Assessment of Acrylamide Levels by Advanced Molecularly Imprinted Polymer-Imprinted Surface Plasmon Resonance (SPR) Sensor Technology and Sensory Quality in Homemade Fried Potatoes
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials, Chemicals, and Apparatus
2.2. SPR Chip Modification with Allyl Mercaptane
2.3. AA-Imprinted SPR Chip Preparation
2.4. AA Removal from MIP/SPR Chips and Analysis Process
2.5. Cooking Condition of Homemade Fried Potatoes and the Procedure of AA Analysis
2.6. Chemical and Physicochemical Parameters of Homemade Fried Potatoes
2.7. Sensory Properties in Homemade Fried Potatoes
2.8. Color Evaluation
3. Results and Discussion
3.1. Chemical Composition of Homemade Fried Potatoes
3.2. Color Attributes of Homemade Fried Potatoes
3.3. Sensory Properties of the Homemade Fried Potatoes
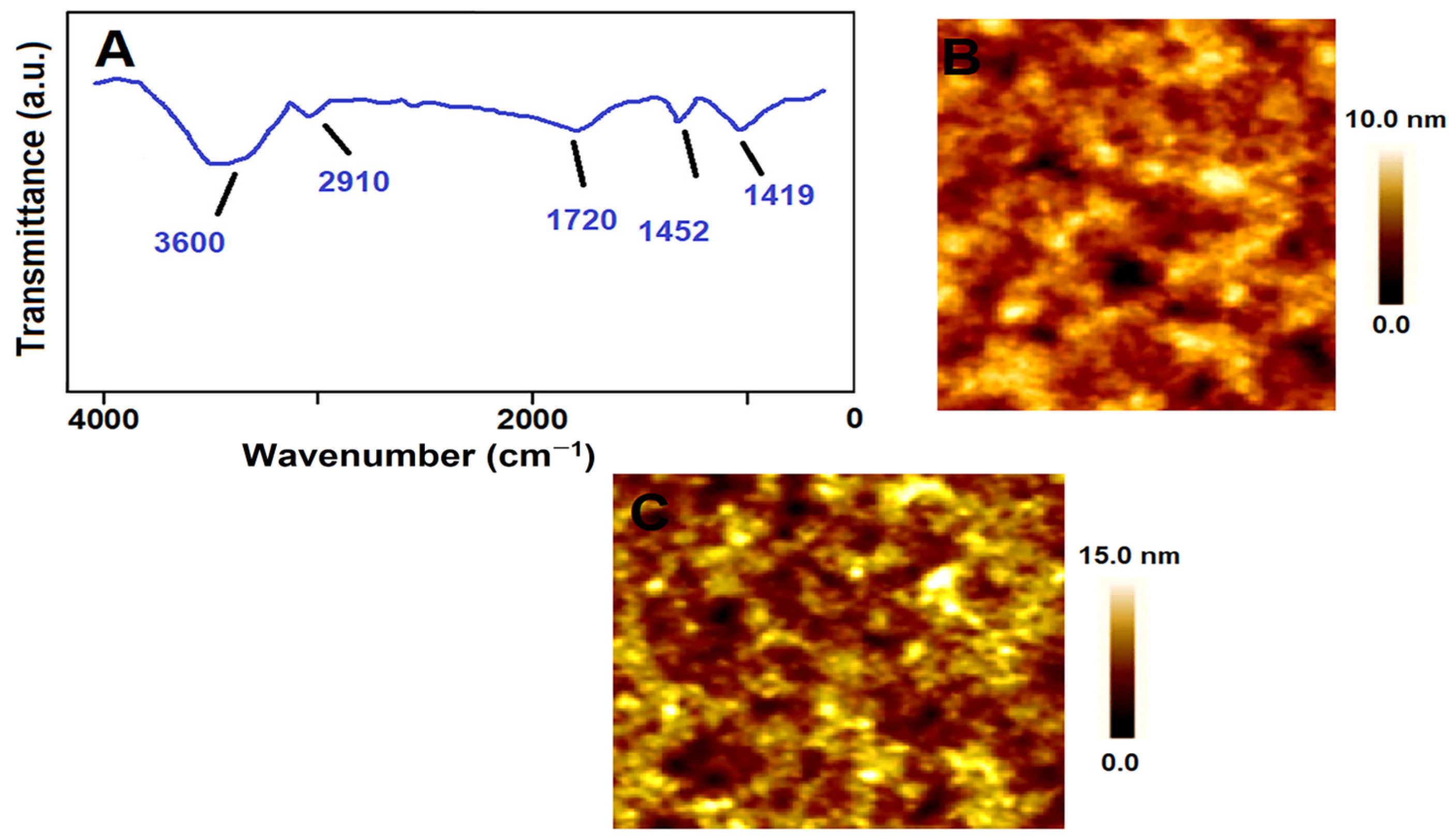
3.4. FTIR, and AFM Characterizations of MIP/SPR
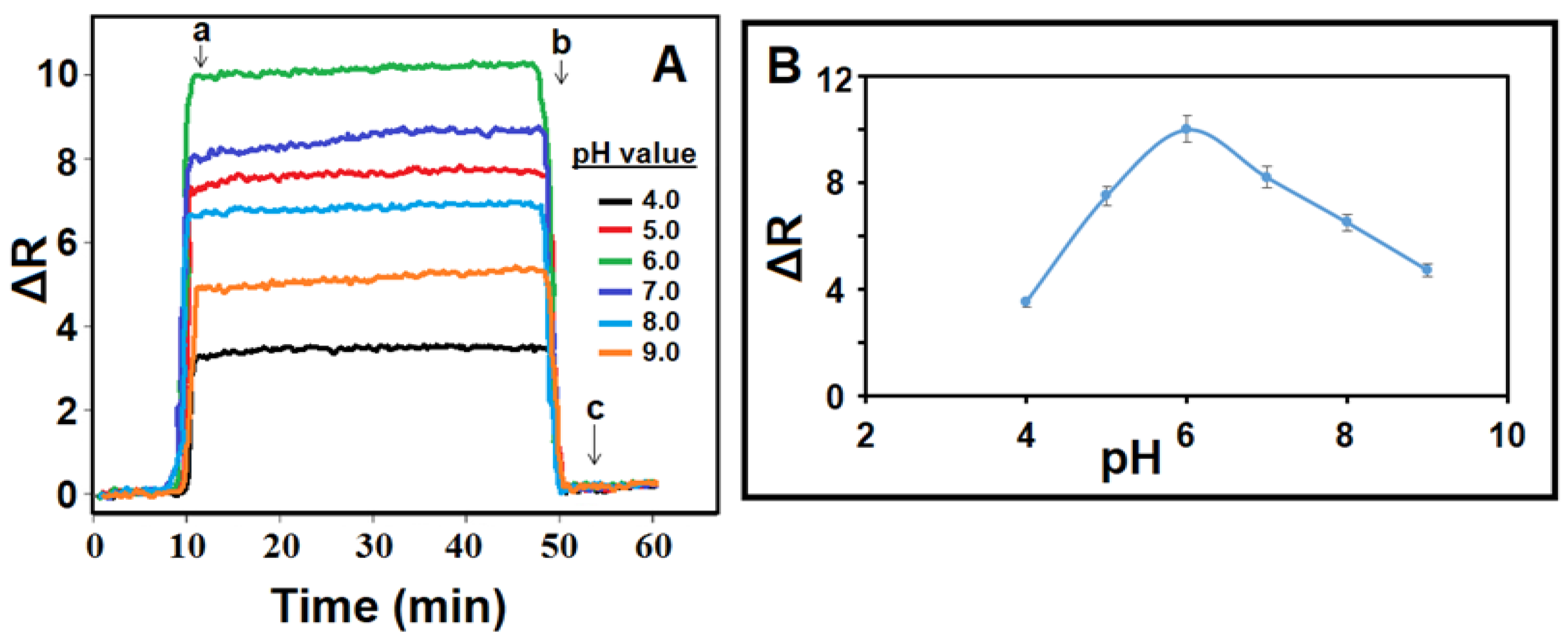
3.5. pH Effect on the MIP/SPR Chip
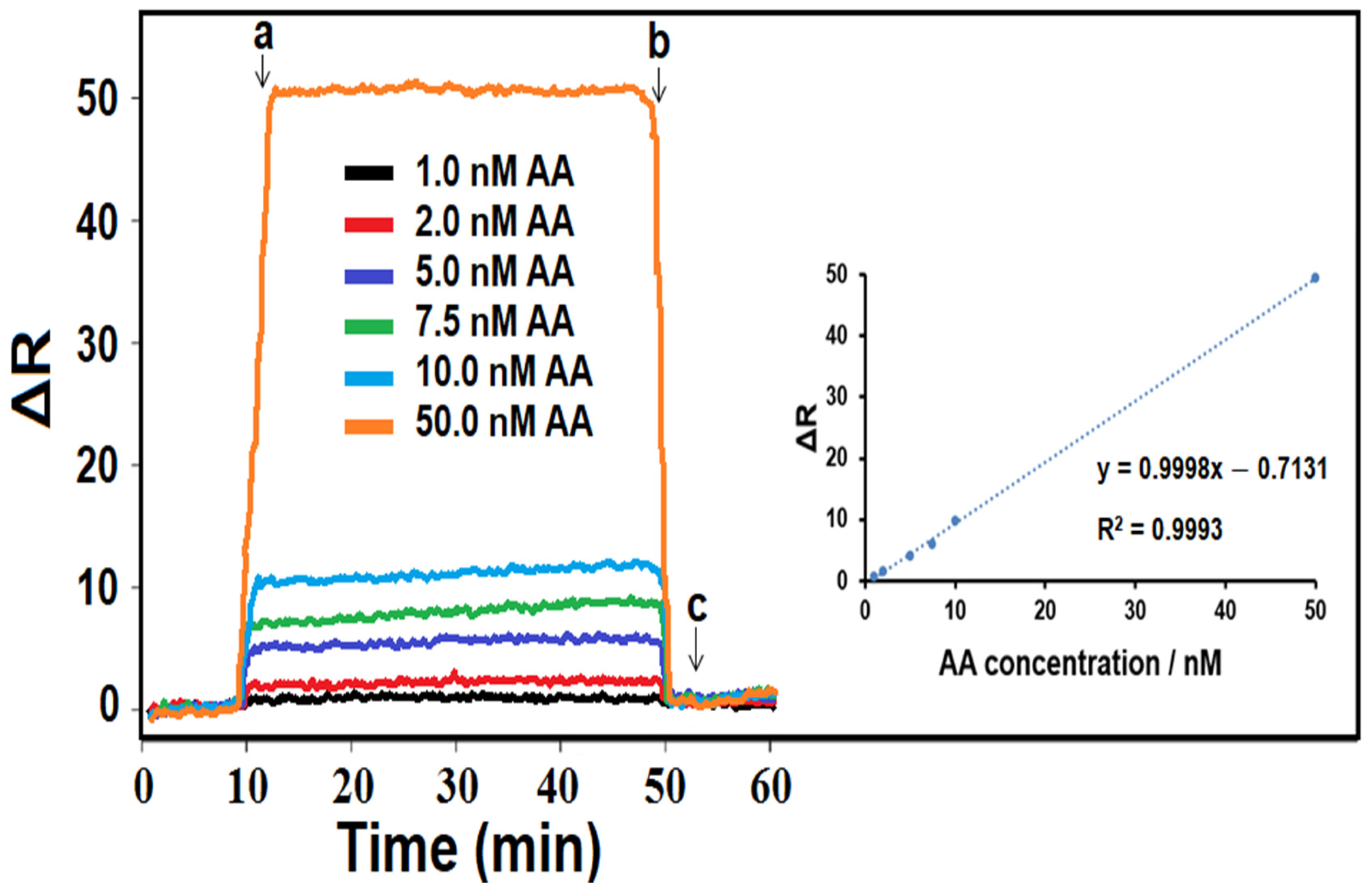
3.6. Linearity Range of the MIP/SPR Chip
3.7. Recovery of MIP/SPR Chip and AA Content
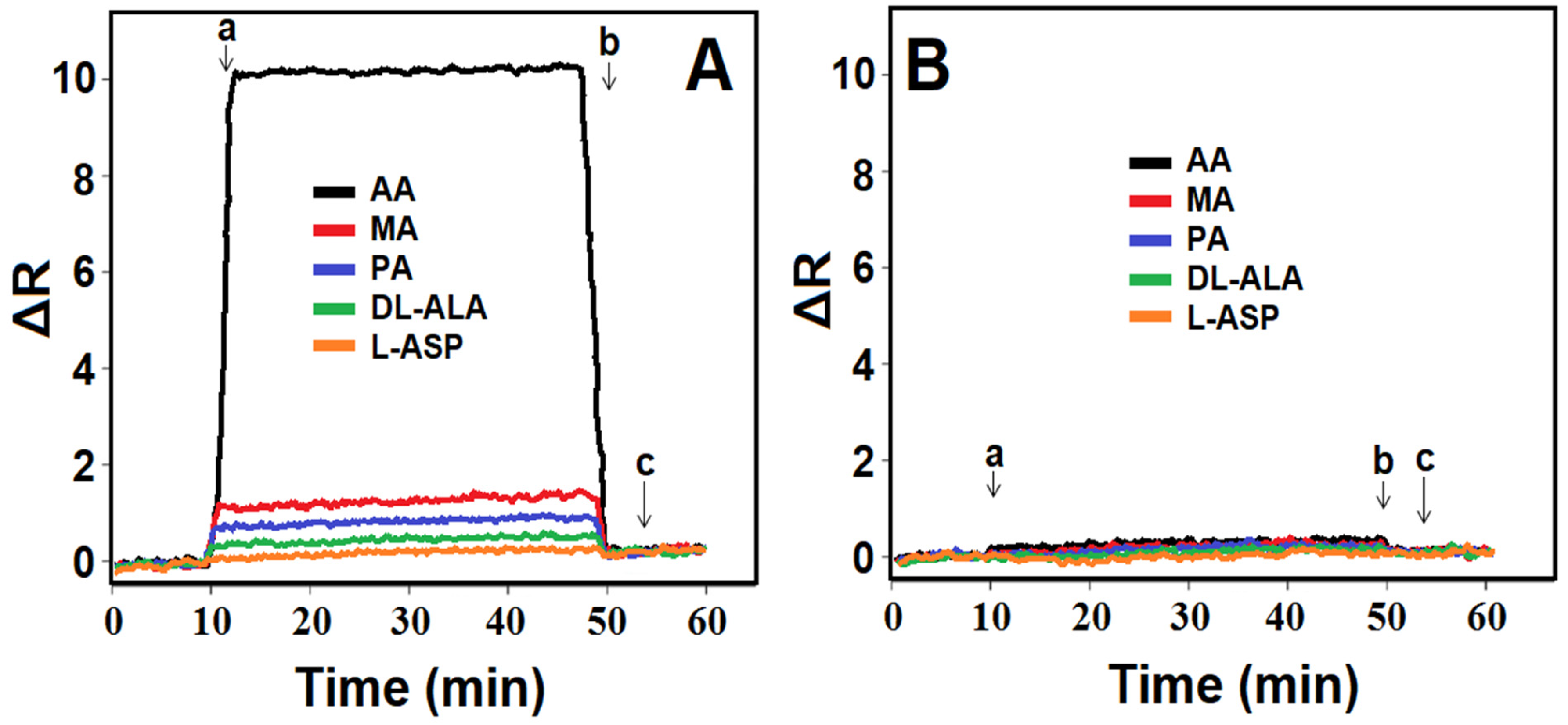
3.8. Selectivity, Repeatability, and Reusability of MIP/SPR Chip
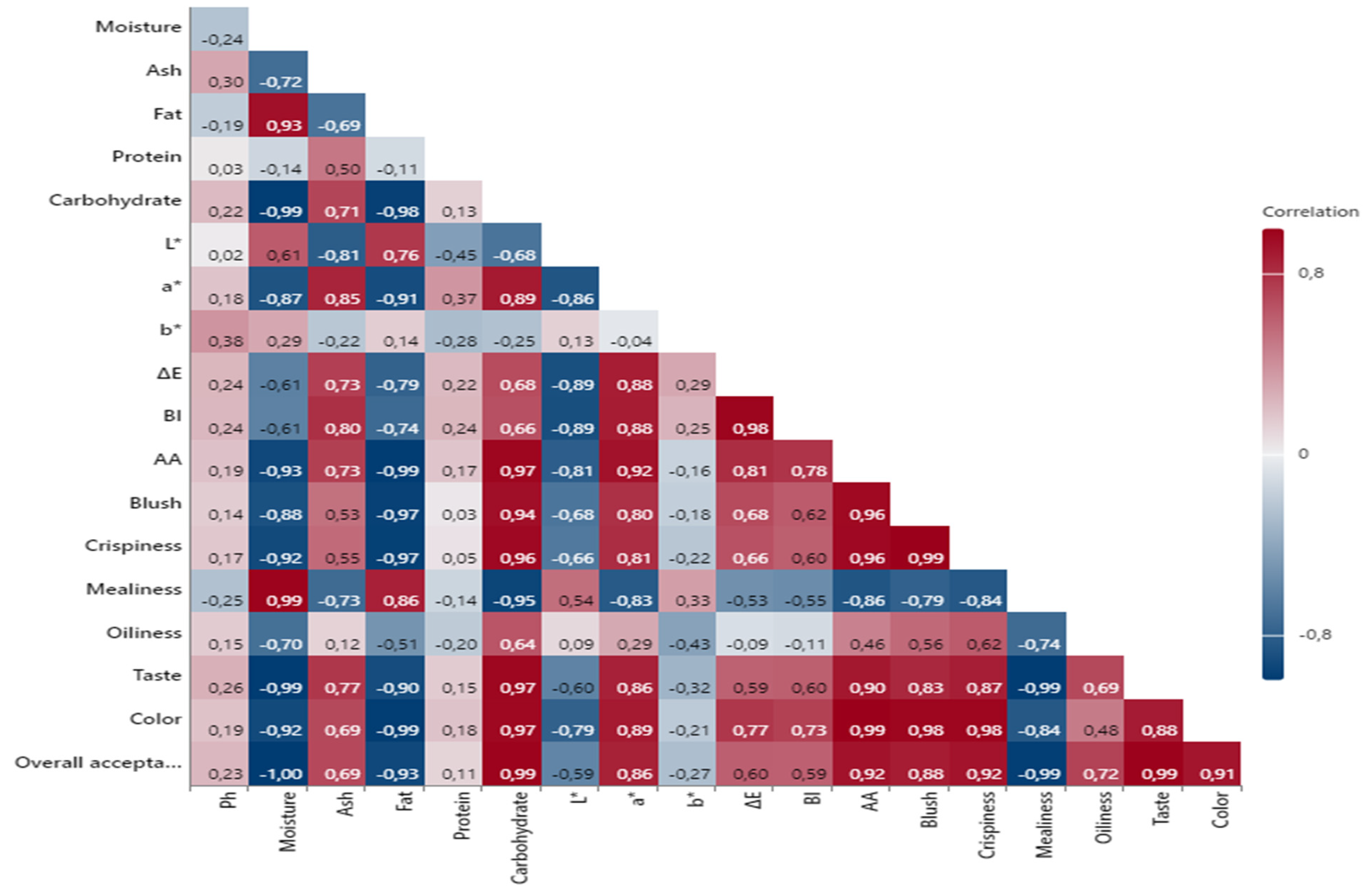
3.9. Pearson Correlation Analysis Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tokucoglu Yumusak, T.; Yilmaz, K.G.; Deligonul, S.Z.; Cavusgil, T. Slow food and the slow food movement: A case study of consumer activism in Turkiye. IJCHM 2024, in press. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Starčević, S.; Marinković, V. Which Attributes Are the Most Important in the Context of the Slow Food Festival? Leis. Sci. 2024, 46, 340–358. [Google Scholar] [CrossRef]
- Petrini, C. Slow Food Nation: Why Our Food Should Be Good, Clean, and Fair; Rizzoli Publications: New York, NY, USA, 2013. [Google Scholar]
- Simonetti, L. The ideology of Slow Food. J. Eur. Stud. 2012, 42, 168–189. [Google Scholar] [CrossRef]
- Sobreira, É.M.C.; Mantovani, D.; Leocádio, Á. Slow Food as an Alternative Food Consumption: Approaches, principles and product attributes. Res. Soc. 2022, 11, e53111326771. [Google Scholar] [CrossRef]
- Özer, Ç.; Bruwier, F.; Olgay, P. Potatoes with its history, usage in turkish-french cuisine and outstanding. Gastroia J. Gastron. Travel Res. 2020, 4, 300–312. [Google Scholar] [CrossRef]
- Çakmak, M.; Sarıışık, M. An investigation on the basic contents of the main dishes of the Turkish cuisine. Anais Bras. Estud. Tur. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Ciccone, M.; Chambers, D.; Chambers, E., IV; Talavera, M. Determining Which Cooking Method Provides the Best Sensory Differentiation of Potatoes. Foods 2020, 9, 451. [Google Scholar] [CrossRef]
- Ahmed, Z.A.; Mohammed, N.K. Investigating influencing factors on acrylamide content in fried potatoes and mitigating measures: A review. FPPN 2024, 6, 24. [Google Scholar] [CrossRef]
