Investigating the Thermal Stability of Omega Fatty Acid-Enriched Vegetable Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Oil Samples and Materials
2.2. Frying Treatment
2.3. Preparation of Fatty Acid Methyl Esters
2.4. Gas Chromatography Analysis
2.5. Multivariate Data Analysis
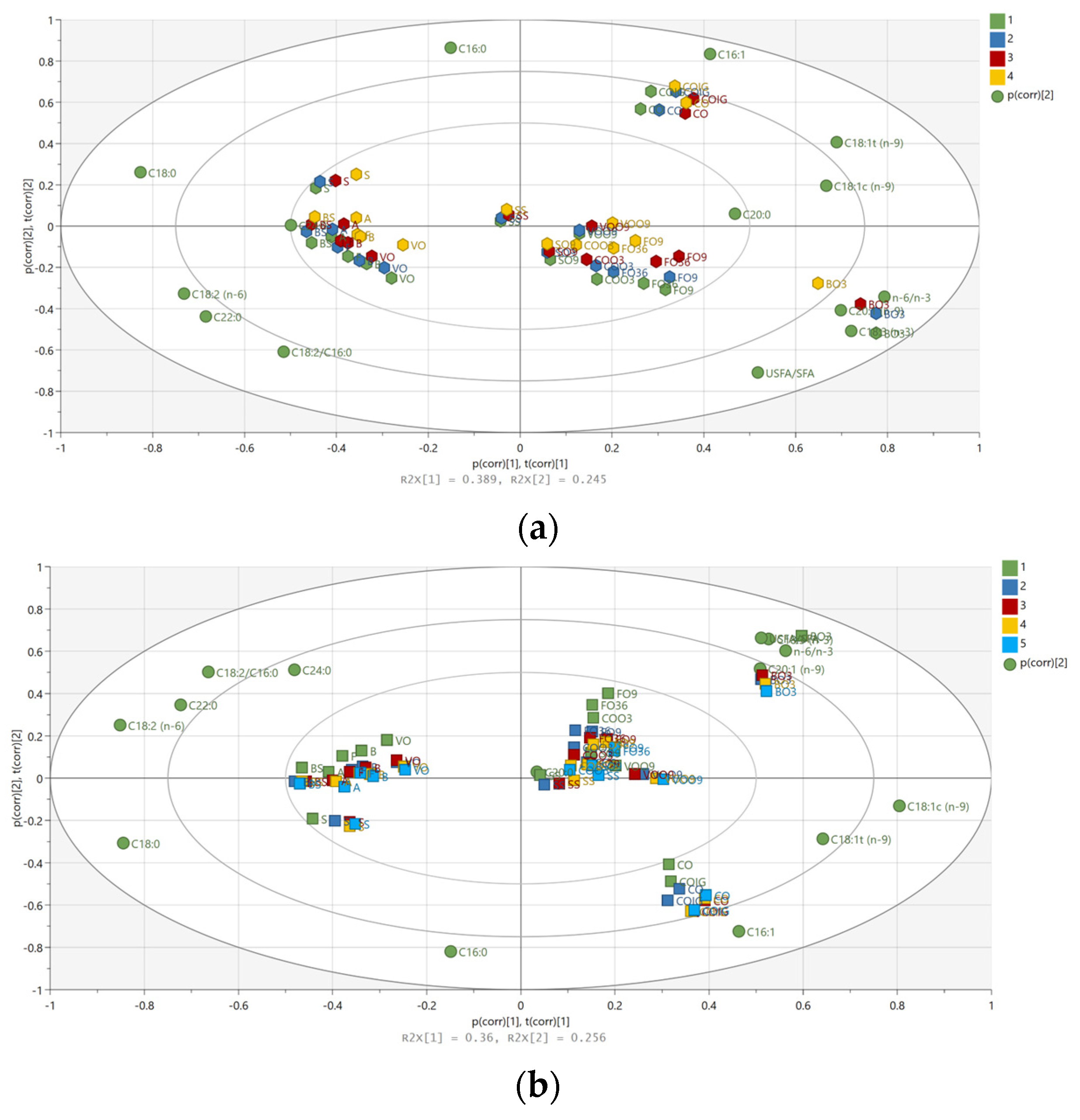
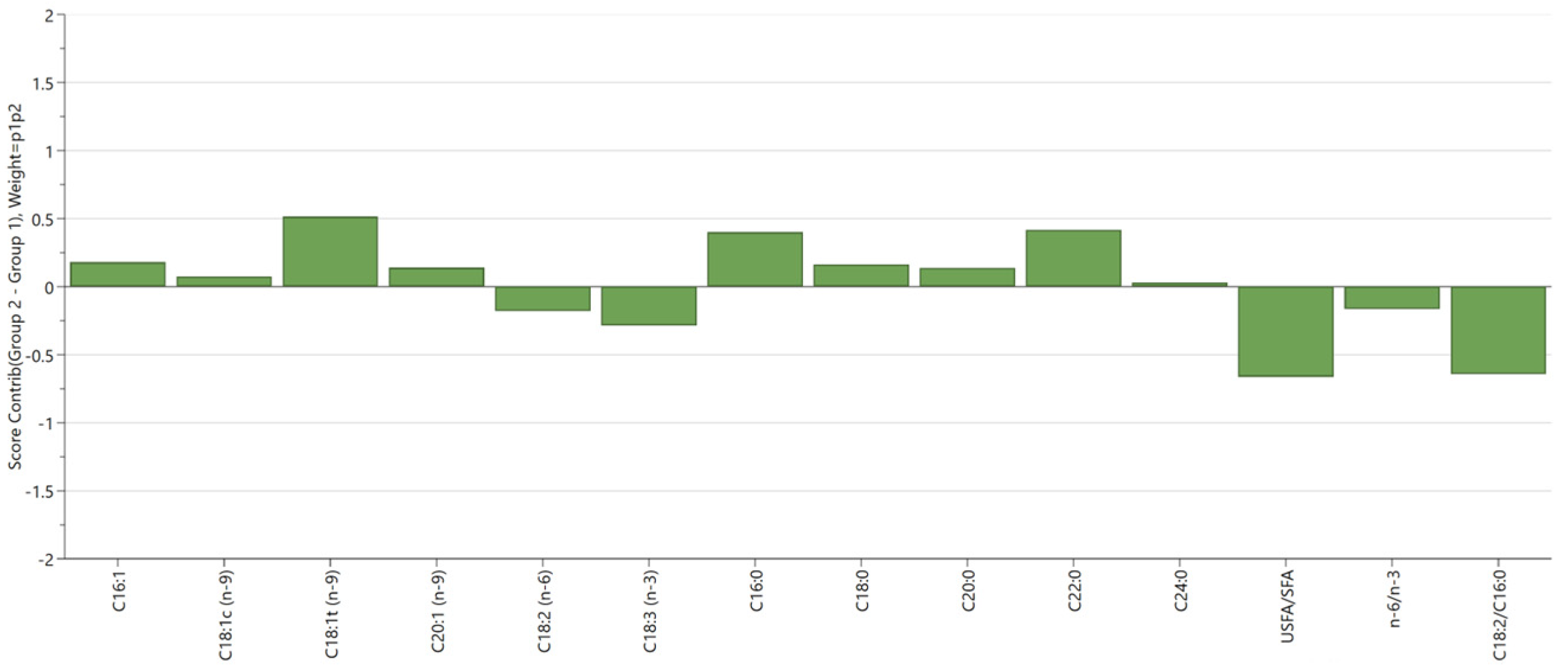
3. Results and Discussion
3.1. Fatty Acid Profile of Vegetable Oils
3.2. Multivariate Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moya Moreno, M.C.M.; Mendoza Olivares, D.; Amézquita López, F.J.; Gimeno Adelantado, J.V.; Bosch Reig, F. Determination of Unsaturation Grade and Trans Isomers Generated during Thermal Oxidation of Edible Oils and Fats by FTIR. J. Mol. Struct. 1999, 482–483, 551–556. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, P.; Liu, B.; Meng, X. Effect of Traditional Chinese Cooking Methods on Fatty Acid Profiles of Vegetable Oils. Food Chem. 2017, 233, 77–84. [Google Scholar] [CrossRef]
- Moulodi, F.; Qajarbeigi, P.; Rahmani, K.; Haj Hosseini Babaei, A.; Mohammadpoorasl, A. Effect of Fatty Acid Composition on Thermal Stability of Extra Virgin Olive Oil. J. Food Qual. Hazards Control 2015, 2, 56–60. [Google Scholar]
- Szabo, Z.; Marosvölgyi, T.; Szabo, E.; Koczka, V.; Verzar, Z.; Figler, M.; Decsi, T. Effects of Repeated Heating on Fatty Acid Composition of Plant-Based Cooking Oils. Foods 2022, 11, 192. [Google Scholar] [CrossRef]
- Endo, J.; Arita, M. Cardioprotective Mechanism of Omega-3 Polyunsaturated Fatty Acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Wu, R.; Yin, T.; You, J.; Hu, B.; Jia, C.; Rong, J.; Liu, R.; Zhang, B.; et al. Changes in the Quality of High-Oleic Sunflower Oil during the Frying of Shrimp (Litopenaeus vannamei). Foods 2023, 12, 1332. [Google Scholar] [CrossRef]
- Jaarin, K.; Kamisah, Y. Repeatedly Heated Vegetable Oils and Lipid Peroxidation. Lipid Peroxidation 2012, 2018, 211–228. [Google Scholar] [CrossRef]
