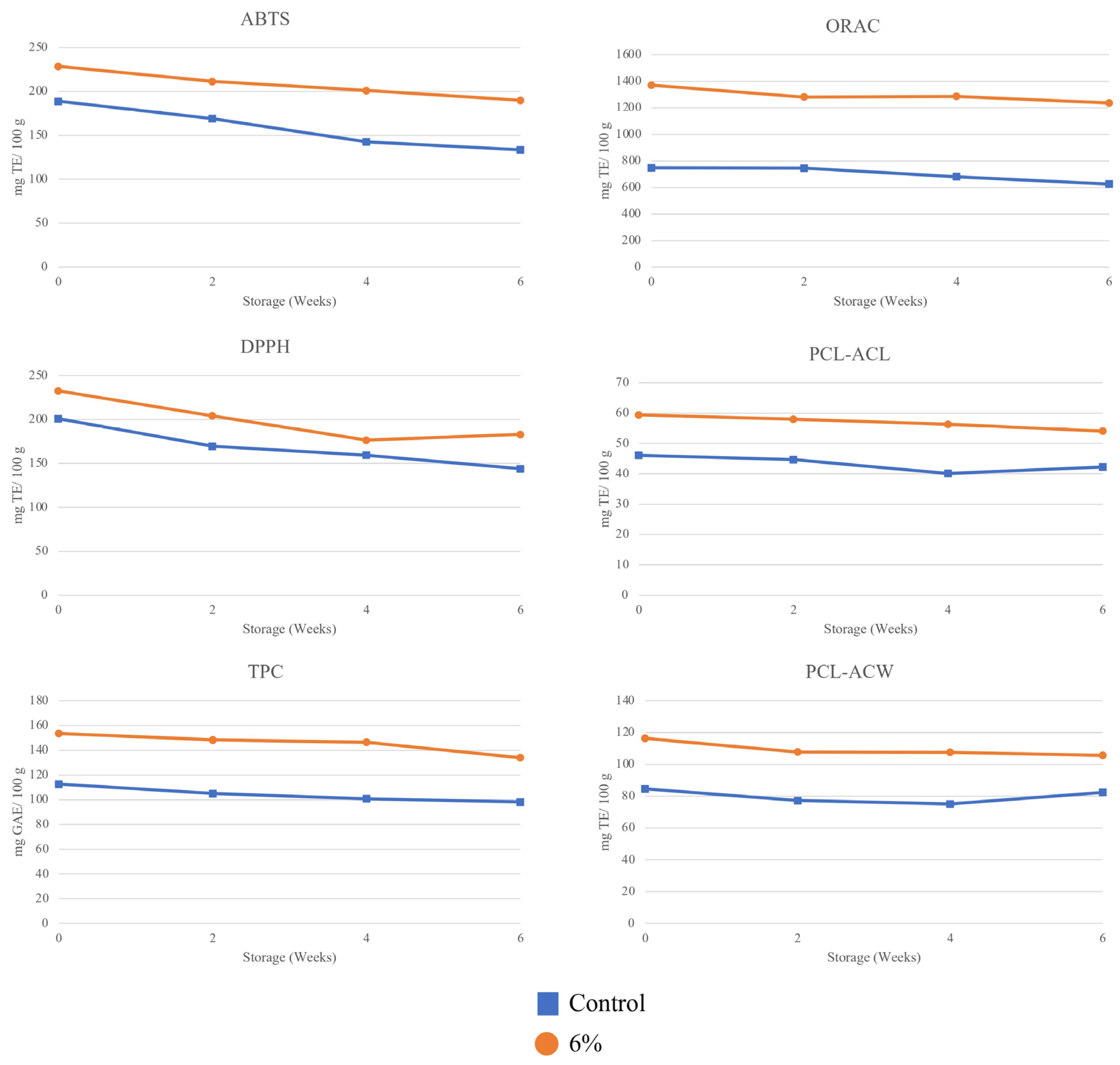
3.4. Total Phenolic Content and Antioxidative Potential
3.5. Lipid Peroxide Value Analysis
3.6. Microbiological Identification
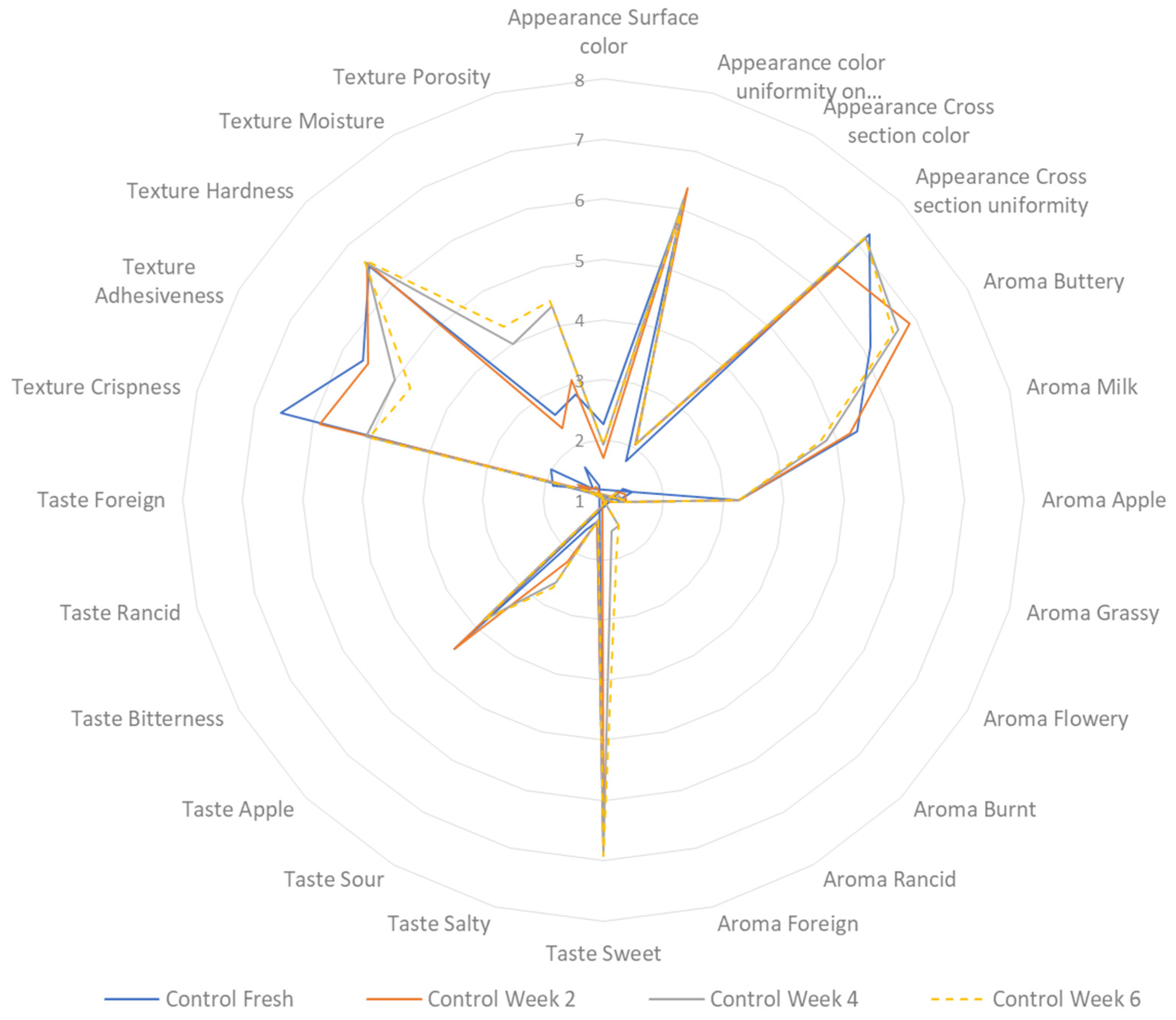
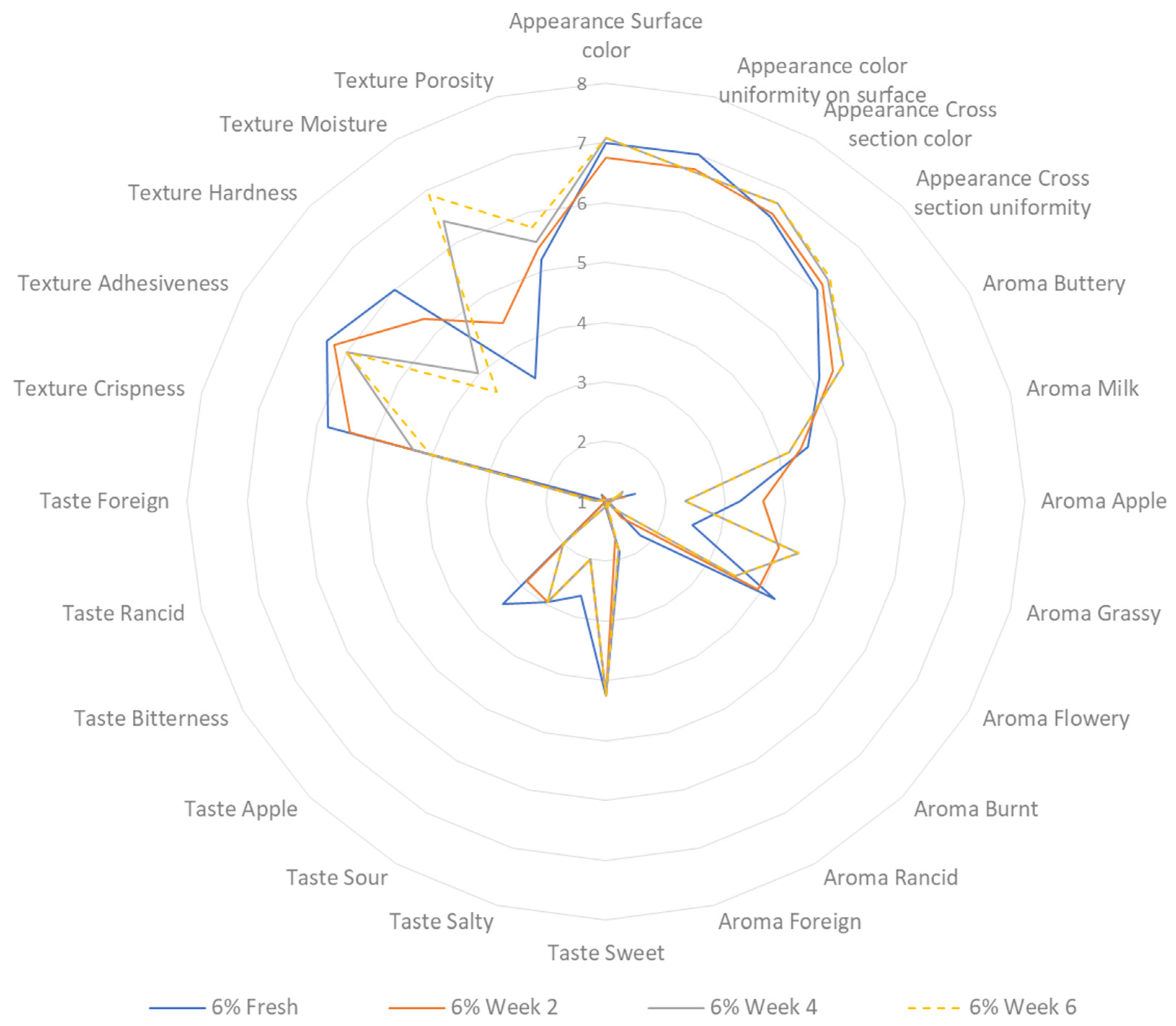
3.7. Sensory Profiling during Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Górecka, D. Antioxidative Potential, Nutritional Value and Sensory Profiles of Confectionery Fortified with Green and Yellow Tea Leaves (Camellia Sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A.; Suliburska, J.; Sidor, A. Puerarin—An Isoflavone with Beneficial Effects on Bone Health. Front. Biosci. -Landmark 2021, 26, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Rajagukguk, Y.V.; Arnold, M.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Schmidt, M.; Gramza-Michałowska, A. Antioxidant Activity, Probiotic Survivability, and Sensory Properties of a Phenolic-Rich Pulse Snack Bar Enriched with Lactiplantibacillus Plantarum. Foods 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rajagukguk, Y.V.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Suliburska, J.; Wawrzyniak, N.; Gramza-Michałowska, A. Innovative Application of Chicken Eggshell Calcium to Improve the Functional Value of Gingerbread. Int. J. Environ. Res. Public Health 2022, 19, 4195. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Concepts and Strategy of Functional Food Science: The European Perspective. Am. J. Clin. Nutr. 2000, 71, 1660S–1664S. [Google Scholar] [CrossRef]