- Bachir, N.; Akkoum, H.; Pujola, M.; Sepulcre, F.; Haddarah, A. Impact of amino acids and sugars after thermal processing on acrylamide formation in synthetic potato models and real potatoes. Food Sci. Nutr. 2024, 12, 1046–1055. [Google Scholar] [CrossRef]
- Rajesh, T.P.; Basheer, V.A.; Packirisamy, A.S.B.; Ravi, S.N.; Vallinayagam, S. Effective inhibition of enzymatic browning and carcinogenic acrylamide in fried food by polyphenols. Top Catal. 2024, 67, 300–312. [Google Scholar] [CrossRef]
- Perera, D.N.; Hewavitharana, G.G.; Navaratne, S. Comprehensive study on the acrylamide content of high thermally processed foods. Biomed Res. Int. 2021, 2021, 6258508. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, T.; Lachenmeier, D.W.; Gökmen, V. Analysis of heat-induced contaminants (acrylamide, chloropropanols and furan) in carbohydrate-rich food. Anal. Bioanal. Chem. 2007, 389, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.H.; Gökmen, V. Acrylamide formation mechanisms. In Acrylamide in Food; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–17. [Google Scholar]
- Cunha, N.M.; de Souza Chaves, D.H.; da Silveira, T.M.L.; Birchal, V.S.; Garcia, M.A.V.T. Evaluation of vegetable oil blends properties during deep frying process and their influence on the quality of fried potatoes. OLEL 2024, 22, e3264. [Google Scholar] [CrossRef]
- Troise, A.D.; Scaloni, A. Quantitation of acrylamide in foods by high-resolution mass spectrometry. In Acrylamide in Food; Elsevier: Amsterdam, The Netherlands, 2024; pp. 565–579. [Google Scholar]
- Govindaraju, I.; Sana, M.; Chakraborty, I.; Rahman, M.H.; Biswas, R.; Mazumder, N. Dietary Acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts. Foods 2024, 13, 556. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Hong, L.; Xie, X.; Wang, S. Review of Research into the Determination of Acrylamide in Foods. Foods 2020, 9, 524. [Google Scholar] [CrossRef]
- Wang, P.; Ji, R.; Ji, J.; Chen, F. Changes of metabolites of acrylamide and glycidamide in acrylamide-exposed rats pretreated with blueberry anthocyanins extract. Food Chem. 2019, 274, 611–619. [Google Scholar] [CrossRef]
- Halford, N.G.; Curtis, T.Y.; Muttucumaru, N.; Postles, J.; Elmore, J.S.; Mottram, D.S. The acrylamide problem: A plant and agronomic science issue. J. Exp. Bot. 2012, 63, 2841–2851. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A review on acrylamide in food: Occurrence, toxicity, and mitigation strategies. IJT 2020, 39, 93–102. [Google Scholar] [CrossRef]