- Dobarganes, C.; Márquez-Ruiz, G. Possible Adverse Effects of Frying with Vegetable Oils. Br. J. Nutr. 2015, 113, S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.P.; Yang, B. Changes in the Volatile Profile, Fatty Acid Composition and Other Markers of Lipid Oxidation of Six Different Vegetable Oils during Short-Term Deep-Frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Chiang, D.; Li, Y.T.; Perng, T.P.; Lee, S. Thermal Degradation of Vegetable Oils. Foods 2023, 12, 1839. [Google Scholar] [CrossRef]
- Berdeaux, O.; Fontagné, S.; Sémon, E.; Velasco, J.; Sébédio, J.L.; Dobarganes, C. A Detailed Identification Study on High-Temperature Degradation Products of Oleic and Linoleic Acid Methyl Esters by GC-MS and GC-FTIR. Chem. Phys. Lipids 2012, 165, 338–347. [Google Scholar] [CrossRef]
- Goh, K.M.; Wong, Y.H.; Tan, C.P.; Nyam, K.L. A Summary of 2-, 3-MCPD Esters and Glycidyl Ester Occurrence during Frying and Baking Processes: Baking and Frying Process Contaminants. Curr. Res. Food Sci. 2021, 4, 460–469. [Google Scholar] [CrossRef]
- Xu, M.; Thompson, A.; Chen, B. Dynamic Changes of 3-MCPD Esters and Glycidyl Esters Contents as Well as Oil Quality during Repeated Deep-Frying. Lwt 2022, 153, 112568. [Google Scholar] [CrossRef]
- Mitrea, L.; Teleky, B.E.; Leopold, L.F.; Nemes, S.A.; Plamada, D.; Dulf, F.V.; Pop, I.D.; Vodnar, D.C. The Physicochemical Properties of Five Vegetable Oils Exposed at High Temperature for a Short-Time-Interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Alzaa, D.F. Evaluation of Chemical and Physical Changes in Different Commercial Oils during Heating. Acta Sci. Nutr. Health 2018, 2, 2–11. [Google Scholar]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of Consumption of Repeatedly Heated Cooking Oils on the Incidence of Various Cancers- A