- Machado, T.A.D.G.; Pacheco, M.T.B.; do Egypto Queiroga, R.d.C.R.; Cavalcante, L.M.; Bezerril, F.F.; de Cássia Salvucci Celeste Ormenese, R.; de Oliveira Garcia, A.; Nabeshima, E.H.; Pintado, M.M.E.; de Oliveira, M.E.G. Nutritional, Physicochemical and Sensorial Acceptance of Functional Cookies Enriched with Xiquexique (Pilosocereus Gounellei) Flour. PLoS ONE 2021, 16, 1–20. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Antioxidant Potential of Phytochemicals in Pumpkin Varieties Belonging to Cucurbita Moschata and Cucurbita Pepo Species. CyTA-J. Food 2020, 18, 472–484. [Google Scholar] [CrossRef]
- Hnin, K.K.; Zhang, M.; Wang, B. Development of Nutritional Properties in Cookies with the Incorporation of Different Levels of Rose Flower Powder by Microwave-Vacuum Drying. Dry. Technol. 2021, 39, 1136–1148. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Pharmacological Importance of Clitoria Ternatea–A Review. IOSR J. Pharm. 2016, 6, 68–83. [Google Scholar]
- Manjula, P.; Mohan, C.H.; Sreekanth, D.; Keerthi, B.; Devi, B.P. Phytochemicalanalysis of Clitoria Ternatea Linn., a Valuable Medicinal Plant. J. Indian. Bot. 2013, 92, 173–178. [Google Scholar]
- Havananda, T.; Luengwilai, K. Variation in Floral Antioxidant Activities and Phytochemical Properties among Butterfly Pea (Clitoria Ternatea L.) Germplasm. Genet. Resour. Crop Evol. 2019, 66, 645–658. [Google Scholar] [CrossRef]
