Implementation of Cellulose-Based Filtration Aids in Industrial Sunflower Oil Dewaxing (Winterization): Process Monitoring, Prediction, and Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Industrial Winterization (Dewaxing)
2.2. Cellulose-Based Filtration Aids
2.3. Oil Samples
Investigation of Wax Content
2.4. Monitoring of Process Parameters in Oil Filtration
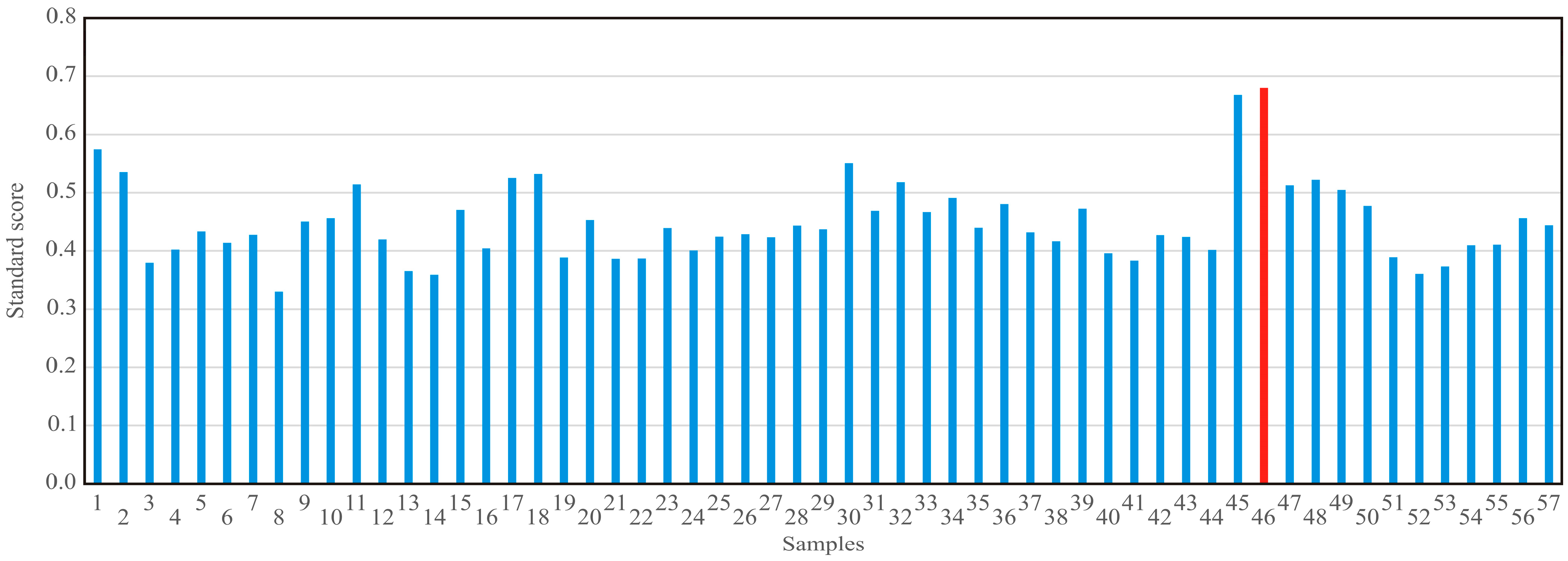
2.5. Standard Scores
2.6. Artificial Neural Network (ANN) Model
Error Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Principal Component Analysis
3.2. Standard Scores and Process Optimization
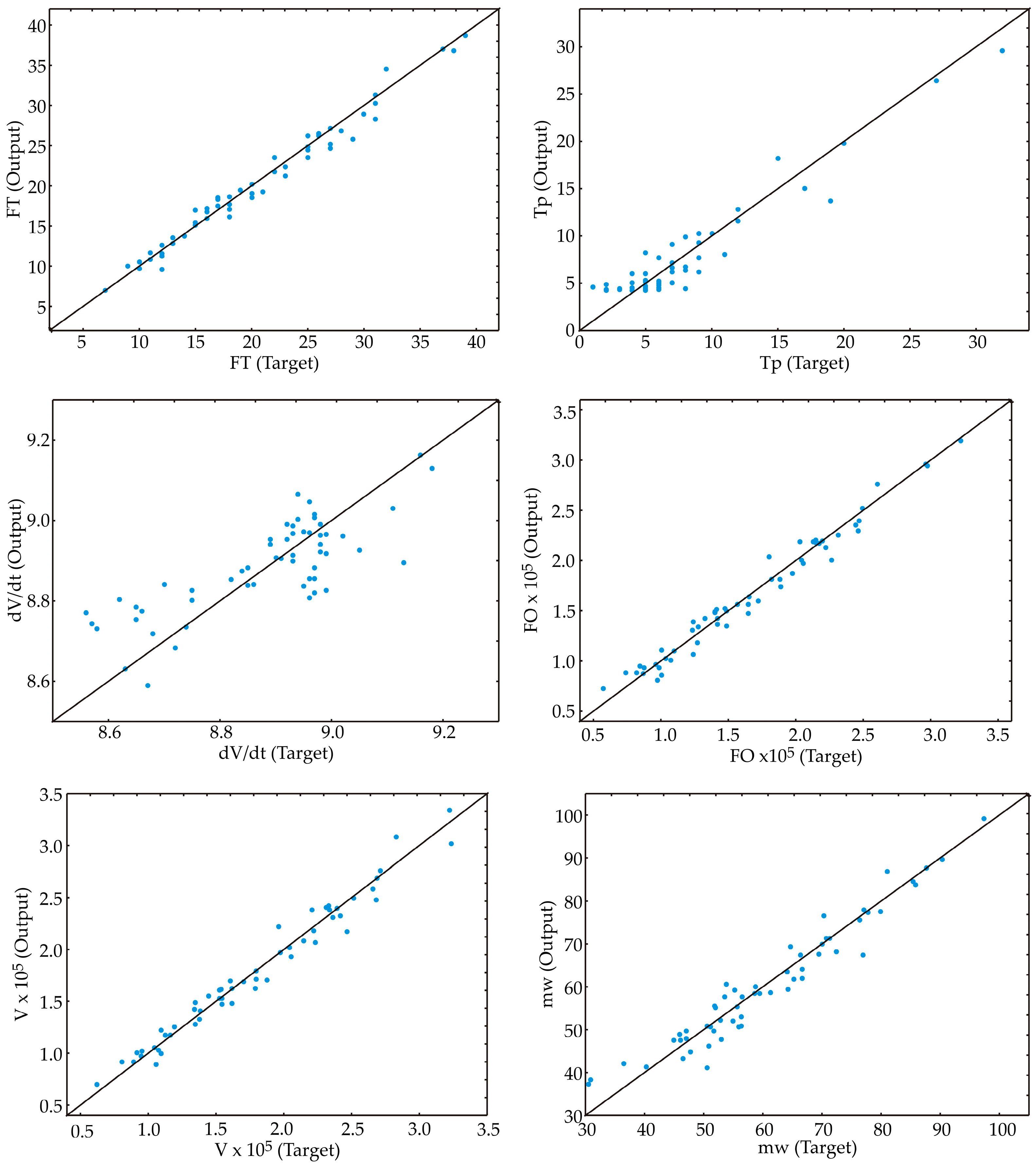
3.3. Artificial Neural Network
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manjula, S.; Subramanian, R. Membrane Technology in Degumming, Dewaxing, Deacidifying, and Decolorizing Edible Oils. Crit. Rev. Food Sci. Nutr. 2006, 46, 569–592. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K. Practical Guide to Vegetable Oil Processing, 2nd ed.; AOCS Press: Urbana, IL, USA, 2017; pp. 1–488. [Google Scholar]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, 6627013. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Añón, M.C. Crystallization of sunflower oil waxes. J. Am. Oil Chem. Soc. 2003, 80, 525–532. [Google Scholar] [CrossRef]
- Nedić Grujin, K.; Lužaić, T.; Pezo, L.; Nikolovski, B.; Maksimović, Z.; Romanić, R. Sunflower Oil Winterization Using the Cellulose-Based Filtration Aid—Investigation of Oil Quality during Industrial Filtration Probe. Foods 2023, 12, 2291. [Google Scholar] [CrossRef]
- Broughton, R.; Ruíz-Lopez, N.; Hassall, K.L.; Martínez-Force, E.; Garcés, R.; Salas, J.J.; Beaudoin, F. New insights in the composition of wax and sterol esters in common and mutant sunflower oils revealed by ESI-MS/MS. Food Chem. 2018, 269, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A. Characterization of waxes and residual oil recovered from sunflower oil winterization waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1500608. [Google Scholar] [CrossRef]
- Bokish, M. The Importance of Fats. In Fats and Oils Handbook; Bokish, M., Ed.; AOCS Press: Urbana, IL, USA, 1998; pp. 1–52. [Google Scholar]