- Abedini, A.H.; Vakili Saatloo, N.; Salimi, M.; Sadighara, P.; Alizadeh Sani, M.; Garcia-Oliviera, P.; Prieto, M.A.; Kharazmi, M.S.; Jafari, S.M. The role of additives on acrylamide formation in food products: A systematic review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2773–2793. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. 5-Hydroxymethylfurfural accumulation plays a critical role on acrylamide formation in coffee during roasting as confirmed by multiresponse kinetic modelling. Food Chem. 2020, 318, 126467. [Google Scholar] [CrossRef]
- Corrêa, C.L.O.; das Merces Penha, E.; Dos Anjos, M.R.; Pacheco, S.; Freitas-Silva, O.; Luna, A.S.; Gottschalk, L.M.F. Use of asparaginase for acrylamide mitigation in coffee and its influence on the content of caffeine, chlorogenic acid, and caffeic acid. Food Chem. 2021, 338, 128045. [Google Scholar] [CrossRef] [PubMed]
- Başaran, B.; Çuvalcı, B.; Kaban, G. Dietary acrylamide exposure and cancer risk: A systematic approach to human epidemiological studies. Foods 2023, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Jozinović, A.; Panak Balentić, J.; Ačkar, Đ.; Benšić, M.; Babić, J.; Barišić, V.; Lončarić, A.; Miličević, B.; Šubarić, D. Nutritionally Valuable Components and Heat-Induced Contaminants in Extruded Snack Products Enriched with Defatted Press Cakes. Molecules 2024, 29, 791. [Google Scholar] [CrossRef] [PubMed]
- Monien, B.H.; Bergau, N.; Gauch, F.; Weikert, C.; Abraham, K. Internal exposure to heat-induced food contaminants in omnivores, vegans and strict raw food eaters: Biomarkers of exposure to acrylamide (hemoglobin adducts, urinary mercapturic acids) and new insights on its endogenous formation. Arch. Toxicol. 2024, 98, 2889–2905. [Google Scholar] [CrossRef]
- Cascos, G.; Montero-Fernández, I.; Marcía-Fuentes, J.A.; Aleman, R.S.; Ruiz-Canales, A.; Martín-Vertedor, D. Electronic Prediction of Chemical Contaminants in Aroma of Brewed Roasted Coffee and Quantification of Acrylamide Levels. Foods 2024, 13, 768. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- Başaran, B.; Aydın, F.; Kaban, G. The determination of acrylamide content in brewed coffee samples marketed in Turkey. Food Addit. Contam. Part A 2020, 37, 280–287. [Google Scholar] [CrossRef]
- Mesias, M.; Delgado-Andrade, C.; Morales, F.J. An updated view of acrylamide in cereal products. Curr. Opin. Food Sci. 2022, 46, 100847. [Google Scholar] [CrossRef]
- Dağoğlu, I.; Keskin Alkaç, Z.; Korkak, F.A.; Kazdal, S.M.; Dağ, A. Acrylamide in heat-treated carbohydrate-rich foods in Turkey. Food Addit. Contam. Part B 2024, 17, 122–128. [Google Scholar] [CrossRef]
- Michalak, J.; Gujska, E.; Klepacka, J. The effect of domestic preparation of some potato products on acrylamide content. Plant Foods Hum. Nutr. 2011, 66, 307–312. [Google Scholar] [CrossRef]
- Foot, R.; Haase, N.U.; Grob, K.; Gonde, P. Acrylamide in fried and roasted potato products: A review on progress in mitigation. Food Addit. Contam. Part A 2007, 24, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Bussan, A.J. Acrylamide in processed potato products. AJPR 2013, 90, 403–424. [Google Scholar] [CrossRef]
