Microencapsulation by Complex Coacervation of Lavender Oil Obtained by Steam Distillation at Semi-Industrial Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Core Material
2.2. Shell Materials
2.3. Analytical Standards
2.4. Steam Distillation
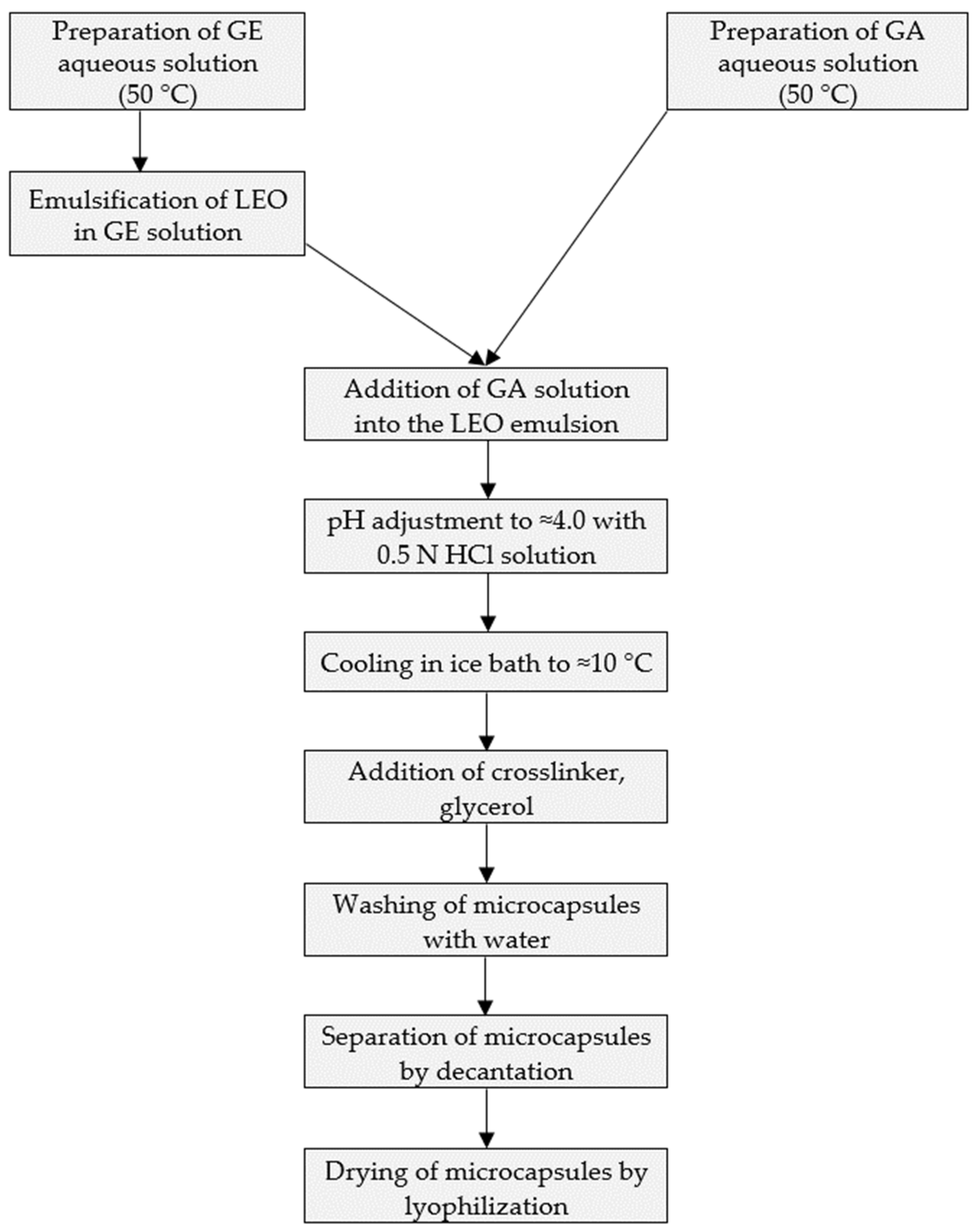
2.5. Microencapsulation Method
2.6. Freeze Drying
2.7. Optical Microscopy
2.8. SEM Investigation
2.9. FT-IR Spectroscopy
2.10. UV-VIS Spectroscopic Analyses
2.11. Essential Oil Content
2.12. GC-MS Investigation
3. Results and Discussion
3.1. GC-MS Investigation of Essential Oil Composition
3.1.1. Free Form of Essential Oil
3.1.2. Microencapsulated Form of Essential Oil
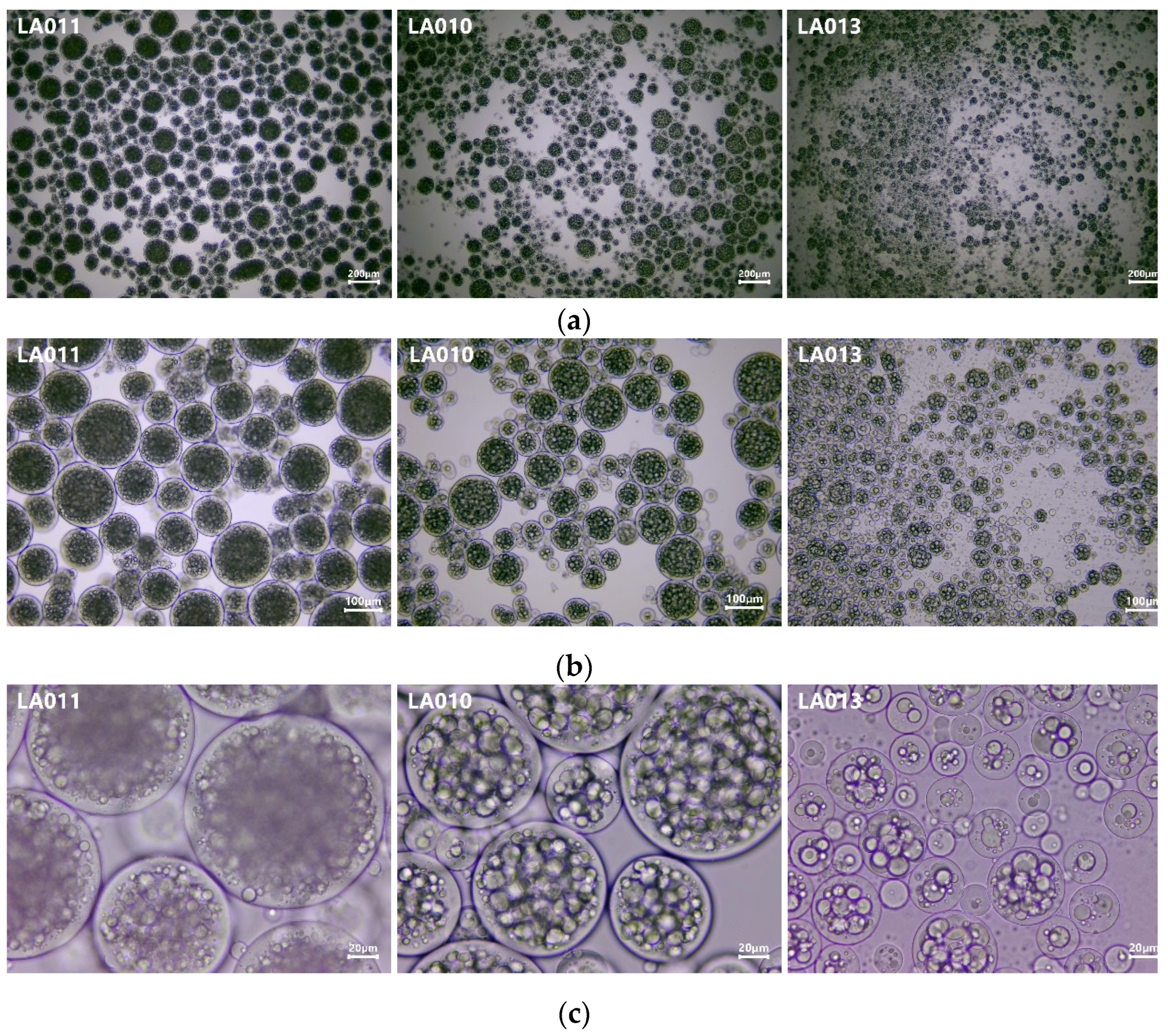
3.2. Results of Optical Microscopic Investigation
3.3. Morphological Investigation of Freeze-Dried Form
3.3.1. Macroscopic Aspect
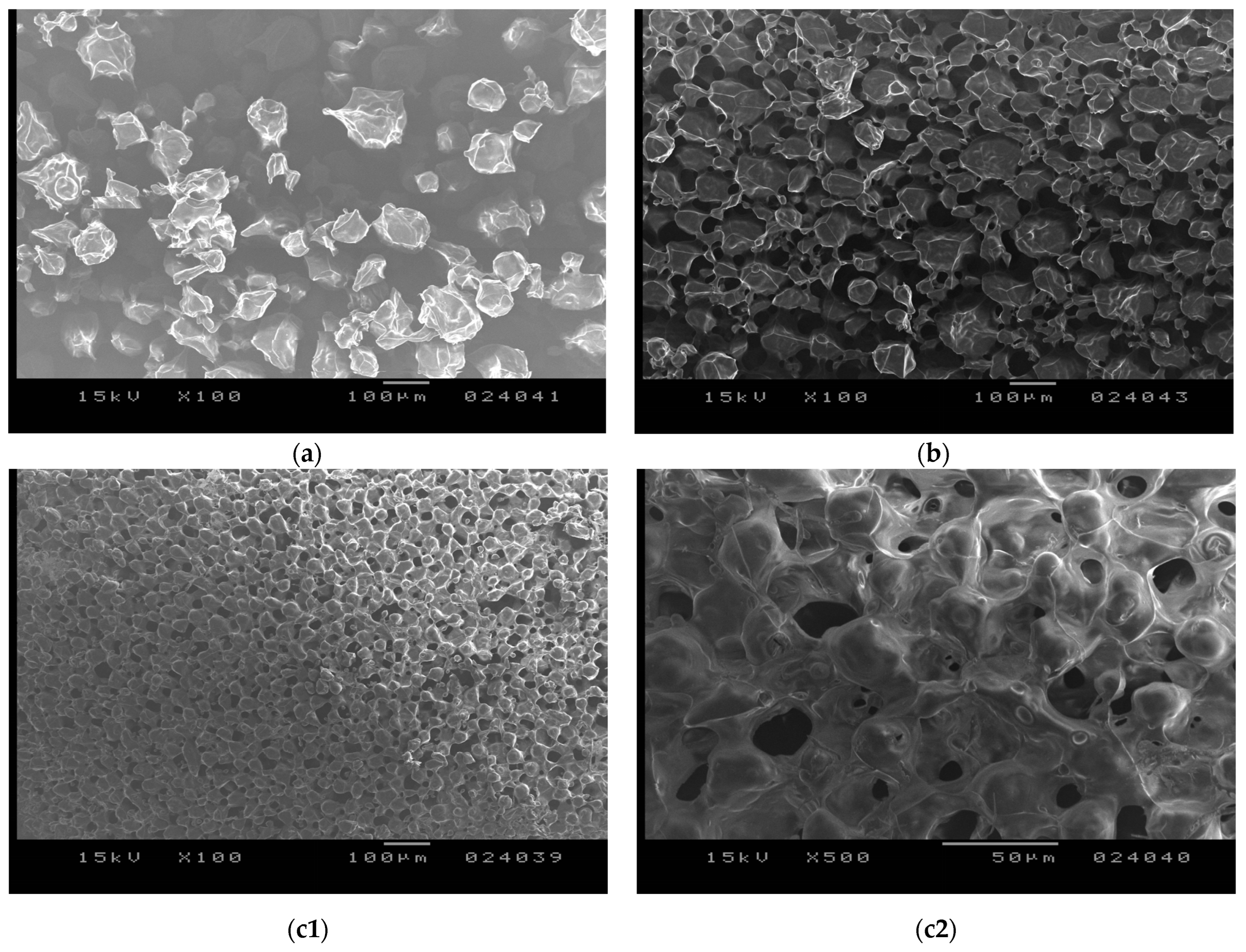
3.3.2. Electronmicroscopic Investigation
3.4. Determination of Encapsulation Efficiency and Loading Capacity by UV Spectroscopy
3.5. Results of FT-IR Spectroscopic Investigations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wells, R.; Truong, F.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. Lavandula Essential Oils: A Current Review of Applications in Medicinal, Food, and Cosmetic Industries of Lavender. Nat. Prod. Commun. 2018, 13, 1403–1417. [Google Scholar]