- Multisona, R.R.; Shirodkar, S.; Arnold, M.; Gramza-Michalowska, A. Clitoria Ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods. Appl. Sci. 2023, 13, 2134. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective Role of Ternatin Anthocyanins and Quercetin Glycosides from Butterfly Pea (Clitoria Ternatea Leguminosae) Blue Flower Petals against Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef]
- Oguis, G.K.; Gilding, E.K.; Jackson, M.A.; Craik, D.J. Butterfly Pea (Clitoria Ternatea), a Cyclotide-Bearing Plant with Applications in Agriculture and Medicine. Front. Plant Sci. 2019, 10, 645. [Google Scholar] [CrossRef]
- Shirodkar, S.M.; Multisona, R.R.; Gramza-Michalowska, A. The Potential for the Implementation of Pea Flower (Clitoria Ternatea) Health Properties in Food Matrix. Appl. Sci. 2023, 13, 7141. [Google Scholar] [CrossRef]
- Neda, G.D.; Rabeta, M.S.; Ong, M.T. Chemical Composition and Anti-Proliferative Properties of Flowers of Clitoria ternatea. Int. Food Res. J. 2013, 20, 1229–1234. [Google Scholar]
- Lauková, M.; Kohajdová, Z.; Karovičová, J. Effect of Hydrated Apple Powder on Dough Rheology and Cookies Quality. Potravinarstvo 2016, 10, 506. [Google Scholar] [CrossRef]
- Socaci, S.A.; Farcas, A.C.; Vodnar, D.C.; Tofana, M. Food Wastes as Valuable Sources of Bioactive Molecules. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; InTech: Rijeka, Croatia, 2017; pp. 75–93. [Google Scholar]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of Apple By-Products as Source of New Ingredients: Current Situation and Perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Jukić, M.; Komlenić, D.K.; Lukinac, J. Influence of Apple Peel Powder Addition on the Physico-Chemical Characteristics and Nutritional Quality of Bread Wheat Cookies. Food Sci. Technol. Int. 2020, 26, 574–582. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T. Microencapsulates and Extracts from Red Beetroot Pomace Modify Antioxidant Capacity, Heat Damage and Colour of Pseudocereals-Enriched Einkorn Water Biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef]
- PN-EN ISO 3947; ISO Starches, Native or Modified—Determination Of Total Fat Content. Polish Committee for Standardization: Warszawa, Poland, 2001.
- AOAC. Official Methods of Analysis: Official Method for Protein; Method No. 920.87; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- AOAC. Official Methods of Analysis: Official Method for Ash; Method No. 936.03; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Dziedzic, K.; Górecka, D.; Kucharska, M.; Przybylska, B. Influence of Technological Process during Buckwheat Groats Production on Dietary Fibre Content and Sorption of Bile Acids. Food Res. Int. 2012, 47, 279–283. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Methods of Analysis and Quantification of Phenolic Compounds. In Food Phenolic: Sources, Chemistry, Effects and Applications; Technomic Publishing Company: Lancaster; PA, USA, 1995; pp. 287–293. