Modeling Behavior of Salmonella spp. and Listeria monocytogenes in Raw and Processed Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of Microbiological Quality, pH, and Water Activity (aw) of Vegetables
2.2. Bacterial Strain Preparation for Predictive Model Development
2.3. Sample Preparation and Inoculation Strains for Model Development
2.4. Model Development and Validation of Model Performance
- N0: log initial number of bacteria (log CFU/g)
- C: difference between initial and final bacteria numbers (log CFU)
- LT: lag time (h)
- SGR: specific growth rate (log CFU/h)
- X: time (h)
- Y: log number of bacteria (log CFU/g)
- Y: lag time (h)
- a, b, c: constants
- T: temperature (°C)
- Y: specific growth rate (log CFU/h)
- b: constant
- T: temperature (°C)
- Tmin: theoretical minimum temperature (°C)
- Y: maximum population density (log CFU/g)
- b0, b1, b2: constants
- T: temperature (°C)
- Y: maximum population density (log CFU/g)
- b0, b1, b2, b3: constants
- T: temperature (°C)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Microbiological Quality, pH, and Water Activity (aw)
3.2. Primary Growth Models of Salmonella spp. and L. monocytogenes
3.2.1. Salmonella spp. Growth in Raw and Processed Vegetables
3.2.2. Listeria Growth in Raw and Processed Vegetables
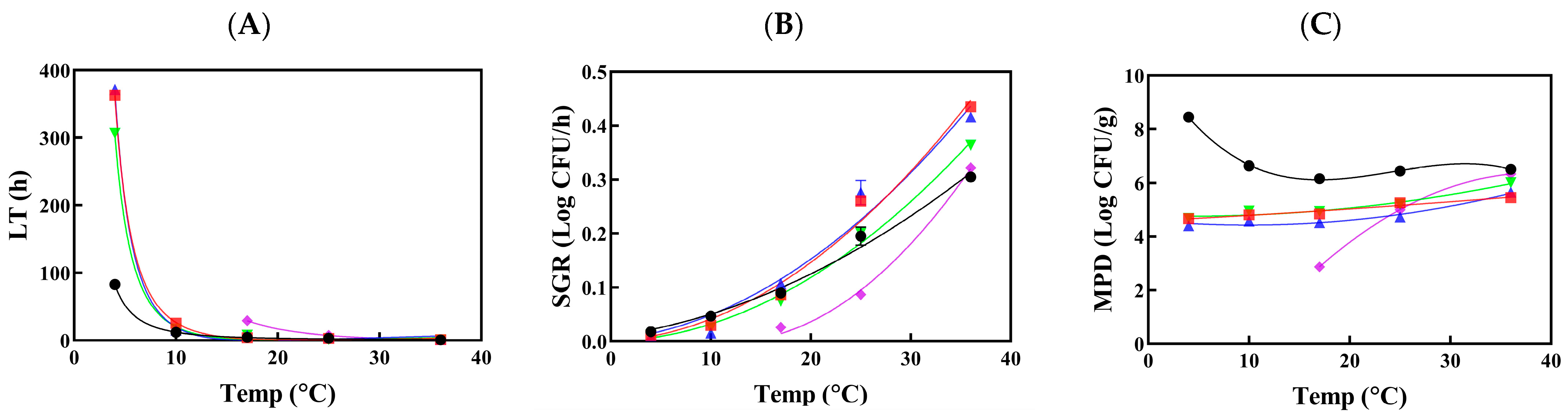
3.3. Secondary Model and Validation of Model Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simon-Kucher & Partners. Sustainability in Restaurants and the Rise of the Eco-Conscious Consumer; Simon-Kucher & Partners: Bonn, Germany, 2023; Available online: https://www.simon-kucher.com/en/insights/sustainability-restaurants-and-rise-eco-conscious-consumer (accessed on 15 August 2024).