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical Composition of the Essential Oil of the New Cultivars of Lavandula Angustifolia Mill. Bred in Ukraine. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef] [PubMed]
- Kozuharova, E.; Simeonov, V.; Batovska, D.; Stoycheva, C.; Valchev, H.; Benbassat, N. Chemical Composition and Comparative Analysis of Lavender Essential Oil Samples from Bulgaria in Relation to the Pharmacological Effects. Pharmacia 2023, 70, 395–403. [Google Scholar] [CrossRef]
- Crișan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current Trends for Lavender (Lavandula angustifolia Mill.) Crops and Products with Emphasis on Essential Oil Quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef]
- Rathore, S.; Kumar, R. Essential Oil Content and Compositional Variability of Lavandula Species Cultivated in the Mid Hill Conditions of the Western Himalaya. Molecules 2022, 27, 3391. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Community Herbal Monograph on Lavender Oil (04/2023:2972). In European Pharmacopoeia (Ph. Eur.), 11th ed.; EDQM Council of Europe: Strasbourg, France, 2022; pp. 4627–4628. [Google Scholar]
- European Medicines Agency (EMA). Community Herbal Monograph on Spike Lavender Oil (07/2018: 2419). In European Pharmacopoeia (Ph. Eur.), 11th ed.; EDQM Council of Europe: Strasbourg, France, 2022; pp. 1735–1736. [Google Scholar]