- Gielecińska, I.; Mojska, H. Trends in the acrylamide content of potato products in Poland in the years 2004–2020. Food Control 2023, 152, 109877. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Visvanathan, R. Acrylamide in food products: A review. J. Food Process Technol. 2014, 5, 344. [Google Scholar]
- Mihai, A.L.; Negoiță, M.; Horneț, G.A. Assessment of acrylamide in potato chips and French fries consumed by the Romanian population. JFPP 2022, 46, e15957. [Google Scholar] [CrossRef]
- Sharafi, K.; Kiani, A.; Massahi, T.; Mansouri, B.; Ebrahimzadeh, G.; Moradi, M.; Fattahi, N.; Omer, A.K. Acrylamide in potato chips in Iran, health risk assessment and mitigation. Food Addit. Contam. Part B 2024, 17, 46–55. [Google Scholar] [CrossRef]
- Shishov, A.; Markova, U.; Ekaterina, D.; Bulatov, A. Automated liquid–liquid deep eutectic solvents based microextraction procedure for determination of acrylamide in foodstuffs by high-performance liquid chromatography with ultraviolet detection. JFST 2024, in press. [Google Scholar] [CrossRef]
- Omar, M.M.A.; Elbashir, A.A.; Schmitz, O.J.; Ziyada, A.K.; Osman, A. Validation of high-performance liquid chromatography coupled with LTQ orbitrap mass spectrometry for analysis of acrylamide. J. Food Meas. Charact. 2024, 18, 930–939. [Google Scholar] [CrossRef]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Špringer, T.; Song, X.C.; Mrázek, J.; Lamačová, J.; Lynn, N.S., Jr.; Šedivák, P.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef]
- Atar, N.; Eren, T.; Yola, M.L. A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice. Food Chem. 2015, 184, 7–11. [Google Scholar] [CrossRef]
- Akıcı, Ş.Y.; Bankoğlu Yola, B.; Karslıoğlu, B.; Polat, İ.; Atar, N.; Yola, M.L. Fenpicoxamid-imprinted surface plasmon resonance (SPR) sensor based on sulfur-doped graphitic carbon nitride and its application to rice samples. Micromachines 2023, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yue, S.; Zhao, Z.; Liu, K.; Wang, P.; Zhan, S. Application of molecularly imprinted polymers in the water environmental field: A review on the detection and efficient removal of emerging contaminants. Mater Today Sustain. 2024, 27, 100904. [Google Scholar] [CrossRef]
- Blackburn, C.; Sullivan, M.V.; Wild, M.I.; O’Connor, A.J.; Turner, N.W. Utilisation of molecularly imprinting technology for the detection of glucocorticoids for a point of care surface plasmon resonance (SPR) device. Anal. Chim. Acta 2024, 1285, 342004. [Google Scholar] [CrossRef] [PubMed]
- Capar, N.; Yola, B.B.; Polat, İ.; Bekerecioğlu, S.; Atar, N.; Yola, M.L. A zearalenone detection based on molecularly imprinted surface plasmon resonance sensor including sulfur-doped g-C3N4/Bi2S3 nanocomposite. Microchem. J. 2023, 193, 109141. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Li, H.; Zhao, L.; Ma, Y.; Zhang, Y.; Liu, J.; Wei, Y. Rigorous recognition mode analysis of molecularly imprinted polymers—Rational design, challenges, and opportunities. Prog. Polym. Sci. 2024, 150, 101790. [Google Scholar] [CrossRef]