- Ripperger, S.; Gösele, W.; Alt, C. Filtration, 1. Fundamentals. In Ullmann’s Encyclopedia of Industrial Chemistry; Goldmann, C., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 14, pp. 678–708. [Google Scholar]
- Buyel, J.F.; Opdensteinen, P.; Fischer, R. Cellulose-based filter aids increase the capacity of depth filters during the downstream processing of plant-derived biopharmaceutical proteins. Biotechnol. J. 2015, 10, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Smith, G. Filter aid filtration. In Filtration in the Biopharmaceutical Industry, 1st ed.; Meltzer, T.H., Jornitz, M.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1998; Volume 1, pp. 1–69. [Google Scholar]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Dahlstrom, D.A.; Bennett, R.C.; Emmett, R.C.; Harriott, P., Jr.; Laros, T.; Leung, W.; McCleary, C.S.; Morey, A.M.B.; Oldshue, J.Y.; Priday, G. Liquid-Solid Operations and Equipment. In Perry’s Chemical Engineers’ Handbook, 7th ed.; Perry, R.H., Green, D.W., Eds.; McGraw-Hill Companies Inc.: New York, NY, USA, 1997; pp. 1–133. [Google Scholar]
- Di Pretoro, A.; Manenti, F. Filtration. In Non-conventional Unit Operations: Solving Practical Issues, 1st ed.; Di Pretoro, A., Manenti, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 47–57. [Google Scholar]
- Du, L.; Chen, X.; Li, W.; Zhu, Q. A study on enhancement of filtration process with filter aids diatomaceous earth and wood pulp cellulose. Chin. J. Chem. Eng. 2011, 19, 792–798. [Google Scholar] [CrossRef]
- Oštrić-Matijašević, B.; Turkulov, J. Determination of Waxes in Sunflower Grains, Shells and Oil. Modified Method of Extraction. Rev. Fr. Corps Gras 1973, 20, 5–10. [Google Scholar]
- Brlek, T.; Pezo, L.; Voća, N.; Krička, T.; Vukmirović, Đ.; Čolović, R.; Bodroža-Solarov, M. Chemometric approach for assessing the quality of olive cake pellets. Fuel Process. Technol. 2013, 116, 250–256. [Google Scholar] [CrossRef]
- Kavuncuoglu, H.; Kavuncuoglu, E.; Karatas, S.M.; Benli, B.; Sagdic, O.; Yalcin, H. Prediction of the antimicrobial activity of walnut (Juglans regia L.) kernel aqueous extracts using artificial neural network and multiple linear regression. J. Microbiol. Methods 2018, 148, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Pezo, L.; Marić, B.; Tukuljac, L.P.; Zeković, Z.; Solarov, M.B.; Teslić, N. Supercritical fluid extraction of raspberry seed oil: Experiments and modelling. J. Supercrit. Fluids 2020, 157, 104687. [Google Scholar] [CrossRef]