- European Medicines Agency (EMA). Community Herbal Monograph on Lavender Flower (07/2018:1534). In European Pharmacopoeia (Ph. Eur.), 11th ed.; EDQM Council of Europe: Strasbourg, France, 2022; pp. 1579–1580. [Google Scholar]
- Wang, M.; Zhao, J.; Ali, Z.; Avonto, C.; Khan, I.A. A Novel Approach for Lavender Essential Oil Authentication and Quality Assessment. J. Pharm. Biomed. Anal. 2021, 199, 114050. [Google Scholar] [CrossRef]
- Beale, D.J.; Morrison, P.D.; Karpe, A.V.; Dunn, M.S. Chemometric Analysis of Lavender Essential Oils Using Targeted and Untargeted GC-MS Acquired Data for the Rapid Identification and Characterization of Oil Quality. Molecules 2017, 22, 1339. [Google Scholar] [CrossRef]
- Hedayati, S.; Tarahi, M.; Iraji, A.; Hashempur, M.H. Recent Developments in the Encapsulation of Lavender Essential Oil. Adv. Colloid Interface Sci. 2024, 331, 103229. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef]
- Fukushima, S.; Cohen, S.M.; Eisenbrand, G.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Rosol, T.J.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS Assessment of Natural Flavor Complexes: Lavender, Guaiac Coriander-Derived and Related Flavoring Ingredients. Food Chem. Toxicol. 2020, 145, 111584. [Google Scholar] [CrossRef]
- Pilicheva, B.; Uzunova, Y.; Katsarov, P. Comparative Study on Microencapsulation of Lavender (Lavandula Angustifolia Mill.) and Peppermint (Mentha piperita L.) Essential Oils via Spray-Drying Technique. Molecules 2021, 26, 7467. [Google Scholar] [CrossRef] [PubMed]