- Wang, L.; Pagett, M.; Zhang, W. Molecularly imprinted polymer (MIP) based electrochemical sensors and their recent advances in health applications. Sens. Actuators Rep. 2023, 5, 100153. [Google Scholar] [CrossRef]
- Gamal, M.; Abdelwahab, N.S.; Imam, M.S.; Albugami, A.S.; Hunjur, S.A.; Aldhalmi, A.K.; AbdElrahman, M.; Ghoneim, M.M.; Ali, H.M.; Eissa, M.S. Current advances in the implementation of magnetic molecularly imprinted polymers tailored for enrichment of target analytes in different environmental samples: An overview from a comprehensive perspective. Trends Environ. Anal. 2024, 43, e00236. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; Li, Y.; Wang, Q.; Wang, M.; Li, Y.; Davenport, A.; Li, B. Recent advances in molecularly imprinted polymer-based electrochemical sensors. Biosens. Bioelectron. 2024, 249, 116018. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Ji, S.; Lu, Y.; Bai, X.; Yin, M.; Huang, C.; Jia, N. Molecularly imprinted photoelectrochemical sensing based on ZnO/polypyrrole nanocomposites for acrylamide detection. Biosens. Bioelectron. 2021, 173, 112816. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Yu, F.; Quan, Y. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef]
- Verma, V.; Yadav, N. Acrylamide content in starch based commercial foods by using high performance liquid chromatography and its association with browning index. CRFS 2022, 5, 464–470. [Google Scholar] [CrossRef] [PubMed]
- International, A. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Nateghi, L.; Hosseini, E.; Fakheri, M.A. The effect of cold atmospheric plasma pretreatment on oil absorption, acrylamide content and sensory characteristics of deep-fried potato strips. Food Chem. X 2024, 21, 101194. [Google Scholar] [CrossRef] [PubMed]
- Alimi, B.A.; Shittu, T.A.; Sanni, L.O.; Arowolo, T. Effect of pre-drying and hydrocolloid type on colour and textural properties of coated fried yam chips. NIFOJ 2013, 31, 97–102. [Google Scholar] [CrossRef]
- Jaggan, M.; Mu, T.; Sun, H. The effect of potato (Solanum tuberosum L.) cultivars on the sensory, nutritional, functional, and safety properties of French fries. JFPP 2020, 44, e14912. [Google Scholar] [CrossRef]
- Pedreschi, F. Frying of potatoes: Physical, chemical, and microstructural changes. Dry.Technol. 2012, 30, 707–725. [Google Scholar] [CrossRef]
- Arisseto, A.P.; Silva, W.C.; Marcolino, P.F.C.; Scaranelo, G.R.; Berbari, S.A.G.; de Oliveira Miguel, A.M.R.; Vicente, E. Influence of potato cultivar, frying oil and sample pre-treatments on the contamination of French fries by 3-monochloropropane-1, 2-diol fatty acid esters. Int. Food Res. 2019, 124, 43–48. [Google Scholar] [CrossRef]