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the Assessment of Antioxidant Activity in Foods; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782420972. [Google Scholar]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Korczak, J. Oxygen Radical Absorbance Capacity of Selected Food Products. Acta Sci. Pol. Technol. Aliment. 2013, 12, 175–180. [Google Scholar]
- Besco, E.; Braccioli, E.; Vertuani, S.; Ziosi, P.; Brazzo, F.; Bruni, R.; Sacchetti, G.; Manfredini, S. The Use of Photochemiluminescence for the Measurement of the Integral Antioxidant Capacity of Baobab Products. Food Chem. 2007, 102, 1352–1356. [Google Scholar] [CrossRef]
- PN ISO 3960:2010; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. Polish Committee for Standardization: Warszawa, Poland, 2010.
- PN-EN ISO 6887-1:2017-05; Microbiology of the Food Chain. Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination. Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. Polish Committee for Standardization: Warszawa, Poland, 2017.
- PN-EN ISO 21257-1:2009; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Polish Committee for Standardization: Warszawa, Poland, 2009.
- PN-ISO 21528-2:2017; Microbiology of the Food Chain. Horizontal Method for the Detection and Enumeration of Enterobacteriaceae Part 2: Colony-Count Technique. Polish Committee for Standardization: Warszawa, Poland, 2017.
- PN ISO 6888-2:2001/A1:2004; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species). Part 1: Method Using Baird-Parker Agar Medium. Polish Committee for Standardization: Warszawa, Poland, 2004.