- Son, S.B.; Lee, S.Y.; Oh, S.W.; Lee, E.Y.; Bae, J.Y.; Yoon, K.S. Purchase and intake of vegetables and processed vegetable products and perception of foodborne illness risk in Korea. Korean J. Food Sci. Technol. 2023, 55, 502–514. [Google Scholar] [CrossRef]
- Korea Rural Economic Institute (KREI). Research on Fresh-Cut Fruits and Vegetables; Korea Rural Economic Institute (KREI): Naju-si, Republic of Korea, 2019; Available online: https://krei.re.kr/krei/researchReportView.do?key=67&pageType=010101&biblioId=522067 (accessed on 15 August 2024).
- Ministry of Food and Drug Safety (MFDS). 2021 Food Production Performance Statistics; Ministry of Food and Drug Safety (MFDS): Cheongju-si, Republic of Korea, 2022; Available online: https://www.mfds.go.kr/brd/m_374/view.do?seq=30207&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed on 15 August 2024).
- Centers for Disease Control and Prevention (CDC). List of Multistate Foodborne Outbreak Notices; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2023. Available online: https://www.cdc.gov/foodborne-outbreaks/active-investigations/all-foodborne-outbreak-notices.html?CDC_AAref_Val=https://www.cdc.gov/foodsafety/outbreaks/lists/outbreaks-list.html (accessed on 15 August 2024).
- Aiyedun, S.O.; Onarinde, B.A.; Swainson, M.; Dixon, R.A. Foodborne outbreaks of microbial infection from fresh produce in Europe and North America: A systematic review of data from this millennium. Int. J. Food Sci. Technol. 2021, 56, 2215–2223. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Statistics for Foodborne Illness Outbreaks; Ministry of Food and Drug Safety (MFDS): Cheongju-si, Republic of Korea, 2023; Available online: https://www.foodsafetykorea.go.kr/portal/healthyfoodlife/foodPoisoningStat.do?menu_no=4425&menu_grp=MENU_NEW02 (accessed on 15 August 2024).
- Jeon, E.B.; Kim, J.Y.; Choi, M.S.; Choi, S.; Bang, H.J.; Park, S.Y. Microbial contamination levels in the raw materials of home meal replacement shabu-shabu meal kit distributed in markets. J. Food Hyg. Saf. 2020, 35, 375–381. [Google Scholar] [CrossRef]
- Health Canada. Recalls and Safety Alerts: Food Recalls; Health Canada: Ottawa, ON, Canada, 2023; Available online: https://recalls-rappels.canada.ca/en/search/site?f%5B0%5D=category%3A144 (accessed on 15 August 2024).
- U.S. Food & Drug Administration (FDA). Recalls, Market Withdrawals, & Safety Alerts; U.S. Food & Drug Administration (FDA): Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts (accessed on 15 August 2024).
- Han, S.H.; Park, S.H.; Choi, S.S.; Jin, Y.H.; Kim, H.S.; Kim, J.S.; Park, J.H.; Ryu, J.; Kang, M.J.; Jeon, S.J.; et al. Food-borne outbreak of Listeria monocytogenes in school students in Seoul, Korea. J. Food Saf. Hyg. 2019, 5, 146–154. [Google Scholar] [CrossRef]