- Valle, R.d.C.S.C.; Valle, J.A.B.; Bezerra, F.M.; Correia, J.; da Costa, C.; Martí, M.; Coderch, L.; López, A.; Arias, M.J.L. Application of Lavender-Oil Microcapsules to Functionalized PET Fibers. Polymers 2023, 15, 917. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, W.; Zhu, G.; Zhou, R.; Niu, Y. Production and Characterization of Multinuclear Microcapsules Encapsulating Lavender Oil by Complex Coacervation. Flavour Fragr. J. 2014, 29, 166–172. [Google Scholar] [CrossRef]
- Gonçalves, N.D.; Grosso, C.R.F.; Rabelo, R.S.; Hubinger, M.D.; Prata, A.S. Comparison of Microparticles Produced with Combinations of Gelatin, Chitosan and Gum Arabic. Carbohydr. Polym. 2018, 196, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Kuś, P.M.; Carbonell-Barrachina, Á.A. Volatile Organic Compounds as Artefacts Derived from Natural Phytochemicals Sourced Form Plants and Honey. Phytochem. Rev. 2019, 18, 871–891. [Google Scholar] [CrossRef]
- Jakab, E.; Blazsó, M.; Barta-Rajnai, E.; Babinszki, B.; Sebestyén, Z.; Czégény, Z.; Nicol, J.; Clayton, P.; McAdam, K.; Liu, C. Thermo-Oxidative Decomposition of Lime, Bergamot and Cardamom Essential Oils. J. Anal. Appl. Pyrolysis 2018, 134, 552–561. [Google Scholar] [CrossRef]
- PubChem (National Library of Medicine). Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 July 2024).