- PN-EN ISO 4833-1:2013; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms. Polish Committee for Standardization: Warszawa, Poland, 2013.
- PN-EN ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Part 1: Detection of Salmonella spp. Polish Committee for Standardization: Warszawa, Poland, 2017.
- Bugaud, C.; Maraval, I.; Daribo, M.O.; Leclerc, N.; Salmon, F. Optimal and Acceptable Levels of Sweetness, Sourness, Firmness, Mealiness and Banana Aroma in Dessert Banana (Musa sp.). Sci. Hortic. 2016, 211, 399–409. [Google Scholar] [CrossRef]
- Liew, S.Y.; Mohd Zin, Z.; Mohd Maidin, N.M.; Mamat, H.; Zainol, M.K. Effect of the Different Encapsulation Methods on the Physicochemical and Biological Properties of Clitoria Ternatea Flowers Microencapsulated in Gelatine. Food Res. 2020, 4, 1098–1108. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Pumalee, T.; Sanguansuk, N.; Chumyen, C.; Wongvasu, P.; Adisakwattana, S.; Ngamukote, S. Physicochemical, Antioxidant and Sensory Characteristics of Sponge Cakes Fortified with Clitoria Ternatea Extract. J. Food Sci. Technol. 2018, 55, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ma, J.; Cheng, K.-W.; Jiang, Y.; Chen, F.; Wang, M. The Effects of Grape Seed Extract Fortification on the Antioxidant Activity and Quality Attributes of Bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Oxidative Stability of Cooked Pork Patties Incorporated with Clitoria Ternatea Extract (Blue Pea Flower Petal) During Refrigerated Storage. J. Food Process Preserv. 2017, 41, e12751. [Google Scholar] [CrossRef]
- Khumkhom, S. Effect of additional dried sesbania (Sesbania Javanica Miq.) flowers powder on physical, nutritional and organoleptic characteristics of butter cookies. Res. J. Phranakhon Rajabhat Sci. Technol. 2018, 13, 139–154. [Google Scholar]
- Thanh, V.T.; Tran, N.Y.T.; Linh, N.T.V.; Vy, T.A.; Truc, T.T. Application of Anthocyanin Natural Colors from Butterfly Pea (Clitoria ternatea L.) Extracts to Cupcake. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, England, 2020; Volume 736. [Google Scholar]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and Functional Characteristics of Dietary Fibre in Beans, Lentils, Peas and Chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Shiau, S.; Yu, Y.; Pan, W.; Li, G. Colorful and Health Improving Chinese Steamed Bread Fortified by Anthocyanin-rich Extract of Butterfly Pea Flower. J. Food Process Preserv. 2022, 46, e16925. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Thakur, R.J.; Shaikh, H.; Gat, Y.; Waghmare, R.B. Effect of Calcium Chloride Extracted from Eggshell in Maintaining Quality of Selected Fresh-Cut Fruits. Int. J. Recycl. Org. Waste Agric. 2019, 8, 27–36. [Google Scholar] [CrossRef]
- Fu, X.; Wu, Q.; Wang, J.; Chen, Y.; Zhu, G.; Zhu, Z. Spectral Characteristic, Storage Stability and Antioxidant Properties of Anthocyanin Extracts from Flowers of Butterfly Pea (Clitoria ternatea L.). Molecules 2021, 26, 7000. [Google Scholar] [CrossRef]