- Adimas, M.A.; Abera, B.D.; Adimas, Z.T.; Woldemariam, H.W.; Delele, M.A. Traditional food processing and Acrylamide formation: A review. Heliyon 2024, 10, e30258. [Google Scholar] [CrossRef]
- Vaitkevičienė, N.; Jarienė, E.; Kulaitienė, J.; Levickienė, D. The physico-chemical and sensory characteristics of coloured-flesh potato chips: Influence of cultivar, slice thickness and frying temperature. Appl. Sci. 2022, 12, 1211. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. Acrylamide content and color development in fried potato strips. Int. Food Res. 2006, 39, 40–46. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Figiel, A.; Carbonell-Barrachina, A.A. Effects of potato strip size and pre-drying method on French fries quality. Eur. Food Res. Technol. 2008, 227, 757–766. [Google Scholar] [CrossRef]
- Dite Hunjek, D.; Pranjić, T.; Repajić, M.; Levaj, B. Fresh-cut potato quality and sensory: Effect of cultivar, age, processing, and cooking during storage. J. Food Sci. 2020, 85, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.; Varela, P.; Sanz, T.; Fiszman, S. Understanding potato chips crispy texture by simultaneous fracture and acoustic measurements, and sensory analysis. LWT Food Sci. Technol. 2009, 42, 763–767. [Google Scholar] [CrossRef]
- Abong, G.O.; Okoth, M.W.; Imungi, J.K.; Kabira, J.N. Effect of slice thickness and frying temperature on color, texture and sensory properties of crisps made from four kenyan potato cultivars. Am. J. Food Technol. 2011, 6, 753–762. [Google Scholar] [CrossRef]
- Kaundal, B.; Sharma, V.; Bansal, D.; Khagwal, A.; Singh, B. Comparative Study of Frying to Different Slice Thickness of Potato: Effect on Nutritive Value. Braz. Arch. Biol. Technol. 2022, 65, e22220033. [Google Scholar] [CrossRef]
- Yola, M.L.; Uzun, L.; Özaltın, N.; Denizli, A. Development of molecular imprinted nanosensor for determination of tobramycin in pharmaceuticals and foods. Talanta 2014, 120, 318–324. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, C.; Waterhouse, G.I.; Sun, Y.; Xu, Z. SERS Sensor Based on Core–Shell Au@ Ag Nanoparticles for the Sensitive Detection of Acrylamide in Foods. Food Anal. Methods 2024, 17, 585–593. [Google Scholar] [CrossRef]
- Guo, K.; Lin, X.; Duan, N.; Lu, C.; Wang, Z.; Wu, S. Detection of acrylamide in food based on MIL-glucose oxidase cascade colorimetric aptasensor. Anal. Chim. Acta 2024, 1288, 342150. [Google Scholar] [CrossRef]
- Cheng, B.; Xia, X.; Han, Z.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W.; Qian, H.; Cheng, Y. A ratiometric fluorescent “off-on” sensor for acrylamide detection in toast based on red-emitting copper nanoclusters stabilized by bovine serum albumin. Food Chem. 2024, 437, 137878. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, P.; Liu, T.; Pu, H.; Sun, D.-W. A fluorescence biosensor based on single-stranded DNA and carbon quantum dots for acrylamide detection. Food Chem. 2021, 356, 129668. [Google Scholar] [CrossRef]