Critical Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.; Zubair, M.; Sumrra, S.H.; Nazar, M.F.; Zafar, M.N.; Jabeen, K.; Hassan, M.B.; Rashid, U. Heating Effect on Quality Characteristics of Mixed Canola Cooking Oils. BMC Chem. 2022, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Brühl, L. Fatty Acid Alterations in Oils and Fats during Heating and Frying. Eur. J. Lipid Sci. Technol. 2014, 116, 707–715. [Google Scholar] [CrossRef]
- Gharby, S.; Hicham, H.; Bouzoubaâ, Z.; Harhar, H.; Boulbaroud, S.; El Madani, N.; Chafchaouni, I.; Charrouf, Z. The Stability of Vegetable Oils (Sunflower, Rapeseed and Palm) Sold on the Moroccan Market at High Temperature. Ijcbs 2014, 5, 47–54. [Google Scholar]
- Abbas Ali, M.; Anowarul Islam, M.; Othman, N.H.; Noor, A.M. Effect of Heating on Oxidation Stability and Fatty Acid Composition of Microwave Roasted Groundnut Seed Oil. J. Food Sci. Technol. 2017, 54, 4335–4343. [Google Scholar] [CrossRef]
- Çelebi, K.; Cerit, I.; Demirkol, O. Effect of Hops Oil on Sunflower Oil Thermal Stability. Progress. Nutr. 2021, 23. [Google Scholar] [CrossRef]
- Flores, M.; Avendaño, V.; Bravo, J.; Valdés, C.; Forero-Doria, O.; Quitral, V.; Vilcanqui, Y.; Ortiz-Viedma, J. Edible Oil Parameters during Deterioration Processes. Int. J. Food Sci. 2021, 2021, 7105170. [Google Scholar] [CrossRef]
- Liu, W.H.; Stephen Inbaraj, B.; Chen, B.H. Analysis and Formation of Trans Fatty Acids in Hydrogenated Soybean Oil during Heating. Food Chem. 2007, 104, 1740–1749. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Jung, J.; Lee, C.; Gim, S.Y.; Ka, H.J.; Yi, B.R.; Kim, M.J.; Kim, C.; Lee, J.H. Analysis of Trans Fat in Edible Oils with Cooking Process. Toxicol. Res. 2015, 31, 307–312. [Google Scholar] [CrossRef]
- Cherif, A.; Slama, A. Stability and Change in Fatty Acids Composition of Soybean, Corn, and Sunflower Oils during the Heating Process. J. Food Qual. 2022, 2022, 6761029. [Google Scholar] [CrossRef]