- ChemSpider (Royal Society of Chemistry). Available online: https://www.chemspider.com/ (accessed on 29 July 2024).
- The Good Scent Company Informational System. Available online: https://www.thegoodscentscompany.com/ (accessed on 29 July 2024).
- Peters, H.J.W.; van Bommel, E.M.; Fokkens, J.G. Effect of Gelatin Properties in Complex Coacervation Processes. Drug Dev. Ind. Pharm. 1992, 18, 123–134. [Google Scholar]
- Comunian, T.A.; Thomazini, M.; Alves, A.J.G.; Junior, F.E.d.M.; Balieiro, J.C.d.C.; Favaro-Trindade, C.S. Microencapsulation of Ascorbic Acid by Complex Coacervation: Protection and Controlled Release. Food Res. Int. 2013, 52, 373–379. [Google Scholar] [CrossRef]
- Alvim, I.D.; Grosso, C.R.F. Microparticles Obtained by Complex Coacervation: Influence of the Type of Reticulation and the Drying Process on the Release of the Core Material. Ciência e Tecnol. Aliment. 2010, 30, 1069–1076. [Google Scholar] [CrossRef]
- Rocha-Selmi, G.A.; Bozza, F.T.; Thomazini, M.; Bolini, H.M.A.; Fávaro-Trindade, C.S. Microencapsulation of Aspartame by Double Emulsion Followed by Complex Coacervation to Provide Protection and Prolong Sweetness. Food Chem. 2013, 139, 72–78. [Google Scholar] [CrossRef]
- Ocak, B. Complex Coacervation of Collagen Hydrolysate Extracted from Leather Solid Wastes and Chitosan for Controlled Release of Lavender Oil. J. Environ. Manag. 2012, 100, 22–28. [Google Scholar] [CrossRef]
- Burhan, A.M.; Abdel-Hamid, S.M.; Soliman, M.E.; Sammour, O.A. Optimisation of the Microencapsulation of Lavender Oil by Spray Drying. J. Microencapsul. 2019, 36, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Rungwasantisuk, A.; Raibhu, S. Application of Encapsulating Lavender Essential Oil in Gelatin/Gum-Arabic Complex Coacervate and Varnish Screen-Printing in Making Fragrant Gift-Wrapping Paper. Prog. Org. Coat. 2020, 149, 105924. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, L.; Xiong, X.; Qian, M.C.; Ji, H. Preparation and Release Mechanism of Lavender Oil Microcapsules with Different Combinations of Coating Materials. Flavour Fragr. J. 2020, 35, 157–166. [Google Scholar] [CrossRef]
- Musa, H.H.; Ahmed, A.A.; Musa, T.H. Chemistry, Biological, and Pharmacological Properties of Gum Arabic. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2019; pp. 797–814. [Google Scholar]
- Rousi, Z.; Malhiac, C.; Fatouros, D.G.; Paraskevopoulou, A. Complex Coacervates Formation between Gelatin and Gum Arabic with Different Arabinogalactan Protein Fraction Content and Their Characterization. Food Hydrocoll. 2019, 96, 577–588. [Google Scholar] [CrossRef]
- Napiórkowska, A.; Szpicer, A.; Wojtasik-Kalinowska, I.; Perez, M.D.T.; González, H.D.; Kurek, M.A. Microencapsulation of Juniper and Black Pepper Essential Oil Using the Coacervation Method and Its Properties after Freeze-Drying. Foods 2023, 12, 4345. [Google Scholar] [CrossRef]