- Bajić, A.; Pezo, L.; Stupar, A.; Filipčev, B.; Cvetković, B.R.; Horecki, A.T.; Mastilović, J. Application of lyophilized plum pomace as a functional ingredient in a plum spread: Optimizing texture, colour and phenol antioxidants by ANN modelling. LWT-Food Sci. Technol. 2020, 130, 109588. [Google Scholar] [CrossRef]
- Aćimović, M.; Pezo, L.; Tešević, V.; Čabarkapa, I.; Todosijević, M. QSRR Model for predicting retention indices of Satureja kitaibelii Wierzb. ex Heuff. essential oil composition. Ind. Crops Prod. 2020, 154, 112752. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Nergiz, C. The effect of different filter aid materials and winterization periods on the oxidative stability of sunflower and corn oils. CyTA-J. Food 2015, 13, 174–180. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Nergiz, C. A research on the turbidity and wax composition of sunflower and corn oils during winterization. Bulg. J. Agric. Sci. 2013, 19, 497–502. [Google Scholar]
- Heertjes, P.M.; Zuideveld, P.L. Clarification of liquids using filter aids Part III. Cake Resistance in surface filtration. Powder Technol. 1978, 19, 45–64. [Google Scholar] [CrossRef]
- Pergam, P.; Kuhn, M.; Briesen, H. Optimal dosage strategies for filter aid filtration processes with compressible cakes. Chem. Eng. Sci. 2022, 262, 117989. [Google Scholar] [CrossRef]
- Otto, M. Chemometrics Statistics and Computer Application in Analytical, Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2017; pp. 1–369. [Google Scholar]
- Kaiser, H.F.; Rice, J. Little Jiffy, mark IV. Educ. Psychol. Meas. 1974, 34, 111–117. [Google Scholar] [CrossRef]

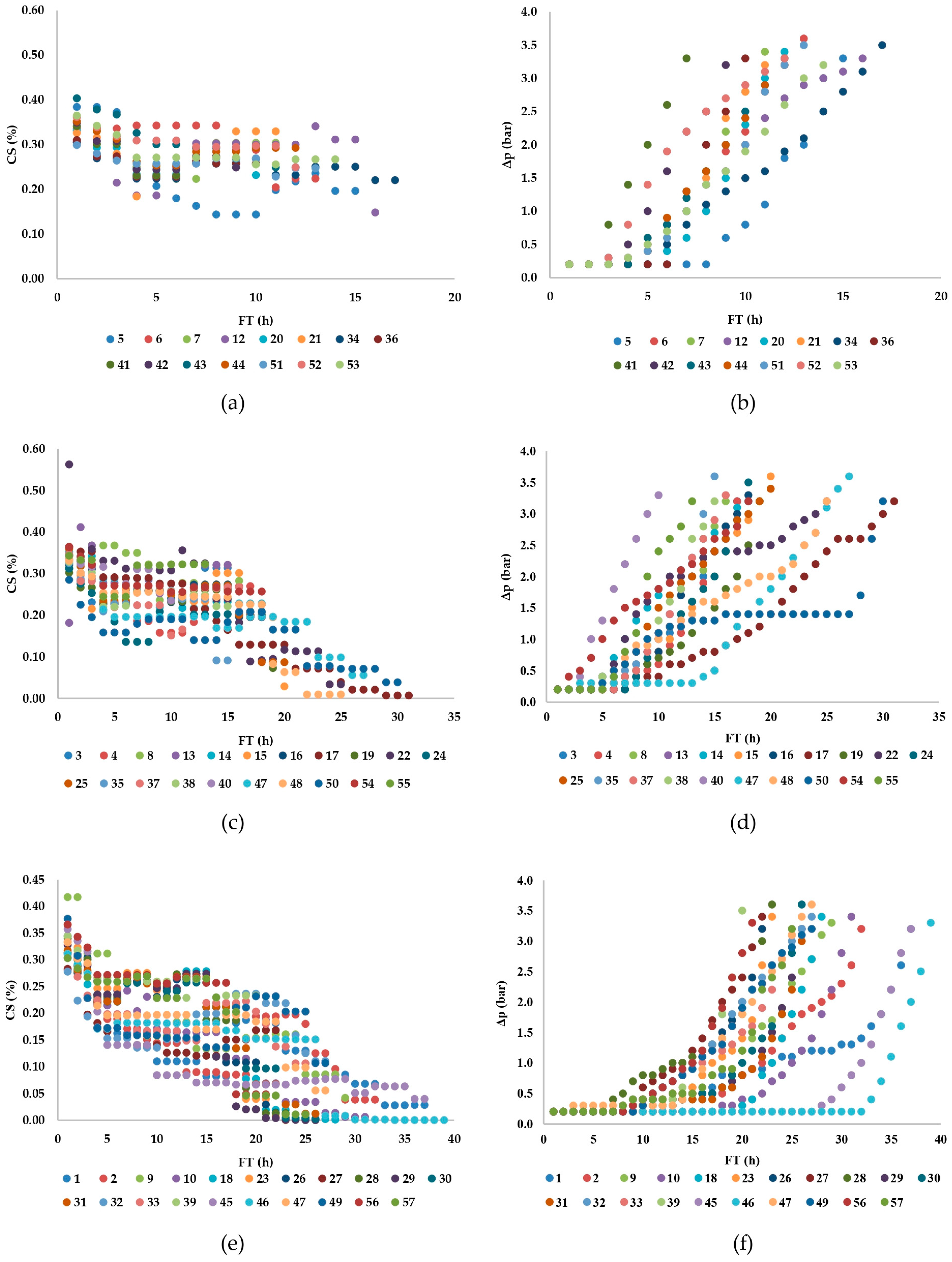

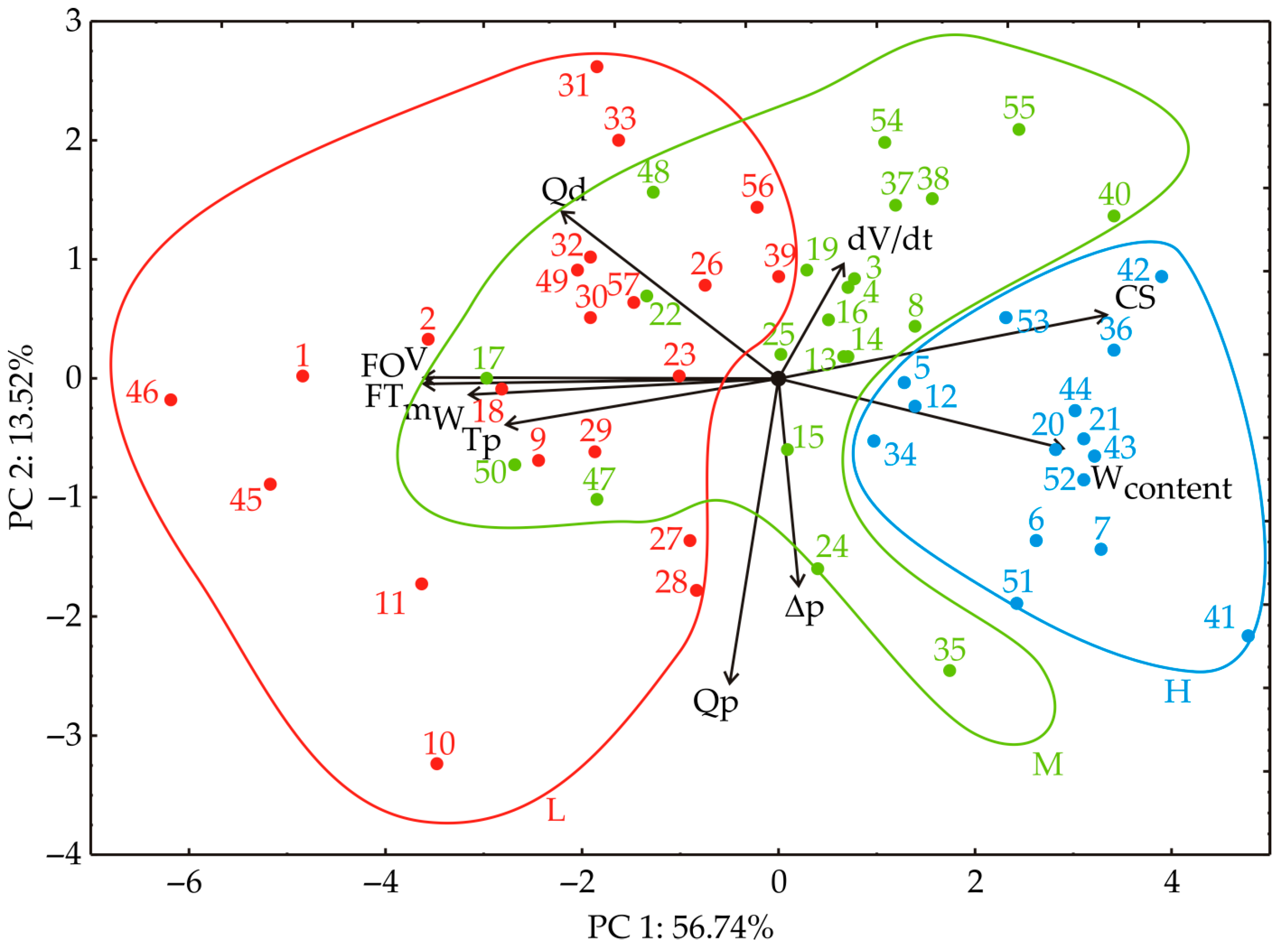
| Filtration Cycle | Dosing Filtration Aid | Qp (kg) | Qd (kg) | CS (% w/w) | Δp (bar) | Tp const. (h) ∆p = 0.2 | dV/dt (m3/h) | FO (kg) | V (m3) | mW (kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FA-I | 80 | 375 | 0.13 | 3.2 | 15 | 8.70 | 296,237 | 321.997 | 87.69 |
| 2 | FA-I | 80 | 375 | 0.14 | 3.2 | 12 | 8.85 | 260,581 | 283.240 | 81.04 |
| 3 | FA-I | 80 | 375 | 0.25 | 3.2 | 6 | 8.96 | 140,083 | 152.264 | 53.51 |