- Kato, S.; Shimizu, N.; Otoki, Y.; Ito, J.; Sakaino, M.; Sano, T.; Takeuchi, S.; Imagi, J.; Nakagawa, K. Determination of Acrolein Generation Pathways from Linoleic Acid and Linolenic Acid: Increment by Photo Irradiation. NPJ Sci. Food 2022, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Passi, S.J.; Misra, A.; Pant, K.K.; Anwar, K.; Pandey, R.M.; Kardam, V. Effect of Heating/Reheating of Fats/Oils, as Used by Asian Indians, on Trans Fatty Acid Formation. Food Chem. 2016, 212, 663–670. [Google Scholar] [CrossRef]
- Martin, C.A.; Milinsk, M.C.; Visentainer, J.V.; Matsushita, M.; De-Souza, N.E. Trans Fatty Acid-Forming Processes in Foods: A Review. An. Acad. Bras. Cienc. 2007, 79, 343–350. [Google Scholar] [CrossRef]
- Bansal, G.; Zhou, W.; Tan, T.W.; Neo, F.L.; Lo, H.L. Analysis of Trans Fatty Acids in Deep Frying Oils by Three Different Approaches. Food Chem. 2009, 116, 535–541. [Google Scholar] [CrossRef]
- Amine, E.K.; Baba, N.H.; Belhadj, M.; Deurenberg-Yap, M.; Djazayery, A.; Forrestre, T.; Galuska, D.A.; Herman, S.; James, W.P.T.; M’Buyamba Kabangu, J.R.; et al. Diet, Nutrition and the Prevention of Chronic Diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Nie, S.; Yang, X.; Wang, Y.; Yang, M.; Li, C.; Xie, M. The Analysis of Trans Fatty Acid Profiles in Deep Frying Palm Oil and Chicken Fillets with an Improved Gas Chromatography Method. Food Control 2014, 44, 191–197. [Google Scholar] [CrossRef]
- Tavani, A.; Negri, E.; D’Avanzo, B.; La Vecchia, C. Margarine Intake and Risk of Nonfatal Acute Myocardial Infarction in Italian Women. Eur. J. Clin. Nutr. 1997, 51, 30–32. [Google Scholar] [CrossRef][Green Version]
- Oteng, A.B.; Kersten, S. Mechanisms of Action of Trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Kummerow, F.A. The Negative Effects of Hydrogenated Trans Fats and What to Do about Them. Atherosclerosis 2009, 205, 458–465. [Google Scholar] [CrossRef]