- Abdullakasim, P.; Songchitsomboon, S.; Techagumpuch, M.; Balee, N.; Swatsitang, P.; Sungpuag, P. Antioxidant Capacity, Total Phenolics and Sugar Content of Selected Thai Health Beverages. Int. J. Food Sci. Nutr. 2007, 58, 77–85. [Google Scholar] [CrossRef]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Total Phenolic Content and Total Flavonoid Content in Sweet Chestnut (Castanea Sativa Mill.) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, A.E.; Kusnadi, J. Optimization of Fermentation Time and Grain Concentration for Water Kefir Production from Butterfly Pea Flower (Clitoria Ternatea). In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Philadelphia, PA, USA, 2021; Volume 924. [Google Scholar]
- Gebreselassie, E.; Clifford, H. Oxidative Stability and Shelf Life of Crackers, Cookies, and Biscuits. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Elsevier: Amsterdam, The Netherlands, 2016; pp. 461–478. [Google Scholar]
- Kamkaen, N.; Wilkinson, J.M. The Antioxidant Activity of Clitoria Ternatea Flower Petal Extracts and Eye Gel. Phytother. Res. 2009, 23, 1624–1625. [Google Scholar] [CrossRef] [PubMed]
- Raphaelli, C.O.; Dannenberg, G.; Dalmazo, G.O.; Pereira, E.S.; Radünz, M.; Vizzotto, M.; Fiorentini, A.M.; Gandra, E.A.; Nora, L. Antibacterial and Antioxidant Properties of Phenolic-Rich Extracts from Apple (Malus Domestica Cv. Gala). Int. Food Res. J. 2019, 26, 1133–1142. [Google Scholar]
- Eldesoukey, R.; El-Desoukey, M.; Almuhsin, S.; Almuhsin, A. The Phytochemical and Antimicrobial Effect of Mallus Domestica (Apple) Dried Peel Powder Extracts on Some Animal Pathogens as Eco-Friendly. Int. J. Vet. Sci. 2018, 7, 88–92. [Google Scholar]
- Ab Rashid, S.; Tong, W.Y.; Leong, C.R.; Abdul Ghazali, N.M.; Taher, M.A.; Ahmad, N.; Tan, W.N.; Teo, S.H. Anthocyanin Microcapsule from Clitoria Ternatea: Potential Bio-Preservative and Blue Colorant for Baked Food Products. Arab. J. Sci. Eng. 2021, 46, 65–72. [Google Scholar] [CrossRef]
- Michalska, A.; Amigo-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of Bread Making on Formation of Maillard Reaction Products Contributing to the Overall Antioxidant Activity of Rye Bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Purlis, E. Browning Development in Bakery Products—A Review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
| Ingredients [g] | Control Sample | Dried CT Flower Powder Concentration | |||
|---|---|---|---|---|---|
| 2% | 4% | 6% | 8% | ||
| Wheat flour | 38.00 | 37.24 | 36.48 | 35.72 | 34.96 |
| Apple peel powder | 12.00 | 11.76 | 11.52 | 11.28 | 11.04 |
| CT flower powder | 0.00 | 1.00 | 2.00 | 3.00 | 4.00 |
| Coconut oil | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Butter | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Sweetened condensed milk | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Salt | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Total | 100.50 | 100.50 | 100.50 | 100.50 | 100.50 |
| Treatment | L* | a* | b* | E |
|---|---|---|---|---|
| Control | 46.12 ± 1.35 a | 15.35 ± 0.30 a | 32.08 ± 0.93 a | 58.24 ± 1.65 a |
| 2% | 32.61 ± 0.57 bc | 10.74 ± 0.11 b | 22.31 ± 0.40 b | 40.94 ± 0.64 b |
| 4% | 34.79 ± 1.06 b | 1.99 ± 0.14 c | 12.61 ± 0.30 c | 37.06 ± 1.09 c |