| 4 | FA-I | 80 | 350 | 0.23 | 3.2 | 7 | 9.05 | 141,554 | 153.863 | 51.81 |
| 5 | FA-I | 80 | 325 | 0.23 | 3.3 | 8 | 8.97 | 123,827 | 134.595 | 53.86 |
| 6 | FA-I | 80 | 325 | 0.30 | 3.6 | 3 | 8.68 | 103,829 | 112.858 | 52.85 |
| 7 | FA-I | 80 | 250 | 0.28 | 3.4 | 4 | 8.62 | 87,232 | 94.817 | 47.72 |
| 8 | FA-I | 80 | 375 | 0.31 | 3.2 | 6 | 8.63 | 126,976 | 138.017 | 51.17 |
| 9 | FA-I | 80 | 375 | 0.18 | 3.3 | 9 | 8.32 | 222,101 | 241.414 | 72.40 |
| 10 | FA-I | 125 | 325 | 0.13 | 3.4 | 17 | 8.66 | 247,125 | 268.614 | 69.44 |
| 11 | FA-II | 100 | 350 | 0.14 | 3.4 | 20 | 8.75 | 249,442 | 271.133 | 70.34 |
| 12 | FA-I | 80 | 375 | 0.27 | 3.3 | 4 | 8.67 | 127,570 | 138.663 | 70.04 |
| 13 | FA-I | 80 | 375 | 0.27 | 3.2 | 6 | 8.57 | 141,880 | 154.217 | 59.45 |
| 14 | FA-I | 80 | 375 | 0.26 | 3.2 | 4 | 8.56 | 141,797 | 154.127 | 58.70 |
| 15 | FA-I | 80 | 375 | 0.24 | 3.6 | 6 | 8.97 | 164,980 | 179.326 | 66.65 |
| 16 | FA-I | 80 | 375 | 0.25 | 3.3 | 7 | 8.97 | 148,604 | 161.526 | 56.47 |
| 17 | FA-I | 80 | 375 | 0.17 | 3.2 | 6 | 8.65 | 246,634 | 268.080 | 97.42 |
| 18 | FA-I | 80 | 375 | 0.17 | 3.4 | 19 | 8.99 | 231,483 | 251.612 | 66.67 |
| 19 | FA-I | 80 | 375 | 0.24 | 3.2 | 5 | 8.98 | 156,903 | 170.547 | 55.70 |
| 20 | FA-I | 80 | 250 | 0.26 | 3.3 | 5 | 8.95 | 98,810 | 107.402 | 50.89 |
| 21 | FA-I | 80 | 275 | 0.28 | 3.2 | 4 | 8.65 | 87,567 | 95.182 | 46.06 |
| 22 | FA-I | 80 | 450 | 0.24 | 3.2 | 4 | 8.58 | 197,439 | 214.608 | 76.41 |
| 23 | FA-II | 80 | 375 | 0.21 | 3.4 | 10 | 8.89 | 188,064 | 204.417 | 56.42 |
| 24 | FA-I | 100 | 325 | 0.21 | 3.5 | 7 | 8.93 | 147,808 | 160.661 | 52.03 |
| 25 | FA-I | 80 | 375 | 0.23 | 3.4 | 5 | 8.98 | 165,202 | 179.567 | 58.65 |
| 26 | FA-II | 80 | 375 | 0.21 | 3.2 | 8 | 8.98 | 181,800 | 197.609 | 61.27 |
| 27 | FA-I | 100 | 325 | 0.16 | 3.4 | 8 | 8.91 | 180,385 | 196.071 | 55.20 |
| 28 | FA-I | 100 | 350 | 0.20 | 3.6 | 6 | 8.93 | 189,063 | 205.503 | 64.28 |
| 29 | FA-I | 100 | 350 | 0.16 | 3.2 | 7 | 8.90 | 212,924 | 231.439 | 70.69 |
| 30 | FA-I | 60 | 400 | 0.19 | 3.6 | 12 | 8.98 | 214,737 | 233.410 | 66.35 |
| 31 | FA-II | 60 | 400 | 0.19 | 3.0 | 9 | 8.99 | 214,996 | 233.691 | 64.07 |
| 32 | FA-I | 60 | 400 | 0.19 | 3.4 | 7 | 8.85 | 219,891 | 239.012 | 71.24 |