- Bhat, S.; Maganja, D.; Huang, L.; Wu, J.H.Y.; Marklund, M. Influence of Heating during Cooking on Trans Fatty Acid Content of Edible Oils: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1489. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, S.; Moslehishad, M. Advantages of Thermal Stability of Virgin Olive Oil over Canola and Frying Oil. J. Food Bioprocess. Eng. 2020, 3, 41–46. [Google Scholar]
- Liu, Y.; Li, J.; Cheng, Y.; Liu, Y. Effect of Frying Oils’ Fatty Acid Profile on Quality, Free Radical and Volatiles over Deep-Frying Process: A Comparative Study Using Chemometrics; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 101, ISBN 0510858767. [Google Scholar]
- Ganesan, B.; Brothersen, C.; McMahon, D.J. Fortification of Foods with Omega-3 Polyunsaturated Fatty Acids. Crit. Rev. Food Sci. Nutr. 2014, 54, 98–114. [Google Scholar] [CrossRef]
- Hernandez, E.M. Issues in Fortification and Analysis of Omega-3 Fatty Acids in Foods. Lipid Technol. 2014, 26, 103–106. [Google Scholar] [CrossRef]
- Alireza, S.; Tan, C.P.; Hamed, M.; Che Man, Y.B. Effect of Frying Process on Fatty Acid Composition and Iodine Value of Selected Vegetable Oils and Their Blends. Int. Food Res. J. 2010, 17, 295–302. [Google Scholar]
- Molina-Garcia, L.; Santos, C.S.P.; Cunha, S.C.; Casal, S.; Fernandes, J.O. Comparative Fingerprint Changes of Toxic Volatiles in Low PUFA Vegetable Oils Under Deep-Frying. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 271–284. [Google Scholar] [CrossRef]
- Patel, A.; Desai, S.S.; Mane, V.K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic Food Fortification with a Balanced Ratio of Dietary ω-3/ω-6 Omega Fatty Acids for the Prevention of Lifestyle Diseases. Trends Food Sci. Technol. 2022, 120, 140–153. [Google Scholar] [CrossRef]