| 6% | 33.50 ± 0.16 b | 0.40 ± 0.05 d | 8.44 ± 0.11 d | 34.55 ± 0.17 c |
| 8% | 30.43 ± 0.47 c | 0.94 ± 0.11 e | 8.87 ± 0.02 d | 31.71 ± 0.45 d |
| Nutrient | Sample | ||
|---|---|---|---|
| 0% | 6% | ||
| Protein | [%] | 8.41 ± 0.31 a | 8.40 ± 0.32 a |
| Lipid | 27.71 ± 0.67 a | 27.46 ± 1.30 a | |
| Carbohydrate | 57.58 ± 0.39 a | 57.29 ± 1.40 a | |
| Ash | 1.39 ± 0.05 a | 1.26 ± 0.07 a | |
| Moisture | 4.91 ± 0.03 a | 5.59 ± 0.07 b | |
| Energy | [kcal/100 g] | 513.38 | 509.90 |
| Dietary Fibre (%) | Sample | |
|---|---|---|
| 0% | 6% | |
| IDF | 8.41 ± 0.44 b | 9.20 ± 0.52 a |
| SDF | 6.40 ± 0.41 b | 9.62 ± 0.49 a |
| TDF | 14.82 ± 0.39 b | 18.82 ± 0.03 a |
| Samples | TPC with | Pearson’s r | Sig. (2-Tailed) |
|---|---|---|---|
| Control | ABTS | 0.915 | p < 0.01 |
| DPPH | 0.935 | p < 0.01 | |
| ORAC | 0.715 | p < 0.01 | |
| PCL-ACL | 0.854 | p < 0.01 | |
| PCL-ACW | 0.472 | p < 0.05 | |
| LPV | −0.863 | p < 0.01 | |
| 6% | ABTS | 0.884 | p < 0.01 |
| DPPH | 0.694 | p < 0.05 | |
| ORAC | 0.790 | p < 0.01 | |
| PCL-ACL | 0.777 | p < 0.05 | |
| PCL-ACW | 0.755 | p < 0.05 | |
| LPV | −0.910 | p < 0.01 |
| Peroxide value (meq O2/kg) | Storage time (week) | Sample | |
| 0% | 6% | ||
| 0 | 20.63 ± 0.15 aB | 18.34 ± 0.02 aA | |
| 2 | 21.64 ± 0.14 abB | 18.52 ± 0.64 aA | |
| 4 | 22.70 ± 0.82 bcB | 19.89 ± 0.01 abA | |
| 6 | 24.28 ± 0.45 cB | 21.55 ± 0.96 bA | |
| Aerobic Mesophilic Microorganisms Count [cfu/g] | Aerobic Psychrophilic Microorganisms Count [cfu/g] | Yeasts and Moulds Count [cfu/g] | Enterobacteriaceae Count [cfu/g] | Presence of Staphylococcus spp. | Presence of Salmonella sp. | |
|---|---|---|---|---|---|---|
| C0 | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 25 g |
| C6 | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 25 g |
| S0 | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 25 g |
| S6 | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 25 g |
| CT | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS approx. 20 g |
| AP | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS 0.1 g | ABS approx. 20 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Multisona, R.R.; Myszka, K.; Kulczyński, B.; Arnold, M.; Brzozowska, A.; Gramza-Michałowska, A. Cookies Fortified with Clitoria ternatea Butterfly Pea Flower Petals: Antioxidant Capacity, Nutritional Composition, and Sensory Profile. Foods 2024, 13, 2924. https://doi.org/10.3390/foods13182924
Multisona RR, Myszka K, Kulczyński B, Arnold M, Brzozowska A, Gramza-Michałowska A. Cookies Fortified with Clitoria ternatea Butterfly Pea Flower Petals: Antioxidant Capacity, Nutritional Composition, and Sensory Profile. Foods. 2024; 13(18):2924. https://doi.org/10.3390/foods13182924
Chicago/Turabian StyleMultisona, Ribi Ramadanti, Kamila Myszka, Bartosz Kulczyński, Marcellus Arnold, Anna Brzozowska, and Anna Gramza-Michałowska. 2024. "Cookies Fortified with Clitoria ternatea Butterfly Pea Flower Petals: Antioxidant Capacity, Nutritional Composition, and Sensory Profile" Foods 13, no. 18: 2924. https://doi.org/10.3390/foods13182924