| 33 | FA-II | 60 | 400 | 0.19 | 3.1 | 9 | 8.84 | 203,219 | 220.890 | 64.62 |
| 34 | FA-II | 80 | 375 | 0.25 | 3.5 | 5 | 8.97 | 140,305 | 152.505 | 77.03 |
| 35 | FA-II | 100 | 250 | 0.23 | 3.6 | 5 | 8.96 | 123,707 | 134.464 | 51.71 |
| 36 | FA-I | 60 | 225 | 0.26 | 3.3 | 6 | 8.94 | 82,212 | 89.361 | 40.28 |
| 37 | FA-II | 60 | 350 | 0.25 | 3.3 | 6 | 9.02 | 132,746 | 144.289 | 47.12 |
| 38 | FA-II | 60 | 325 | 0.25 | 3.2 | 5 | 8.94 | 123,411 | 134.142 | 44.92 |
| 39 | FA-I | 60 | 375 | 0.26 | 3.5 | 11 | 8.96 | 164,906 | 179.246 | 52.93 |
| 40 | FA-I | 60 | 275 | 0.29 | 3.3 | 2 | 9.16 | 84,266 | 91.593 | 30.25 |
| 41 | FA-I | 100 | 150 | 0.28 | 3.3 | 2 | 8.92 | 57,426 | 62.420 | 29.86 |
| 42 | FA-I | 60 | 250 | 0.28 | 3.2 | 2 | 8.93 | 73,913 | 80.340 | 36.51 |
| 43 | FA-I | 80 | 250 | 0.31 | 3.3 | 4 | 8.82 | 97,364 | 105.830 | 50.53 |
| 44 | FA-I | 80 | 300 | 0.29 | 3.2 | 2 | 8.72 | 96,254 | 104.624 | 54.96 |
| 45 | FA-II | 80 | 275 | 0.10 | 3.2 | 27 | 8.74 | 297,423 | 323.286 | 85.35 |
| 46 | FA-I | 80 | 375 | 0.12 | 3.3 | 32 | 8.99 | 322,503 | 350.547 | 90.41 |
| 47 | FA-II | 80 | 375 | 0.18 | 3.6 | 5 | 8.75 | 217,384 | 236.287 | 77.85 |
| 48 | FA-II | 60 | 375 | 0.20 | 3.2 | 5 | 8.93 | 205,337 | 223.192 | 79.88 |
| 49 | FA-I | 80 | 375 | 0.19 | 3.2 | 9 | 9.13 | 226,770 | 246.489 | 76.88 |
| 50 | FA-I | 100 | 350 | 0.15 | 3.2 | 4 | 8.86 | 244,400 | 265.652 | 85.78 |
| 51 | FA-I | 100 | 275 | 0.26 | 3.5 | 3 | 8.95 | 107,053 | 116.362 | 47.08 |
| 52 | FA-I | 100 | 275 | 0.31 | 3.3 | 2 | 9.11 | 100,530 | 109.272 | 46.48 |
| 53 | FA-I | 80 | 325 | 0.28 | 3.2 | 3 | 8.96 | 100,530 | 109.272 | 50.53 |
| 54 | FA-II | 60 | 375 | 0.28 | 3.2 | 1 | 8.97 | 148,548 | 161.465 | 56.00 |
| 55 | FA-II | 60 | 350 | 0.31 | 3.2 | 5 | 9.18 | 109,773 | 119.318 | 45.89 |
| 56 | FA-I | 60 | 375 | 0.24 | 3.3 | 8 | 8.92 | 172,409 | 187.401 | 56.38 |
| 57 | FA-I | 80 | 375 | 0.19 | 3.2 | 7 | 8.89 | 204,384 | 222.157 | 65.24 |
| Filtration Cycle | W/A | Qp/A | Qd/A | Qaverage | FT | Tp const./FT | ∆p/∆t | mW/A | V/A |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.93 | 1.33 | 6.25 | 0.13 | 38 | 0.39 | 0.09 | 1.46 | 5366.61 |
| 2 | 5.18 | 1.33 | 6.25 | 0.14 | 32 | 0.38 | 0.14 | 1.35 | 4720.67 |
| 3 | 6.37 | 1.33 | 6.25 | 0.25 | 17 | 0.35 | 0.29 | 0.89 | 2537.74 |