- Zhang, C.; Ou, W.; Zeng, Z.; Liu, H.; Yu, K.; Wang, L.; Zhou, L. Polypyrrole participates in the construction of a polarity-switchable photoelectrochemical molecularly imprinted sensor for the detection of acrylamide in fried foods. Sens. Actuators B Chem. 2024, 402, 135037. [Google Scholar] [CrossRef]
- Asnaashari, M.; Kenari, R.E.; Taghdisi, S.M.; Abnous, K.; Farahmandfar, R. A novel fluorescent DNA sensor for acrylamide detection in food samples based on single-stranded DNA and GelRed. J. Fluoresc. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Palazoğlu, T.K.; Şenyuva, H.Z. Relation between the acrylamide formation and time–temperature history of surface and core regions of French fries. J. Food Eng. 2006, 77, 972–976. [Google Scholar] [CrossRef]
- Norén, L. Acrylamide in Ready-to-Eat Potato Products. Master’s Thesis, Lund University, Lund, Sweden, 2019. [Google Scholar]
- Navruz-Varlı, S.; Mortaş, H. Acrylamide formation in air-fried versus deep and oven-fried potatoes. Front. Nutr. 2024, 10, 1297069. [Google Scholar] [CrossRef]
- Shamla, L.; Nisha, P. Acrylamide in deep-fried snacks of India. Food Addit. Contam. Part B 2014, 7, 220–225. [Google Scholar] [CrossRef]

| Parameters | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| pH | 6.31 ± 0.01 a | 6.30 ± 0.02 a | 6.29 ± 0.01 a | 6.29 ± 0.02 a |
| Moisture (%) | 21.84 ± 0.74 a | 55.08 ± 0.92 b | 47.90 ± 0.29 c | 55.31 ± 0.39 d |
| Protein (%) | 6.98 ± 0.06 a | 7.01 ± 0.08 a | 7.02 ± 0.88 a | 6.90 ± 0.40 a |
| Fat (%) | 28.15 ± 0.04 c | 30.06 ± 0.51 b | 28.96 ± 0.04 bc | 30.18 ± 0.08 a |
| Ash (%) | 2.64 ± 0.01 a | 2.57 ± 0.09 a | 2.50 ± 0.33 a | 2.39 ± 0.12 a |
| Carbohydrate (g/100 g DW) | 51.68 ± 0.05 a | 11.75 ± 0.13 c | 26.14 ± 0.21 b | 11.68 ± 0.05 c |
| L* | a* | b* | ΔE | BI | |
|---|---|---|---|---|---|
| Fresh potatoes | 69.01 ± 0.15 | 0.38 ± 0.05 | 21.05 ± 0.08 | - | - |
| T1 | 51.09 ± 0.12 a | 11.78 ± 0.30 a | 33.58 ± 0.98 a | 24.67 ± 0.73 a | 120.36 ± 1.24 a |
| T2 | 54.82 ± 0.51 b | 7.14 ± 0.30 ab | 35.48 ± 0.41 a | 21.35 ± 0.16 a | 105.39 ± 0.19 a |
| T3 | 51.45 ± 0.85 a | 8.35 ± 1.12 bc | 36.02 ± 2.77 a | 24.57 ± 1.44 a | 115.40 ± 0.12 a |
| T4 | 59.19 ± 1.21 c | 3.94 ± 0.42 c | 34.45 ± 2.48 a | 15.92 ± 1.21 b | 81.55 ± 0.17 b |
| Sensory Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Blush (Scores 1–5) | Crispiness (Scores 1–5) | Mealiness (Scores 1–5) | Oiliness (Scores 1–5) | Taste (Scores 1–5) | Color (Scores 1–5) | Overall Acceptability (Scores 1–5) |
| T1 | 3.53 ± 0.07 a | 4.08 ± 0.07 a | 2.68 ± 0.07 a | 4.04 ± 0.03 a | 4.05 ± 0.01 a | 4.02 ± 0.10 a | 4.41 ± 0.06 a |
| T2 | 2.51 ± 0.02 c | 1.91 ± 0.21 b | 3.72 ± 0.03 a | 2.70 ± 0.01 b | 3.13 ± 0.03 b | 3.09 ± 0.07 b | 2.96 ± 0.04 b |