- Larkin, P.J. IR and Raman Spectra–Structure Correlations: Characteristic Group Frequencies. In Infrared and Raman Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–134. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications. In Analytical Techniques in the Sciences; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 71–80. ISBN 9780470854273. [Google Scholar]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Use of Gelatin and Gum Arabic for Encapsulation of Black Raspberry Anthocyanins by Complex Coacervation. Int. J. Biol. Macromol. 2018, 107, 1800–1810. [Google Scholar] [CrossRef]
- Milano, F.; Masi, A.; Madaghiele, M.; Sannino, A.; Salvatore, L.; Gallo, N. Current Trends in Gelatin-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1499. [Google Scholar] [CrossRef]
- Yang, W.; Gong, Y.; Wang, Y.; Wu, C.; Zhang, X.; Li, J.; Wu, D. Design of Gum Arabic/Gelatin Composite Microcapsules and Their Cosmetic Applications in Encapsulating Tea Tree Essential Oil. RSC Adv. 2024, 14, 4880–4889. [Google Scholar] [CrossRef]
- Santos, M.G.; Bozza, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Microencapsulation of Xylitol by Double Emulsion Followed by Complex Coacervation. Food Chem. 2015, 171, 32–39. [Google Scholar] [CrossRef]





| Component | ISO Requirement (Other Origins) | Ph. Eur. Requirement | Lavender Oil Obtained by Steam Distillation in 2022 | |
|---|---|---|---|---|
| Initial | After 2 Years of Storage | |||
| % | Peak, % | |||
| Linalyl acetate * | 25–47 | 25.0–47.0 | 38.38 | 31.15 |
| Linalool * | 20–43 | 20.0–45.0 | 26.23 | 18.72 |
| (Z)-β-ocimene ** | 1–10 | N/A | 0.07 | N/A |
| Lavandulyl acetate | ≤8 | ≥0.2 | 3.54 | 2.57 |
| Terpinen-4-ol | ≤8 | 0.1–8.0 | 7.38 | 5.58 |
| (E)-β-ocimene ** | 0.5–6.0 | N/A | 0.09 | 9.24 |
| 3-Octanone | ≤3 | 0.1–5.0 | 0.38 | 0.41 |
| 1,8-Cineole | ≤3 | ≤2.5 | 0.26 | 0.50 |
| Lavandulol | ≤3 | ≥ 0.1 | 0.39 | 0.18 |
| α-terpineol | ≤2 | ≤2.0 | 1.16 | 1.07 |
| Camphor | ≤1.5 | ≤1.2 | 0.57 | 0.27 |
| Limonene | ≤1 | ≤1.0 | 0.19 | 1.10 |
| β-phellandrene ** | ≤1 | N/A | 0.06 | N/A |
| No. | Compound * | Retention Time (min) | LEO | LA010 | LA011 | LA013 |
|---|---|---|---|---|---|---|
| Peak, % | ||||||
| 1 | Thujene | 5.225 | 0.32 | - | - | - |
| 2 | Alpha-pinene | 5.452 | 0.53 | - | - | - |
| 3 | Camphene | 5.942 | 0.40 | - | - | - |
| 4 | Beta-pinene | 6.951 | 0.30 | - | - | - |
| 5 | 3-Octanone | 7.287 | 0.41 | - | - | - |
| 6 | Beta-myrcene | 7.507 | 1.18 | - | - | - |
| 7 | 3-Carene | 8.610 | 0.23 | - | - | - |
| 8 | P-Cymene | 9.259 | 0.49 | - | - | - |
| 9 | Limonene | 9.507 | 1.10 | - | - | - |
| 10 | 1,8 Cineole | 9.658 | 0.50 | - | - | - |
| 11 | Trans-beta-ocimene | 10.155 | 9.24 | 6.31 | - | 2.39 |
| 12 | Beta-ocimene | 10.769 | 2.53 | 1.50 | - | - |
| 13 | Gamma-terpinene | 11.369 | 0.43 | - | - | - |
| 14 | Terpinolene | 13.080 | 0.30 | - | - | - |
| 15 | Linalool | 13.906 | 18.72 | 18.60 | 22.01 | 19.67 |