- Rodríguez, G.; Squeo, G.; Estivi, L.; Quezada Berru, S.; Buleje, D.; Caponio, F.; Brandolini, A.; Hidalgo, A. Changes in Stability, Tocopherols, Fatty Acids and Antioxidant Capacity of Sacha Inchi (Plukenetia volubilis) Oil during French Fries Deep-Frying. Food Chem. 2021, 340, 127942. [Google Scholar] [CrossRef]
- Alimentarius, C. Standard for Named Vegetable Oils Codex Stan 210-1999. Codex Aliment. 1999, 210, 1–13. [Google Scholar]
- Ratnayake, W.M.N.; Gagnon, C.; Dumais, L.; Lillycrop, W.; Wong, L.; Meleta, M.; Calway, P. Trans Fatty Acid Content of Canadian Margarines Prior to Mandatory Trans Fat Labelling. JAOCS J. Am. Oil Chem. Soc. 2007, 84, 817–825. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.M.; Martínez, B.; Pérez, B.; Antón, X.; Vázquez, B.I.; Fente, C.A.; Franco, C.M.; Rodríguez, J.L.; Cepeda, A. The Effects of Industrial Pre-Frying and Domestic Cooking Methods on the Nutritional Compositions and Fatty Acid Profiles of Two Different Frozen Breaded Foods. Lwt 2010, 43, 1271–1276. [Google Scholar] [CrossRef]

| Oil ID | Composition | Brand | Abbreviation |
|---|---|---|---|
| A | Refined sunflower oil | Brand 6 | RS |
| BS | Unrefined sunflower oil (extra virgin) | Brand 7 | URS |
| BO3 | Refined rapeseed oil | Brand 1 | RR |
| CO | Extra virgin olive oil | Brand 3 | EVOO |
| COO3 | Oil blend (sunflower, grapeseed, corn, flaxseed, rice bran) | Brand 3 | MX |
| FO36 | Refined rapeseed oil (60%) and sunflower oil (40%) | Brand 2 | MX |
| F | Refined sunflower oil | Brand 2 | RS |
| COIG | Extra virgin olive oil | Brand 3 | EVOO |
| SS | Unrefined sunflower oil (extra virgin) | Brand 8 | URS |
| S | Refined sunflower oil | Brand 4 | RS |
| SO9 | Refined high-oleic sunflower oil | Brand 4 | RS |
| B | Refined sunflower oil | Brand 1 | RS |
| FO9 | Refined rapeseed oil (70%) and refined sunflower oil (high-oleic) | Brand 2 | MX |
| VO | Refined sunflower oil | Brand 5 | RS |
| VOO9 | Refined high-oleic sunflower oil | Brand 5 | RS |