- Mishra, A.; Guo, M.; Buchanan, R.L.; Schaffner, D.W.; Pradhan, A.K. Development of growth and survival models for Salmonella and Listeria monocytogenes during non-isothermal time-temperature profiles in leafy greens. Food Control 2017, 71, 32–41. [Google Scholar] [CrossRef]
- Kim, W.I.; Ryu, S.D.; Kim, S.R.; Kim, H.J.; Lee, S.; Kim, J. Population changes and growth modeling of Salmonella enterica during alfalfa seed germination and early sprout development. Food Sci. Biotechnol. 2018, 27, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Tarlak, F.; Johannessen, G.; Bascón Villegas, I.; Bolívar, A.; Posada-Izquierdo, G.D.; Pérez-Rodríguez, F. Modelling of the behaviour of Salmonella enterica serovar Reading on commercial fresh-cut iceberg lettuce stored at different temperatures. Foods 2020, 9, 946. [Google Scholar] [CrossRef]
- Ndraha, N.; Goh, A.P.; Tran, G.D.; Chen, C.Q.; Hsiao, H.I. Predictive models for the growth of Salmonella spp., Listeria spp., and Escherichia coli in lettuce harvested on Taiwanese farms. J. Food Sci. 2022, 87, 3599–3610. [Google Scholar] [CrossRef]
- Jung, J.; Schaffner, D.W. Modeling the survival of Salmonella on whole cucumbers as a function of temperature and relative humidity. Food Microbiol. 2021, 100, 103840. [Google Scholar] [CrossRef]
- Jung, J.; Schaffner, D.W. Modeling the growth of Salmonella on sliced cucumbers as a function of temperature and relative humidity. J. Food Prot. 2022, 85, 1122–1127. [Google Scholar] [CrossRef]
- Kim, G.H.; Lim, J.Y.; Kim, Y.H.; Yang, S.Y.; Yoon, K.S. Development of predictive models of Listeria monocytogenes in fresh-cut fruits and vegetables. J. Food Hyg. Saf. 2020, 35, 495–502. [Google Scholar] [CrossRef]
- Feng, K.; Sarengaowa; Ma, J.; Hu, W. Modeling the growth of Listeria monocytogenes on fresh-cut cucumbers at various storage temperatures. Horticulturae 2024, 10, 667. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Risk Analysis of Salmonella spp. and Listeria monocytogenes in Vegetables and Vegetable Products; Ministry of Food and Drug Safety (MFDS): Cheongju-si, Republic of Korea, 2023; (Unpublished work). [Google Scholar]
- Kim, J.; Ro, E.; Yoon, K. Comparison of growth kinetics of various pathogenic E. coli on fresh perilla leaf. Foods 2013, 2, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.M.; Bratchell, N.; Roberts, T.A. The effect of sodium chloride and temperature on the rate and extent of growth of Clostridium botulinum type A in pasteurized pork slurry. J. Appl. Microbiol. 1987, 62, 479–490. [Google Scholar] [CrossRef]
- Davey, K.R. A predictive model for combined temperature and water activity on microbial growth during the growth phase. J. Appl. Microbiol. 1989, 67, 483–488. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef]