| 16 | Neo-allo-ocimene | 14.506 | 0.39 | - | - | - |
| 17 | Camphor | 15.306 | 0.27 | - | - | - |
| 18 | 1-Menthone | 16.464 | 1.45 | - | - | - |
| 19 | Endo-borneol | 17.002 | 1.58 | - | 1.57 | - |
| 20 | Lavandulol | 17.193 | 0.18 | - | - | - |
| 21 | Menthol | 17.395 | 2.89 | - | - | - |
| 22 | Terpinen-4-ol | 17.623 | 5.58 | 3.38 | 3.17 | 3.16 |
| 23 | Cryptone | 17.983 | 0.16 | - | - | - |
| 24 | Terpineol | 18.209 | 1.07 | - | 1.37 | |
| 25 | Isobornyl acetate | 18.395 | 0.22 | - | - | |
| 26 | Linalyl acetate | 21.243 | 31.15 | 55.82 | 54.27 | 60.94 |
| 27 | Bornyl acetate | 22.255 | 0.17 | - | - | - |
| 28 | Lavandulyl acetate | 22.511 | 2.57 | 3.01 | 3.11 | 3.31 |
| 29 | Neryl acetate | 25.187 | 0.76 | - | - | - |
| 30 | Geranyl acetate | 25.856 | N/A | 1.35 | 1.16 | 1.41 |
| 31 | Beta-bourbonene | 25.862 | 1.21 | - | - | - |
| 32 | Caryophyllene | 27.069 | 4.67 | 3.80 | - | 2.63 |
| 33 | Trans-alpha-bergamotene | 27.604 | 0.21 | - | - | - |
| 34 | Humulene | 28.183 | 0.10 | - | - | - |
| 35 | Beta-farnesene | 28.324 | 4.35 | 3.41 | - | 2.20 |
| 36 | Germacrene D | 29.096 | 0.33 | - | - | - |
| 37 | Gamma-cadinene | 30.131 | 0.96 | - | - | - |
| 38 | Caryophyllene oxide | 32.234/32.234 | 0.52 | 1.31 | 4.52 | 2.35 |
| 39 | Tau cadinol | 33.902 | 1.13 | 1.51 | 1.48 | 1.93 |
| Total identified | 98.6 | 100 | 92.66 | 99.99 | ||
| Compound | Molecular Weight | Boiling Point * (°C) | Group of Compounds | Terpene Category |
|---|---|---|---|---|
| Thujene | 136.23 | 150–152 | Alkene | Monoterpene |
| Alpha-pinene | 136.23 | 155–157 | Alkene | Monoterpene |
| Camphene | 136.23 | 156–160 | Alkene | Monoterpene |
| Beta-pinene | 136.23 | 165–166 | Alkene | Monoterpene |
| 3-Octanone | 128.21 | 167–170 | Ketone | Ketone (not a terpene) |
| Beta-myrcene | 136.23 | 166–167 | Alkene | Monoterpene |
| 3-Carene | 136.23 | 169–174 | Alkene | Monoterpene |
| P-cymene | 134.22 | 176–178 | Aromatic Hydrocarbon | Monoterpene |
| Limonene | 136.23 | 175–176 | Alkene | Monoterpene |
| 1,8 Cineole | 154.25 | 176–177 | Ether | Monoterpene |
| Trans-beta-ocimene 2 | 136.23 | 174–175 | Alkene | Monoterpene |
| Beta-ocimene 1 | 136.23 | 174–175 | Alkene | Monoterpene |
| Gamma-terpinene | 136.23 | 181–183 | Alkene | Monoterpene |
| Terpinolene | 136.23 | 183–185 | Alkene | Monoterpene |
| Linalool 3 | 154.25 | 197–198 | Alcohol | Monoterpene alcohol |
| Neo-allo-ocimene | 136.23 | 188–189 (predicted) | Alkene | Monoterpene |
| Camphor | 152.23 | 204–209 | Ketone | Monoterpene ketone |
| 1-Menthone | 154.25 | 207–210 | Ketone | Monoterpene ketone |
| Endo-borneol 1 | 154.25 | 210–215 | Alcohol | Monoterpene alcohol |
| Lavandulol | 154.25 | 229–230 | Alcohol | Monoterpene alcohol |
| Menthol | 156.26 | 214–216 | Alcohol | Monoterpene alcohol |
| Terpinen-4-ol 3 | 154.25 | 209–212 | Alcohol | Monoterpene alcohol |
| Cryptone | 138.21 | 198 (experim.) 208 (predicted) | Ketone | Monoterpene ketone |