| 4 | 6.10 | 1.33 | 5.83 | 0.23 | 17 | 0.41 | 0.33 | 0.86 | 2564.38 |
| 5 | 7.25 | 1.33 | 5.42 | 0.23 | 15 | 0.53 | 0.48 | 0.90 | 2243.24 |
| 6 | 8.48 | 1.33 | 5.42 | 0.30 | 13 | 0.23 | 0.38 | 0.88 | 1880.96 |
| 7 | 9.12 | 1.33 | 4.17 | 0.28 | 11 | 0.36 | 0.51 | 0.80 | 1580.29 |
| 8 | 6.72 | 1.33 | 6.25 | 0.27 | 16 | 0.38 | 0.32 | 0.85 | 2300.29 |
| 9 | 5.43 | 1.33 | 6.25 | 0.18 | 29 | 0.31 | 0.16 | 1.21 | 4023.57 |
| 10 | 4.92 | 2.08 | 5.42 | 0.13 | 31 | 0.55 | 0.22 | 1.16 | 4476.90 |
| 11 | 4.77 | 1.67 | 5.83 | 0.14 | 31 | 0.65 | 0.26 | 1.17 | 4518.88 |
| 12 | 9.15 | 1.33 | 6.25 | 0.27 | 16 | 0.25 | 0.28 | 1.17 | 2311.05 |
| 13 | 6.98 | 1.33 | 6.25 | 0.27 | 18 | 0.50 | 0.25 | 0.99 | 2570.29 |
| 14 | 6.90 | 1.33 | 6.25 | 0.26 | 18 | 0.94 | 0.21 | 0.98 | 2568.79 |
| 15 | 6.73 | 1.33 | 6.25 | 0.24 | 20 | 1.00 | 0.25 | 1.11 | 2988.77 |
| 16 | 6.33 | 1.33 | 6.25 | 0.25 | 18 | 0.22 | 0.3 | 0.94 | 2692.10 |
| 17 | 6.58 | 1.33 | 6.25 | 0.17 | 31 | 0.19 | 0.13 | 1.62 | 4468.01 |
| 18 | 4.80 | 1.33 | 6.25 | 0.17 | 28 | 0.68 | 0.38 | 1.11 | 4193.53 |
| 19 | 5.92 | 1.33 | 6.25 | 0.26 | 19 | 0.21 | 0.19 | 0.93 | 2842.45 |
| 20 | 8.58 | 1.33 | 4.17 | 0.28 | 12 | 0.50 | 0.52 | 0.85 | 1790.04 |
| 21 | 8.77 | 1.33 | 4.58 | 0.24 | 11 | 0.36 | 0.46 | 0.77 | 1586.36 |
| 22 | 6.45 | 1.33 | 8.33 | 0.28 | 25 | 0.24 | 0.11 | 1.27 | 3576.79 |
| 23 | 5.00 | 1.33 | 6.25 | 0.21 | 23 | 0.22 | 0.21 | 0.94 | 3406.96 |
| 24 | 5.87 | 1.67 | 5.42 | 0.21 | 18 | 0.39 | 0.28 | 0.87 | 2677.68 |
| 25 | 5.92 | 1.33 | 6.25 | 0.23 | 20 | 0.25 | 0.21 | 0.98 | 2992.79 |
| 26 | 5.62 | 1.33 | 6.25 | 0.21 | 22 | 0.36 | 0.19 | 1.02 | 3293.48 |
| 27 | 5.10 | 1.67 | 5.42 | 0.16 | 22 | 0.36 | 0.21 | 0.92 | 3267.84 |
| 28 | 5.67 | 1.67 | 5.83 | 0.2 | 23 | 0.26 | 0.15 | 1.07 | 3425.05 |
| 29 | 5.53 | 1.67 | 5.83 | 0.16 | 26 | 0.27 | 0.12 | 1.18 | 3857.32 |
| 30 | 5.15 | 1.00 | 6.67 | 0.19 | 26 | 0.46 | 0.21 | 1.11 | 3890.16 |
| 31 | 4.97 | 1.00 | 6.67 | 0.19 | 26 | 0.35 | 0.13 | 1.07 | 3894.86 |
| 32 | 5.40 | 1.00 | 6.67 | 0.19 | 27 | 0.26 | 0.17 | 1.19 | 3983.53 |
| 33 | 5.30 | 1.00 | 6.67 | 0.19 | 25 | 0.36 | 0.16 | 1.08 | 3681.50 |
| 34 | 9.15 | 1.33 | 6.25 | 0.25 | 17 | 0.29 | 0.26 | 1.28 | 2541.76 |
| 35 | 6.97 | 1.67 | 4.17 | 0.23 | 15 | 0.33 | 0.35 | 0.86 | 2241.07 |