| Vegetable Oils/Fatty Acids | Rapeseed Oil (%) | Sunflower Oil (%) | High-Oleic Sunflower Oil (%) | EVOO (%) | Oil Blends (%) |
|---|---|---|---|---|---|
| saturated fatty acid (SFA) | |||||
| C16:0 | 4.26 | 5.89–12.38 | 4.09–4.33 | 12.56–14.77 | 5.03–5.74 |
| C18:0 | 1.48 | 3.12–6.63 | 2.50–2.84 | 2.98–3.16 | 2.55–2.78 |
| C20:0 | 0.62 | 0.28–0.36 | 0.26–0.33 | 0.49–0.52 | 0.30–0.58 |
| C22:0 | 0.48 | 0.91–1.10 | 0.94–1.20 | 0.23–0.24 | 0.74–0.80 |
| C24:0 | 0.29 | 0.37–0.49 | 0.37–0.48 | 0.54–0.59 | 0.34–0.38 |
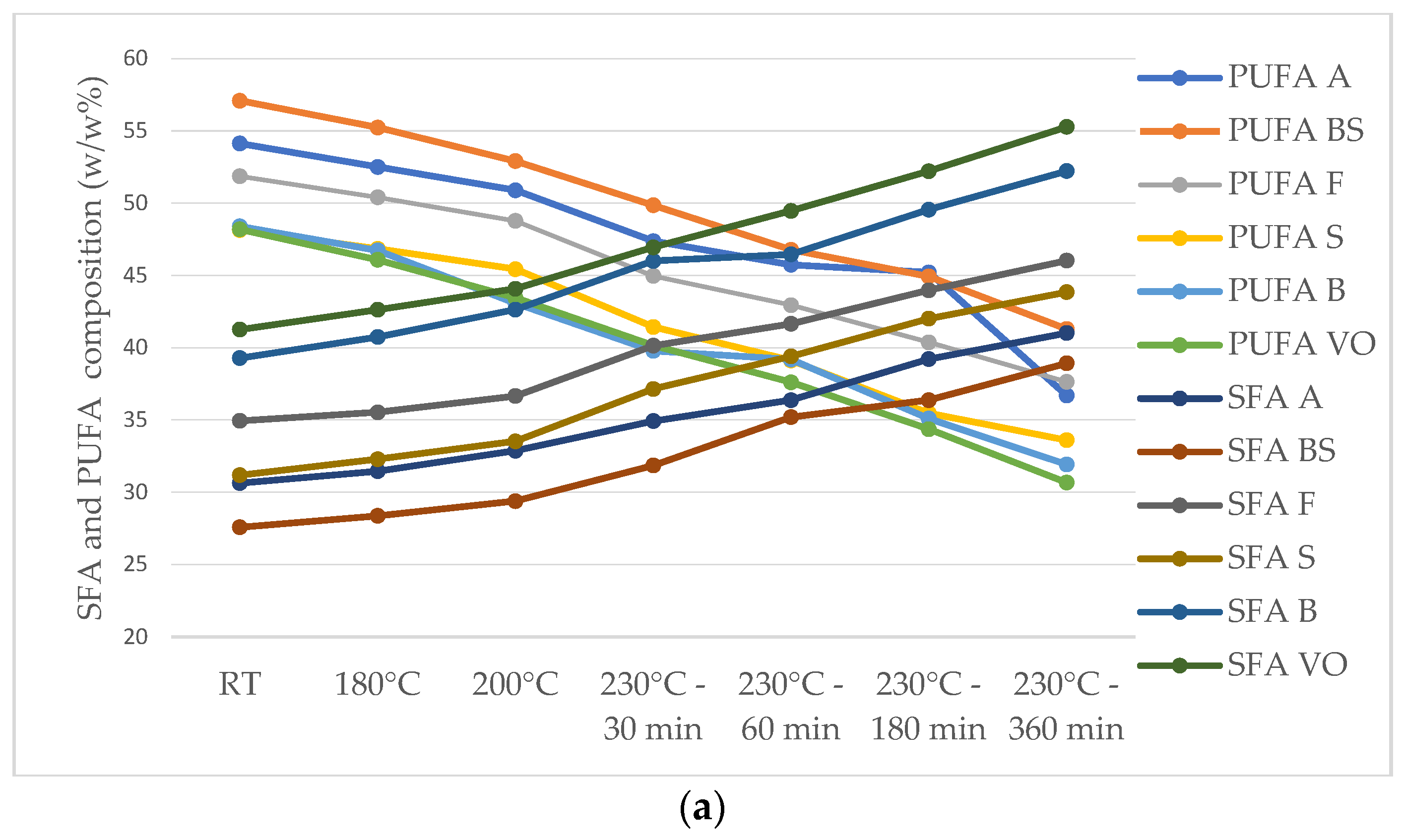
| SFA TOTAL | 7.17 | 10.53–20.66 | 8.19–9.19 | 16.86–19.24 | 8.98–10.02 |
| monounsaturated fatty acid (MUFA) | |||||
| C16:1 | 0.27 | 0.14–0.18 | 0.15–0. 17 | 1.15–1.28 | 0.15–0.24 |
| C18:1c (n-9) | 60.55 | 26.18–40.17 | 81.30–83.65 | 68.26–71.08 | 47.43–60.79 |
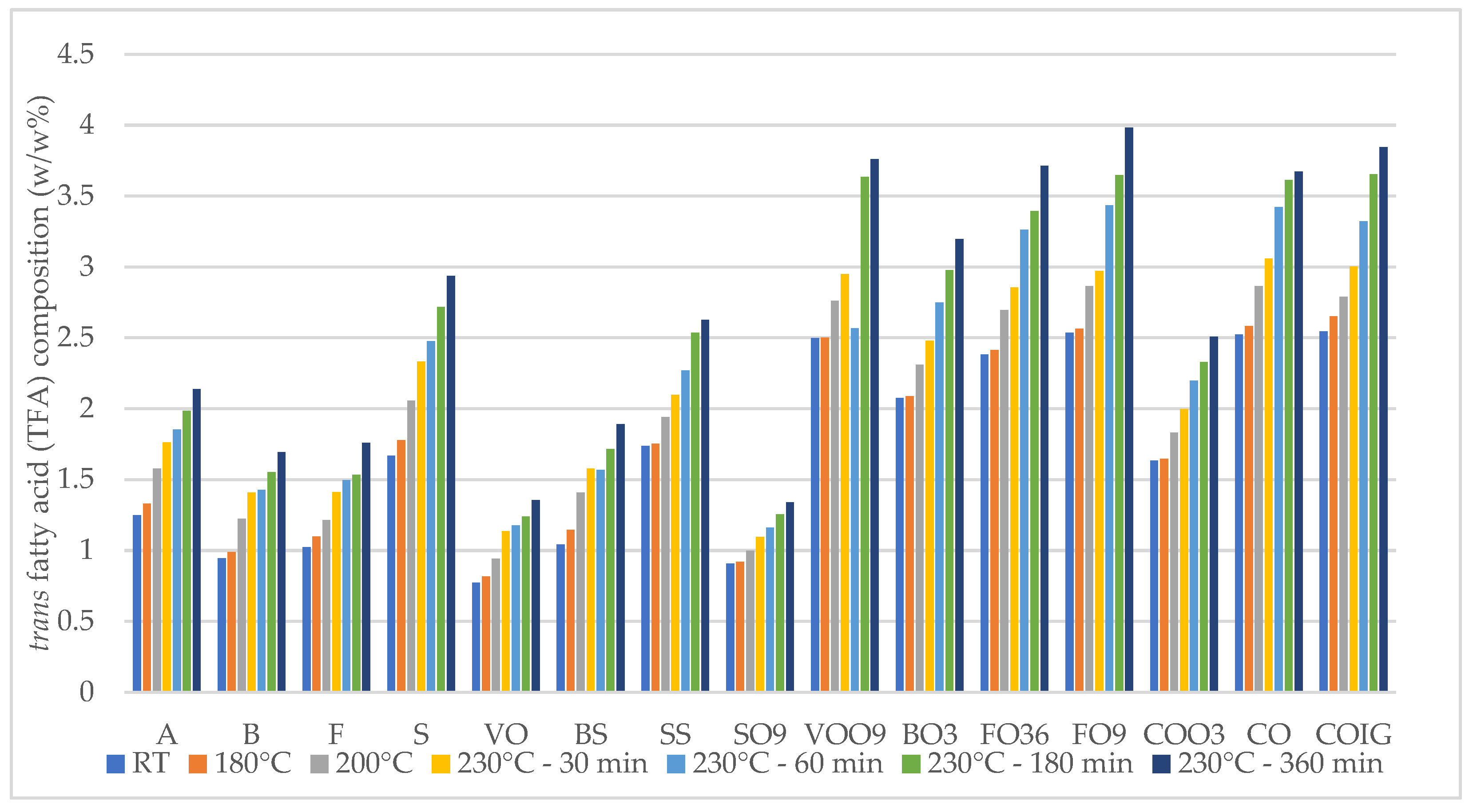
| C18:1t (n-9) | 2.07 | 0.77–1.66 | 0.90–2. 49 | 2.52–2.54 | 1.63–2.53 |
| C20:1 (n-9) | 1.30 | 0.16–0.20 | 0.20–0.26 | 0.26–0.35 | 0.24–0.90 |
| MUFA TOTAL | 64.20 | 27.58–41.25 | 82.58–86.60 | 72.44–75.02 | 50.87–62.83 |
| polyunsaturated fatty acid (PUFA) | |||||
| C18:2 (n-6) | 21.02 | 48.07–56.99 | 4.14–9.17 | 7.46–7.57 | 24.70–34.65 |
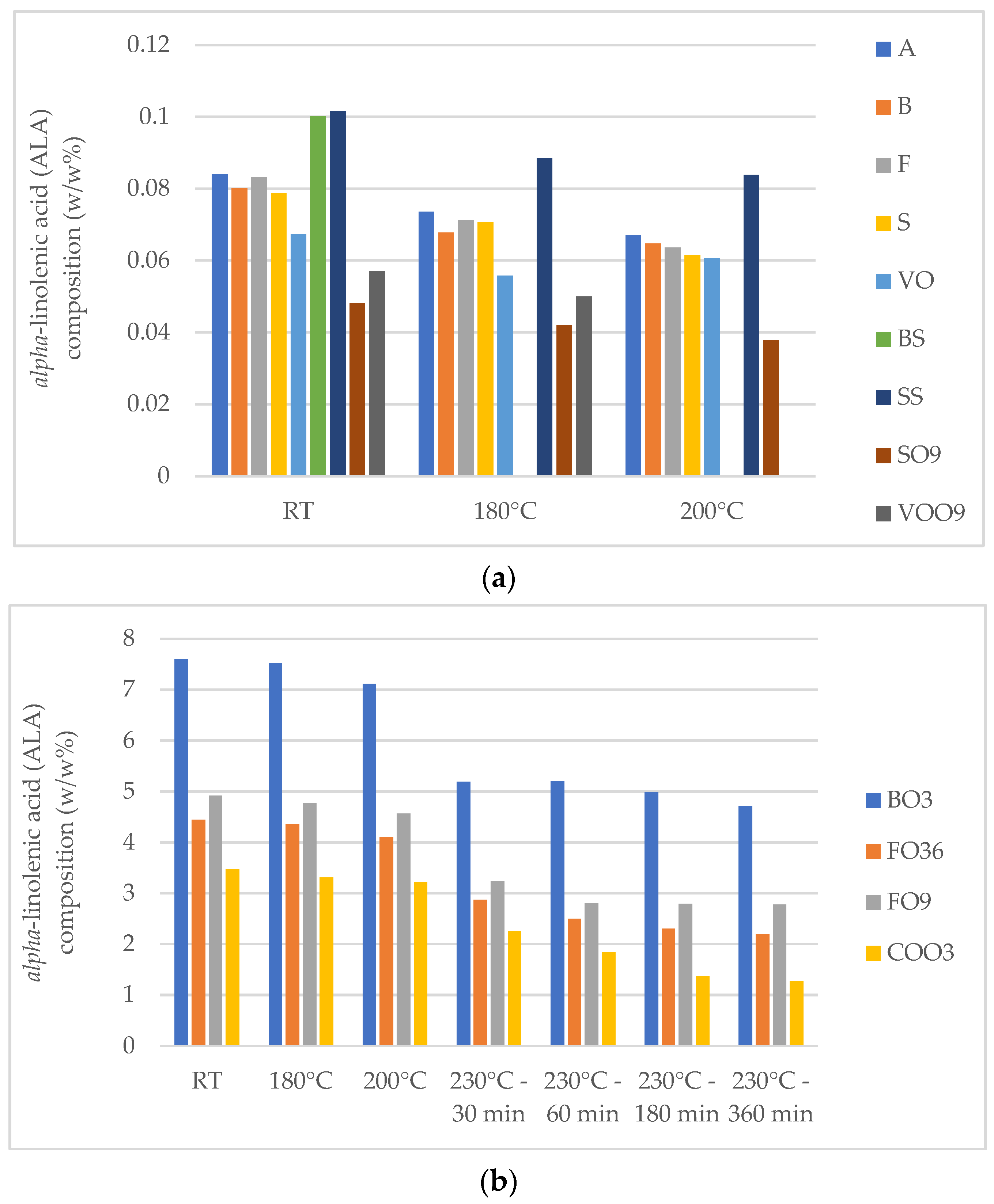
| C18:3 (n-3) | 7.60 | 0.06–0.10 | 0.04–0.05 | 0.64–0.73 | 3.47–4.91 |
| PUFA TOTAL | 28.62 | 48.15–57.09 | 4.19–9.22 | 8.10–8.30 | 28.18–39.09 |
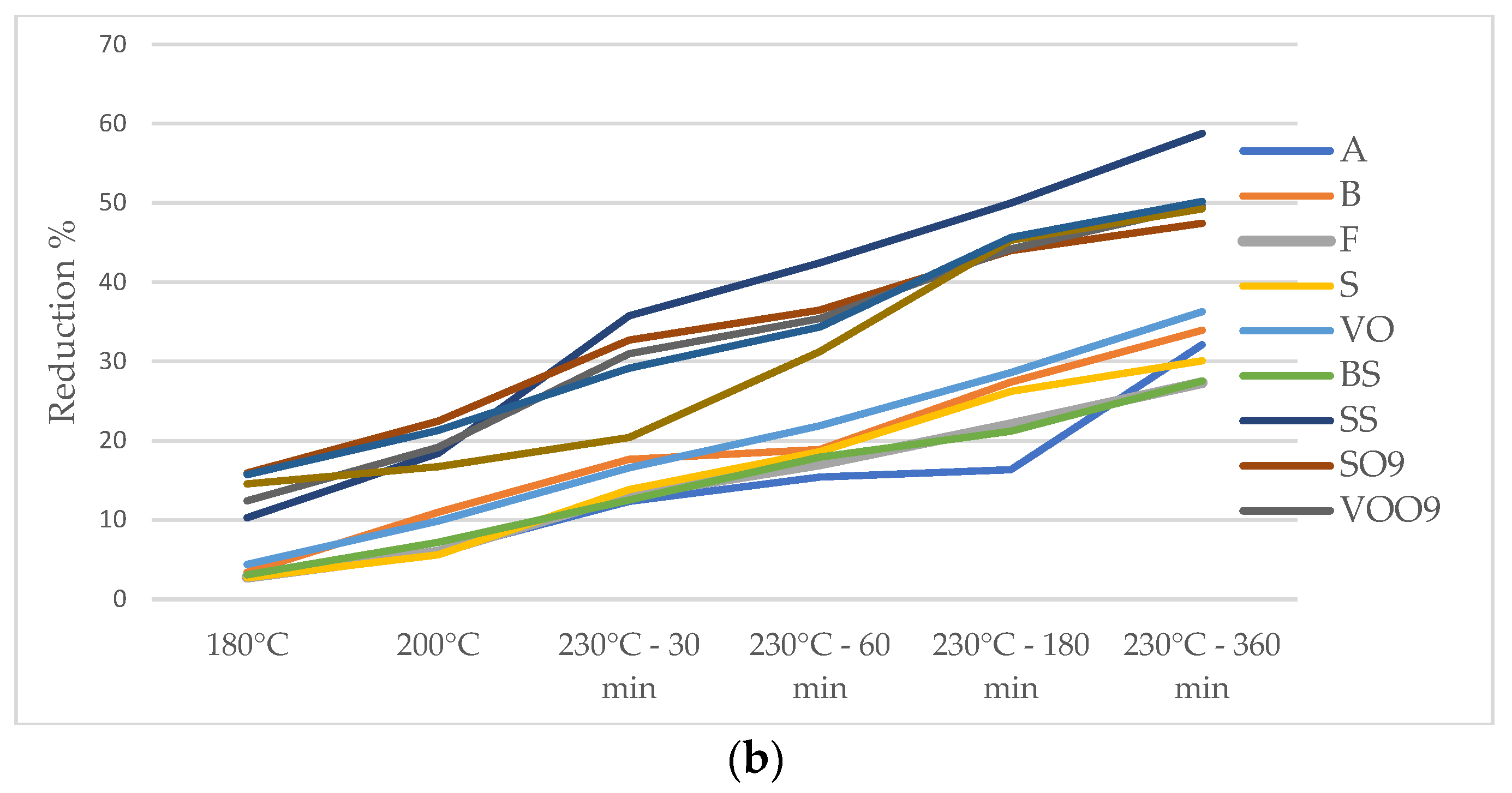
| Vegetable Oil Type | Vegetable Oil ID | RT | 180 °C | 200 °C | 30 min @230 °C | 60 min @230 °C | 180 min @230 °C | 360 min @230 °C |
|---|---|---|---|---|---|---|---|---|
| Sunflower oil | A | 9.44 | 8.95 | 8.83 | 8.62 | 8.50 | 8.60 | 7.71 |
| B | 12.88 | 12.83 | 11.85 | 12.00 | 11.77 | 11.05 | 10.78 | |
| F | 11.75 | 11.10 | 10.62 | 10.38 | 10.19 | 9.99 | 9.74 | |
| S | 6.41 | 6.34 | 6.41 | 6.25 | 6.12 | 5.80 | 5.83 | |
| VO | 15.18 | 14.76 | 13.18 | 12.37 | 12.64 | 12.21 | 12.03 | |