- McMeekin, T.A.; Olley, J.N.; Ross, T.; Ratkowsky, D.A. Predictive Microbiology: Theory and Application; Research Studies Press Ltd.: Taunton, UK, 1993; p. 340. [Google Scholar]
- Fujimoto, A.; Watanabe, Y. Polynomial evolution equations of not normal type admitting nontrivial symmetries. Phys. Lett. A 1989, 136, 294–299. [Google Scholar] [CrossRef]
- Abou-Zeid, K.A.; Oscar, T.P.; Schwarz, J.G.; Hashem, F.M.; Whiting, R.C.; Yoon, K. Development and validation of a predictive model for Listeria monocytogenes Scott A as a function of temperature, pH, and commercial mixture of potassium lactate and sodium diacetate. J. Microbiol. Biotechnol. 2009, 19, 718–726. [Google Scholar]
- Baranyi, J.; Ross, T.; McMeekin, T.A.; Roberts, T.A. Effects of parameterization on the performance of empirical models used in ‘predictive microbiology’. Food Microbiol. 1996, 13, 83–91. [Google Scholar] [CrossRef]
- Ross, T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 1996, 81, 501–508. [Google Scholar]
- Seo, Y.H.; Jang, J.H.; Moon, K.D. Microbial evaluation of minimally processed vegetables and sprouts produced in Seoul, Korea. Food Sci. Biotechnol. 2010, 19, 1283–1288. [Google Scholar] [CrossRef]
- Waje, C.K.; Jun, S.Y.; Lee, Y.K.; Kim, B.N.; Han, D.H.; Jo, C.; Kwon, J.H. Microbial quality assessment and pathogen inactivation by electron beam and gamma irradiation of commercial seed sprouts. Food Control 2009, 20, 200–204. [Google Scholar] [CrossRef]
- Park, H.; Puligundla, P.; Mok, C. Microbial decontamination of mung bean sprouts using electrolyzed water and its effects on the physicochemical and sensory properties of the sprouts. Chiang Mai J. Sci. 2020, 47, 28–38. [Google Scholar]
- Alegbeleye, O.; Sant’Ana, A.S. Survival and growth behavior of Listeria monocytogenes in ready-to-eat vegetable salads. Food Control 2022, 138, 109023. [Google Scholar] [CrossRef]
- Kyung, K.H.; Kim, M.H.; Park, M.S.; Kim, Y.S. Alliinase-independent Inhibition of Staphylococcus aureus B33 by Heated Garlic. J. Food Sci. 2002, 67, 780–785. [Google Scholar] [CrossRef]
- Chin, H.W.; Lindsay, R.C. Mechanisms of formation of volatile sulfur compounds following the action of cysteine sulfoxide lyases. J. Agric. Food Chem. 1994, 42, 1529–1536. [Google Scholar] [CrossRef]
- Kyung, K.H.; Fleming, H.P. Antimicrobial activity-of sulfur compounds derived from cabbage. J. Food Prot. 1997, 60, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, A.; Yoon, J.H.; Lee, S.Y. Growth evaluation of Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes in fresh fruit and vegetable juices via predictive modeling. LWT 2022, 162, 113485. [Google Scholar] [CrossRef]
- Sant’Ana, A.S.; Franco, B.D.; Schaffner, D.W. Modeling the growth rate and lag time of different strains of Salmonella enterica and Listeria monocytogenes in ready-to-eat lettuce. Food Microbiol. 2012, 30, 267–273. [Google Scholar] [CrossRef]