| Terpineol 1 | 154.25 | 218–221 | Alcohol | Monoterpene alcohol |
| Isobornyl acetate | 196.29 | 220–227 | Ester | Monoterpene ester |
| Linalyl acetate 3 | 196.29 | 220–221 | Ester | Monoterpene ester |
| Bornyl acetate | 196.29 | 220–227 | Ester | Monoterpene ester |
| Lavandulyl acetate 3 | 196.29 | 228.7 ± 19.0 (predicted) | Ester | Monoterpene ester |
| Neryl acetate | 196.29 | 262–265 | Ester | Monoterpene ester |
| Beta-bourbonene | 204.35 | 255.9 ± 7.0 (predicted) | Alkene | Sesquiterpene |
| Caryophyllene 2 | 204.35 | 256–259 | Alkene | Sesquiterpene |
| Trans-alpha-bergamotene | 204.35 | 259–260 | Alkene | Sesquiterpene |
| Humulene | 204.35 | 166–168 | Alkene | Sesquiterpene |
| Beta-farnesene 2 | 204.35 | 206 | Alkene | Sesquiterpene |
| Germacrene D | 204.35 | 279.7 ± 20.0 (predicted) | alkene | Sesquiterpene |
| Gamma-cadinene | 204.35 | 271–276 | alkene | Sesquiterpene |
| Caryophyllene oxide 3 | 220.35 | 279.7 ± 19.0 (predicted) | Epoxide | Sesquiterpene oxide |
| Tau cadinol 3 | 222.37 | 303.4 ± 31.0 (predicted) | Alcohol | Sesquiterpene alcohol |
| Particle Size (µm) | SD | RSD% | Median (µm) | Polydispersity Index | |||
|---|---|---|---|---|---|---|---|
| Exp. No. | Average | Min | Max | ||||
| LA011 | 101 1 | 25 | 273.5 | 43.24 | 42.69 | 98.1 | 0.18 |
| LA010 | 71 2 | 25.8 | 197.6 | 32.17 | 45.20 | 62.6 | 0.20 |
| LA013 | 31 3 | 8.5 | 100.7 | 13.15 | 42.94 | 28.3 | 0.18 |
| Exp. No. | LEO in Microcapsules | LEO Concentration mg/mL | Concentration of MC Solution mg/mL | Theoretical Oil Content mg/mL MC | EE% | LC% |
|---|---|---|---|---|---|---|
| LA011 | Total oil | 4.00 | 10 | 4.03 | 91.71 | 36.96 |
| Surface oil | 0.31 | |||||
| LA010 | Total oil | 3.49 | 10 | 4.03 | 81.46 | 32.83 |
| Surface oil | 0.21 | |||||
| LA013 | Total oil | 2.79 | 10 | 4.03 | 56.36 | 22.71 |
| Surface oil | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Székely-Szentmiklósi, I.; Rédai, E.M.; Szabó, Z.-I.; Kovács, B.; Albert, C.; Gergely, A.-L.; Székely-Szentmiklósi, B.; Sipos, E. Microencapsulation by Complex Coacervation of Lavender Oil Obtained by Steam Distillation at Semi-Industrial Scale. Foods 2024, 13, 2935. https://doi.org/10.3390/foods13182935
Székely-Szentmiklósi I, Rédai EM, Szabó Z-I, Kovács B, Albert C, Gergely A-L, Székely-Szentmiklósi B, Sipos E. Microencapsulation by Complex Coacervation of Lavender Oil Obtained by Steam Distillation at Semi-Industrial Scale. Foods. 2024; 13(18):2935. https://doi.org/10.3390/foods13182935
Chicago/Turabian StyleSzékely-Szentmiklósi, István, Emőke Margit Rédai, Zoltán-István Szabó, Béla Kovács, Csilla Albert, Attila-Levente Gergely, Blanka Székely-Szentmiklósi, and Emese Sipos. 2024. "Microencapsulation by Complex Coacervation of Lavender Oil Obtained by Steam Distillation at Semi-Industrial Scale" Foods 13, no. 18: 2935. https://doi.org/10.3390/foods13182935