| T3 | 3.24 ± 0.01 b | 3.24 ± 0.08 a | 3.71 ± 0.06 a | 2.87 ± 0.03 b | 3.18 ± 0.02 b | 3.66 ± 0.04 a | 3.29 ± 0.21 b |
| T4 | 2.70 ± 0.01 c | 2.30 ± 0.06 b | 3.78 ± 0.01 b | 3.65 ± 0.11 a | 3.06 ± 0.14 b | 3.04 ± 0.11 b | 2.98 ± 0.13 b |
| Method | Linear Range (M) | LOD (M) | Ref. |
|---|---|---|---|
| Au@Ag NPs SERS sensor | 1.0 × 10−8 –1.0 × 10−3 | 1.27 × 10−9 | [71] |
| Colorimetric aptasensor | 1.0 × 10−8–1.0 × 10−4 | 1.53 × 10−6 | [72] |
| CuNCs/GSH | 5.0 × 10−6–3.0 × 10−4 | 1.48 × 10−6 | [73] |
| CDs/DNA | 1.0 × 10−7–1.0 × 10−3 | 2.41 × 10−8 | [74] |
| PEC-MIP sensor | 2.50 × 10−10–1.04 × 10−7 | 1.70 × 10−9 | [75] |
| Fluorescent sensor based on ssDNA and GelRed | 1.0 × 10−5–9.5 × 10−3 | 3.0 × 10−6 | [76] |
| MIP/SPR chip | 1.0 × 10−9–5.0 × 10−8 | 3.0 × 10−10 | This study |
| Treatment | Added AA (nM) | Found AA (nM) | * Recovery (%) |
|---|---|---|---|
| T1 | - | 15.37 ± 0.01 a | - |
| 2.00 | 17.38 ± 0.02 | 100.06 ± 0.06 | |
| 4.00 | 19.36 ± 0.06 | 99.95 ± 0.03 | |
| 6.00 | 21.36 ± 0.08 | 99.95 ± 0.02 | |
| T2 | - | 5.31 ± 0.03 b | - |
| 2.00 | 7.32 ± 0.04 | 100.14 ± 0.03 | |
| 4.00 | 9.30 ± 0.05 | 99.89 ± 0.01 | |
| 6.00 | 11.32 ± 0.07 | 100.09 ± 0.01 | |
| T3 | - | 10.79 ± 0.04 c | - |
| 2.00 | 12.78 ± 0.05 | 99.92 ± 0.07 | |
| 4.00 | 14.80 ± 0.05 | 100.07 ± 0.09 | |
| 6.00 | 16.80 ± 0.03 | 100.06 ± 0.04 | |
| T4 | - | 3.89 ± 0.08 d | - |
| 2.00 | 5.90 ± 0.02 | 100.17 ± 0.07 | |
| 4.00 | 6.90 ± 0.07 | 100.15 ± 0.07 | |
| 6.00 | 9.88 ± 0.03 | 99.90 ± 0.03 |
| MIP | NIP | ||||
|---|---|---|---|---|---|
| ∆R | k | ∆R | k | k′ | |
| AA | 10.0 ± 0.02 | - | 0.20 ± 0.03 | - | - |
| MA | 1.00 ± 0.01 | 10.00 | 0.15 ± 0.01 | 1.33 | 7.52 |
| PA | 0.75 ± 0.03 | 13.00 | 0.10 ± 0.02 | 2.00 | 6.50 |
| DL-ALA | 0.50 ± 0.06 | 20.00 | 0.05 ± 0.01 | 4.00 | 5.00 |
| L-ASP | 0.25 ± 0.02 | 40.00 | 0.01 ± 0.01 | 20.00 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karslıoğlu, B.; Bankoğlu Yola, B.; Polat, İ.; Alkan, H.Y.; Yola, M.L. Assessment of Acrylamide Levels by Advanced Molecularly Imprinted Polymer-Imprinted Surface Plasmon Resonance (SPR) Sensor Technology and Sensory Quality in Homemade Fried Potatoes. Foods 2024, 13, 2927. https://doi.org/10.3390/foods13182927
Karslıoğlu B, Bankoğlu Yola B, Polat İ, Alkan HY, Yola ML. Assessment of Acrylamide Levels by Advanced Molecularly Imprinted Polymer-Imprinted Surface Plasmon Resonance (SPR) Sensor Technology and Sensory Quality in Homemade Fried Potatoes. Foods. 2024; 13(18):2927. https://doi.org/10.3390/foods13182927
Chicago/Turabian StyleKarslıoğlu, Betül, Bahar Bankoğlu Yola, İlknur Polat, Harun Yiğit Alkan, and Mehmet Lütfi Yola. 2024. "Assessment of Acrylamide Levels by Advanced Molecularly Imprinted Polymer-Imprinted Surface Plasmon Resonance (SPR) Sensor Technology and Sensory Quality in Homemade Fried Potatoes" Foods 13, no. 18: 2927. https://doi.org/10.3390/foods13182927