- Bystrická, J.; Musilová, J.; Vollmannová, A.; Timoracká, M.; Kavalcová, P. Bioactive components of onion (Allium cepa L.)—A Review. Acta Aliment. 2013, 42, 11–22. [Google Scholar] [CrossRef]
- Elnima, E.I.; Ahmed, S.A.; Mekkawi, A.G.; Mossa, J.S. The antimicrobial activity of garlic and onion extracts. Pharmazie 1983, 38, 747–748. [Google Scholar] [PubMed]
- Hernández, I.; Alfaro, B.; Rodriguez-Ezpeleta, N. Enhancing high throughput sequencing unveils changes in bacterial communities during ready-to-eat lettuce spoilage. J. Hortic. Postharvest Res. 2020, 3, 297–310. [Google Scholar]
- Oscar, T.P. Development and validation of primary, secondary, and tertiary models for growth of Salmonella Typhimurium on sterile chicken. J. Food Prot. 2005, 68, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Luo, Y.; Zhou, B.; Zheng, J.; Nou, X. Growth and survival of Salmonella enterica and Listeria monocytogenes on fresh-cut produce and their juice extracts: Impacts and interactions of food matrices and temperature abuse conditions. Food Control 2019, 100, 300–304. [Google Scholar] [CrossRef]

| Sample | Microbial Populations (Log CFU/g) | pH | aw | ||
|---|---|---|---|---|---|
| TAB | TCC | E. coli | |||
| Mung bean sprouts | 7.23 ± 0.10 | 6.03 ± 0.06 | ND | 6.30 ± 0.08 | 0.951 ± 0.003 |
| Onion | 2.73 ± 0.10 | ND | 6.30 ± 0.04 | 0.955 ± 0.001 | |
| Cabbage | 4.67 ± 0.01 | 6.71 ± 0.02 | 0.948 ± 0.000 | ||
| RTE shredded cabbage salad | 2.02 ± 0.17 | 6.80 ± 0.02 | 0.962 ± 0.001 | ||
| Cabbage juice | ND | 5.20 ± 0.03 | 0.964 ± 0.001 | ||
| Onion juice | 4.74 ± 0.01 | 0.957 ± 0.001 | |||
| Sample | Storage Temperature (°C) | LT | SGR | MPD |
|---|---|---|---|---|
| Mung bean sprouts | 7 | ND | ND | ND |
| 8 | 170.1 ± 3.07 a | 0.012 ± 0.000 e | 8.47 ± 0.01 c | |
| 10 | 16.47 ± 1.93 b | 0.035 ± 0.001 d | 8.31 ± 0.04 d | |
| 17 | 7.11 ± 0.12 c | 0.209 ± 0.001 c | 8.82 ± 0.00 b | |
| 25 | 3.36 ± 0.28 d | 0.467 ± 0.025 b | 9.28 ± 0.13 a | |
| 36 | 1.85 ± 0.07 d | 1.138 ± 0.004 a | 9.30 ± 0.04 a | |
| Onion | 8 | ND | ND | ND |
| 9 | 169.50 ± 4.80 a | 0.012 ± 0.000 d | 3.43 ± 0.05 d | |
| 15 | 24.33 ± 0.26 b | 0.114 ± 0.004 c | 4.70 ± 0.07 c | |
| 25 | 6.18 ± 0.14 c | 0.332 ± 0.004 b | 5.96 ± 0.02 b | |
| 36 | 4.79 ± 0.00 c | 1.308 ± 0.040 a | 7.54 ± 0.08 a | |
| Cabbage | 8 | ND | ND | ND |
| 9 | 292.35 ± 59.25 a | 0.013 ± 0.005 d | 3.89 ± 0.03 d | |
| 10 | 68.48 ± 0.80 b | 0.015 ± 0.000 d | 4.42 ± 0.02 c | |
| 17 | 14.95 ± 0.18 c | 0.120 ± 0.000 c | 6.04 ± 0.06 b | |
| 25 | 5.34 ± 0.11 c | 0.312 ± 0.007 b | 6.69 ± 0.03 a | |
| 36 | 4.10 ± 0.00 c | 0.716 ± 0.000 a | 6.73 ± 0.00 a | |
| RTE shredded cabbage salad | 9 | ND | ND | ND |
| 10 | 121.10 ± 1.80 a | 0.024 ± 0.000 d | 4.42 ± 0.02 d | |
| 17 | 17.11 ± 0.69 b | 0.058 ± 0.000 c | 4.76 ± 0.03 c | |