| BS | 9.77 | 9.00 | 8.48 | 8.25 | 8.48 | 8.15 | 7.62 | |
| SS | 15.36 | 15.19 | 15.00 | 14.76 | 15.67 | 16.91 | 19.45 | |
| High-oleic sunflower oil | SO9 | 22.40 | 20.47 | 20.79 | 19.19 | 19.13 | 19.50 | 19.81 |
| VOO9 | 20.96 | 20.99 | 20.80 | 20.25 | 20.43 | 20.75 | 21.73 | |
| Rapeseed oil | BO3 | 21.77 | 17.55 | 17.21 | 16.52 | 16.23 | 14.13 | 13.65 |
| Oil blend | FO36 | 15.66 | 14.44 | 14.09 | 13.16 | 13.24 | 12.90 | 12.20 |
| FO9 | 17.55 | 15.31 | 13.24 | 12.30 | 12.77 | 12.13 | 11.28 | |
| COO3 | 18.06 | 16.54 | 16.19 | 15.34 | 15.15 | 14.62 | 14.01 | |
| EVOO | CO | 6.61 | 6.52 | 6.54 | 6.30 | 6.24 | 6.21 | 6.24 |
| COIG | 5.47 | 5.35 | 5.63 | 5.37 | 5.56 | 5.43 | 5.36 |
| Temperature Variations | Exposure Time at 230 °C | Vegetable Oil Types |
|---|---|---|
| Room Temperature (RT) | 30 min | Sunflower oil (A, B, BS, F, S, SS, VO) |
| 180 °C | 60 min | Omega-9 oil (FO9, SO9, VOO9) |
| 200 °C | 180 min | Omega-3, 6 oil (BO3, COO3, FO36) |
| 230 °C | 360 min | Olive oil (CO, COIG) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, K.; Iacob, B.-C.; Bodoki, E.; Oprean, R. Investigating the Thermal Stability of Omega Fatty Acid-Enriched Vegetable Oils. Foods 2024, 13, 2961. https://doi.org/10.3390/foods13182961
Nagy K, Iacob B-C, Bodoki E, Oprean R. Investigating the Thermal Stability of Omega Fatty Acid-Enriched Vegetable Oils. Foods. 2024; 13(18):2961. https://doi.org/10.3390/foods13182961
Chicago/Turabian StyleNagy, Katalin, Bogdan-Cezar Iacob, Ede Bodoki, and Radu Oprean. 2024. "Investigating the Thermal Stability of Omega Fatty Acid-Enriched Vegetable Oils" Foods 13, no. 18: 2961. https://doi.org/10.3390/foods13182961
APA StyleNagy, K., Iacob, B.-C., Bodoki, E., & Oprean, R. (2024). Investigating the Thermal Stability of Omega Fatty Acid-Enriched Vegetable Oils. Foods, 13(18), 2961. https://doi.org/10.3390/foods13182961