| 36 | 8.17 | 1.00 | 3.75 | 0.26 | 10 | 0.60 | 0.65 | 0.67 | 1489.35 |
| 37 | 5.92 | 1.00 | 5.83 | 0.25 | 16 | 0.38 | 0.34 | 0.79 | 2404.82 |
| 38 | 6.07 | 1.00 | 5.42 | 0.25 | 15 | 0.27 | 0.29 | 0.75 | 2235.71 |
| 39 | 5.35 | 1.00 | 6.25 | 0.26 | 20 | 0.55 | 0.36 | 0.88 | 2987.43 |
| 40 | 5.98 | 1.00 | 4.58 | 0.29 | 10 | 0.20 | 0.41 | 0.50 | 1526.56 |
| 41 | 8.67 | 1.67 | 2.50 | 0.28 | 7 | 0.29 | 0.62 | 0.50 | 1040.33 |
| 42 | 8.23 | 1.00 | 4.17 | 0.28 | 9 | 0.22 | 0.50 | 0.61 | 1339.00 |
| 43 | 8.65 | 1.33 | 4.17 | 0.31 | 12 | 0.33 | 0.40 | 0.84 | 1763.84 |
| 44 | 9.52 | 1.33 | 5.00 | 0.29 | 12 | 0.17 | 0.38 | 0.92 | 1743.73 |
| 45 | 4.78 | 1.33 | 4.58 | 0.10 | 37 | 0.73 | 0.33 | 1.42 | 5388.10 |
| 46 | 4.68 | 1.33 | 6.25 | 0.12 | 39 | 0.82 | 0.47 | 1.51 | 5842.45 |
| 47 | 5.98 | 1.33 | 6.25 | 0.18 | 27 | 0.19 | 0.17 | 1.30 | 3938.12 |
| 48 | 6.50 | 1.00 | 6.25 | 0.20 | 25 | 0.20 | 0.12 | 1.33 | 3719.87 |
| 49 | 5.67 | 1.33 | 6.25 | 0.19 | 27 | 0.33 | 0.18 | 1.28 | 4108.15 |
| 50 | 5.85 | 1.67 | 5.83 | 0.15 | 30 | 0.13 | 0.06 | 1.43 | 4427.54 |
| 51 | 7.38 | 1.67 | 4.58 | 0.26 | 13 | 0.23 | 0.38 | 0.78 | 1939.37 |
| 52 | 7.77 | 1.67 | 4.58 | 0.31 | 12 | 0.17 | 0.32 | 0.77 | 1821.20 |
| 53 | 7.35 | 1.33 | 5.42 | 0.28 | 14 | 0.21 | 0.30 | 0.84 | 1821.20 |
| 54 | 6.32 | 1.00 | 6.25 | 0.28 | 18 | 0.06 | 0.16 | 0.93 | 2691.09 |
| 55 | 7.00 | 1.00 | 5.83 | 0.31 | 13 | 0.38 | 0.41 | 0.76 | 1988.64 |
| 56 | 5.47 | 1.00 | 6.25 | 0.24 | 21 | 0.38 | 0.24 | 0.94 | 3123.35 |
| 57 | 5.33 | 1.33 | 6.25 | 0.19 | 25 | 0.28 | 0.15 | 1.09 | 3702.61 |
| Performance (r2) | Error | Train. | Error | Activation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Net. Name | Train. | Test | Valid. | Train. | Test | Valid. | algor. | func. | Hidden | Output | |
| FT | MLP 7-5-1 | 0.977 | 0.976 | 0.968 | 0.653 | 0.595 | 1.044 | BFGS 32 | SOS | Exp. | Identity |
| Tp const. | MLP 7-5-1 | 0.927 | 0.905 | 0.721 | 1.252 | 1.215 | 3.886 | BFGS 55 | SOS | Logistic | Logistic |
| dV/dt | MLP 7-10-1 | 0.401 | 0.853 | 0.882 | 0.008 | 0.006 | 0.004 | BFGS 52 | SOS | Exp. | Tanh |
| FO | MLP 7-3-1 | 0.970 | 0.974 | 0.981 | 5.7 × 107 | 4.0 × 107 | 3.7 × 107 | BFGS 21 | SOS | Tanh | Exp. |
| V | MLP 7-8-1 | 0.969 | 0.976 | 0.988 | 7.0 × 107 | 4.3 × 107 | 3.7 × 107 | BFGS 18 | SOS | Exp. | Identity |