| 25 | 4.46 ± 0.37 c | 0.210 ± 0.005 b | 5.59 ± 0.01 b | |
| 36 | 1.02 ± 0.16 d | 0.432 ± 0.000 a | 7.49 ± 0.01 a | |
| Cabbage juice | 16 | ND | ND | ND |
| 17 | 42.37 ± 1.11 a | 0.096 ± 0.005 c | 3.37 ± 0.01 c | |
| 25 | 9.65 ± 0.02 b | 0.240 ± 0.006 b | 4.93 ± 0.00 b | |
| 36 | 6.36 ± 0.03 c | 0.709 ± 0.008 a | 7.08 ± 0.01 a | |
| Onion juice | 36 | ND | ND | ND |
| Sample | Storage Temperature (°C) | LT | SGR | MPD |
|---|---|---|---|---|
| Mung bean sprouts | 4 | 83.86 ± 1.81 a | 0.018 ± 0.000 e | 8.45 ± 0.01 a |
| 10 | 13.08 ± 0.92 b | 0.048 ± 0.001 d | 6.55 ± 0.09 b | |
| 17 | 4.38 ± 0.05 c | 0.090 ± 0.001 c | 6.17 ± 0.01 d | |
| 25 | 3.13 ± 0.19 c | 0.187 ± 0.017 b | 6.38 ± 0.06 c | |
| 36 | 1.05 ± 0.06 d | 0.306 ± 0.001 a | 6.51 ± 0.00 c | |
| Onion | 4 | 363.00 ± 0.50 a | 0.011 ± 0.000 e | 4.67 ± 0.06 d |
| 10 | 25.37 ± 1.37 b | 0.030 ± 0.001 d | 4.81 ± 0.07 c | |
| 17 | 3.89 ± 0.14 c | 0.086 ± 0.001 c | 4.85 ± 0.00 c | |
| 25 | 2.73 ± 0.02 c | 0.260 ± 0.001 b | 5.27 ± 0.00 b | |
| 36 | 1.15 ± 0.12 d | 0.435 ± 0.007 a | 5.45 ± 0.07 a | |
| Cabbage | 4 | 371.57 ± 6.31 a | 0.010 ± 0.001 d | 4.67 ± 0.06 d |
| 10 | 15.37 ± 6.00 b | 0.030 ± 0.001 d | 4.81 ± 0.07 c | |
| 17 | 7.04 ± 0.38 c | 0.086 ± 0.001 c | 4.85 ± 0.00 c | |
| 25 | 4.50 ± 0.51 c | 0.260 ± 0.001 b | 5.27 ± 0.00 b | |
| 36 | 1.19 ± 0.00 c | 0.435 ± 0.007 a | 5.45 ± 0.07 a | |
| RTE shredded cabbage salad | 4 | 306.77 ± 6.32 a | 0.012 ± 0.001 e | 4.68 ± 0.00 d |
| 10 | 16.90 ± 1.87 b | 0.025 ± 0.001 d | 4.95 ± 0.01 c | |
| 17 | 7.75 ± 0.13 c | 0.075 ± 0.001 c | 4.94 ± 0.00 c | |
| 25 | 1.52 ± 0.38 d | 0.200 ± 0.004 b | 5.21 ± 0.03 b | |
| 36 | 1.04 ± 0.38 d | 0.364 ± 0.003 a | 6.01 ± 0.07 a | |
| Cabbage juice | 17 | 29.14 ± 2.46 a | 0.026 ± 0.000 c | 2.87 ± 0.04 c |
| 25 | 7.54 ± 0.92 b | 0.087 ± 0.003 b | 5.00 ± 0.18 b | |
| 36 | 1.06 ± 0.03 c | 0.322 ± 0.001 a | 6.34 ± 0.03 a | |
| Onion juice | 36 | - | ||
| Pathogens | Sample | Parameter | Equation |
|---|---|---|---|
| Salmonella spp. | Mung bean sprouts | LT | Y = 37.37 + (−1874/T) + (23,607/T2) |
| SGR | Y = {0.03344 × (T − 4.169)}2 | ||
| MPD | Y = 7.607 + (0.1005 × T) + (−0.001471 × T2) | ||
| Onion | LT | Y = 53.81 + (−2592/T) + (32,676/T2) | |
| SGR | Y = {0.04756 × (T − 12.07)}2 | ||
| MPD | Y = 1.812 + (0.1986 × T) + (−0.001123 × T2) | ||
| Cabbage | LT | Y = 93.42 + (−4713/T) + (58,501/T2) | |
| SGR | Y = {0.02666 × (T − 4.202)}2 | ||
| MPD | Y = 0.9006 + (0.4112 × T) + (−0.006954 × T2) | ||
| RTE shredded cabbage salad | LT | Y = 32.11 + (−1836/T) + (27,241/T2) | |
| SGR | Y = {0.02014 × (T − 3.198)}2 | ||
| MPD | Y = 4.559 + (−0.05021 × T) + (0.003657 × T2) | ||
| Cabbage juice | LT | Y = 48.97 + (−2806/T) + (45,798/T2) | |
| SGR | Y = {0.02986 × (T − 7.91)}2 | ||
| MPD | Y = 0.09955 + (0.1916 × T) + (0.00006041 × T2) | ||
| L. monocytogenes | Mung bean sprouts | LT | Y = 1.476 + (−35.64/T) + (1444/T2) |
| SGR | Y = {0.01286 × (T + 7.497)}2 | ||
| MPD 1 | Y = 10.54 + (−0.6337 × T) + (0.02869 × T2) + (−0.0003944 × T3) | ||
| Onion | LT | Y = 18.33 + (−780.2/T) + (8634/T2) | |
| SGR | Y = [0.01789 × {T − (−1.381)}]2 | ||
| MPD | Y = 4.581 + (0.01973 × T) + (0.000143 × T2) | ||
| Cabbage | LT | Y = 28.7 + (−1060/T) + (9722/T2) | |
| SGR | Y = [0.01688 × {T − (−3.122)}]2 | ||
| MPD | Y = 4.604 + (−0.03468 × T) + (0.001746 × T2) | ||
| RTE shredded cabbage salad | LT | Y = 19.06 + (−744.7/T) + (7578/T2) | |
| SGR | Y = [0.01649 × {T − (−0.8631)}]2 | ||
| MPD | Y = 4.779 + (−0.009426 × T) + (0.001188 × T2) | ||
| Cabbage juice | LT | Y = 8.426 + (−817.5/T) + (19,884/T2) | |
| SGR | Y = {0.02345 × (T − 11.87)}2 | ||
| MPD | Y = (−4.923) + (0.5887 × T) + (−0.007666 × T2) |
| Pathogens | Sample | Parameter | Bf | Af | RMSE |
|---|---|---|---|---|---|
| Salmonella spp. | Mung bean sprouts | LT | 0.615 | 2.307 | 2.049 |
| SGR | 0.944 | 1.107 | 0.014 | ||
| MPD | 0.978 | 1.032 | 0.471 | ||
| Onion | LT | 0.936 | 1.009 | 10.441 | |
| SGR | 0.754 | 1.388 | 0.023 | ||
| MPD | 0.985 | 1.040 | 0.233 | ||
| Cabbage | LT | 0.503 | 2.594 | 16.637 | |
| SGR | 0.934 | 1.156 | 0.014 | ||
| MPD | 0.949 | 1.074 | 0.708 | ||
| RTE shredded cabbage salad | LT | 1.019 | 1.499 | 1.307 | |
| SGR | 0.976 | 1.169 | 0.022 | ||
| MPD | 1.006 | 1.009 | 0.085 | ||
| Cabbage juice | LT | 0.920 | 1.103 | 1.873 | |
| SGR | 1.084 | 1.200 | 0.034 | ||
| MPD | 1.041 | 1.050 | 0.580 | ||
| L. monocytogenes | Mung bean sprouts | LT | 1.086 | 1.222 | 0.800 |
| SGR | 0.970 | 1.060 | 0.008 | ||
| MPD | 1.010 | 1.021 | 0.222 | ||
| Onion | LT | 0.757 | 1.952 | 2.098 | |
| SGR | 0.925 | 1.141 | 0.019 | ||
| MPD | 0.964 | 1.041 | 0.313 | ||
| Cabbage | LT | 0.858 | 1.765 | 5.675 | |
| SGR | 0.855 | 1.686 | 0.085 | ||
| MPD | 1.003 | 1.034 | 0.187 | ||
| RTE shredded cabbage salad | LT | 1.210 | 2.176 | 4.994 | |
| SGR | 0.944 | 1.249 | 0.026 | ||
| MPD | 1.028 | 1.040 | 0.470 | ||
| Cabbage juice | LT | 0.900 | 1.337 | 4.799 | |
| SGR | 1.324 | 1.442 | 0.070 | ||
| MPD | 0.928 | 1.078 | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, S.B.; Lee, H.K.; Kim, S.J.; Yoon, K.S. Modeling Behavior of Salmonella spp. and Listeria monocytogenes in Raw and Processed Vegetables. Foods 2024, 13, 2972. https://doi.org/10.3390/foods13182972
Son SB, Lee HK, Kim SJ, Yoon KS. Modeling Behavior of Salmonella spp. and Listeria monocytogenes in Raw and Processed Vegetables. Foods. 2024; 13(18):2972. https://doi.org/10.3390/foods13182972
Chicago/Turabian StyleSon, Su Bin, Ha Kyoung Lee, So Jeong Kim, and Ki Sun Yoon. 2024. "Modeling Behavior of Salmonella spp. and Listeria monocytogenes in Raw and Processed Vegetables" Foods 13, no. 18: 2972. https://doi.org/10.3390/foods13182972