| mW | MLP 7-10-1 | 0.940 | 0.837 | 0.990 | 6.730 | 11.387 | 4.065 | BFGS 32 | SOS | Logistic | Exp. |
| χ2 | RMSE | MBE | MPE | SSE | AARD | r2 | Skew | Kurt | Mean | StDev | Var | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FT | 1.424 | 1.183 | 0.205 | 4.613 | 77.372 | 51.474 | 0.967 | 0.364 | 0.162 | 0.205 | 1.175 | 1.382 |
| Tp const. | 3.291 | 1.798 | 0.009 | 33.730 | 184.293 | 81.881 | 0.924 | 0.236 | 0.181 | 0.009 | 1.814 | 3.291 |
| dV/dt | 0.015 | 0.120 | −0.016 | 0.926 | 0.806 | 4.636 | 0.703 | −1.796 | 8.260 | −0.016 | 0.120 | 0.014 |
| FO | 1.1 × 108 | 1.0 × 104 | 924.573 | 5.806 | 5.9 × 109 | 4.6 × 105 | 0.966 | 0.027 | −0.252 | 924.573 | 1.0 × 104 | 1.1 × 108 |
| V | 1.2 × 108 | 1.1 × 104 | −19.177 | 5.164 | 7.0 × 109 | 4.9 × 105 | 0.959 | 0.203 | 0.206 | −19.177 | 1.1 × 104 | 1.2 × 108 |
| mW | 14.269 | 3.744 | 0.206 | 5.450 | 796.652 | 165.644 | 0.953 | 0.075 | 0.061 | 0.206 | 3.772 | 14.226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lužaić, T.; Nedić Grujin, K.; Pezo, L.; Nikolovski, B.; Maksimović, Z.; Romanić, R. Implementation of Cellulose-Based Filtration Aids in Industrial Sunflower Oil Dewaxing (Winterization): Process Monitoring, Prediction, and Optimization. Foods 2024, 13, 2960. https://doi.org/10.3390/foods13182960
Lužaić T, Nedić Grujin K, Pezo L, Nikolovski B, Maksimović Z, Romanić R. Implementation of Cellulose-Based Filtration Aids in Industrial Sunflower Oil Dewaxing (Winterization): Process Monitoring, Prediction, and Optimization. Foods. 2024; 13(18):2960. https://doi.org/10.3390/foods13182960
Chicago/Turabian StyleLužaić, Tanja, Katarina Nedić Grujin, Lato Pezo, Branislava Nikolovski, Zoran Maksimović, and Ranko Romanić. 2024. "Implementation of Cellulose-Based Filtration Aids in Industrial Sunflower Oil Dewaxing (Winterization): Process Monitoring, Prediction, and Optimization" Foods 13, no. 18: 2960. https://doi.org/10.3390/foods13182960
APA StyleLužaić, T., Nedić Grujin, K., Pezo, L., Nikolovski, B., Maksimović, Z., & Romanić, R. (2024). Implementation of Cellulose-Based Filtration Aids in Industrial Sunflower Oil Dewaxing (Winterization): Process Monitoring, Prediction, and Optimization. Foods, 13(18), 2960. https://doi.org/10.3390/foods13182960







