Variation and Abundance of Resistant Starch in Selected Banana Cultivars in Uganda
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Resistant Starch
2.3. Total Starch, Polymer, and Resistant Starch Quantification
2.4. Structural and Morphological Analysis
2.5. Analysis of Functional Properties
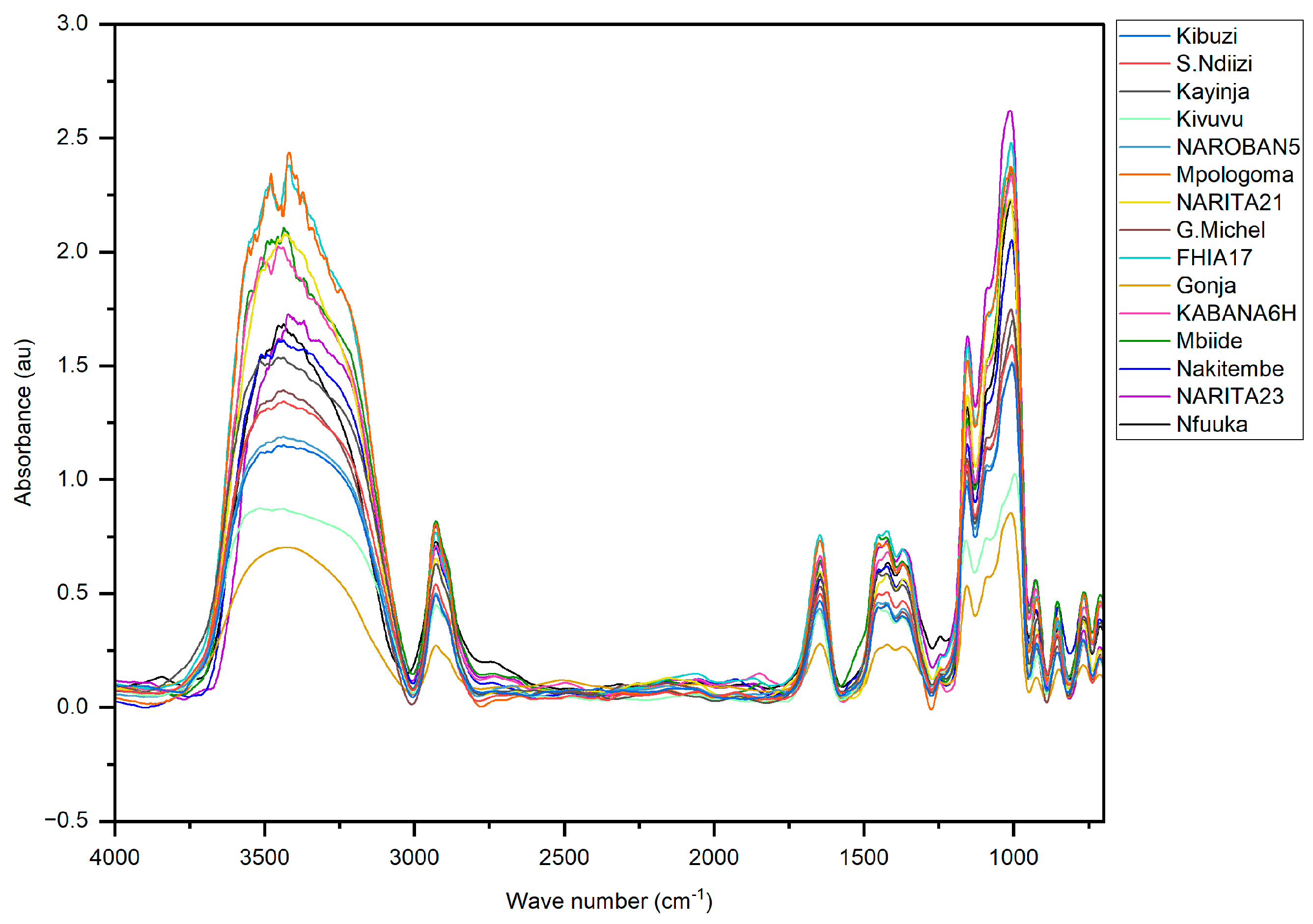
2.6. Fourier Transform Infrared Spectrometry
2.7. Statistical Analysis
3. Results and Discussion
3.1. Resistant Starch Content and Polymer Composition
3.2. Structural Analysis
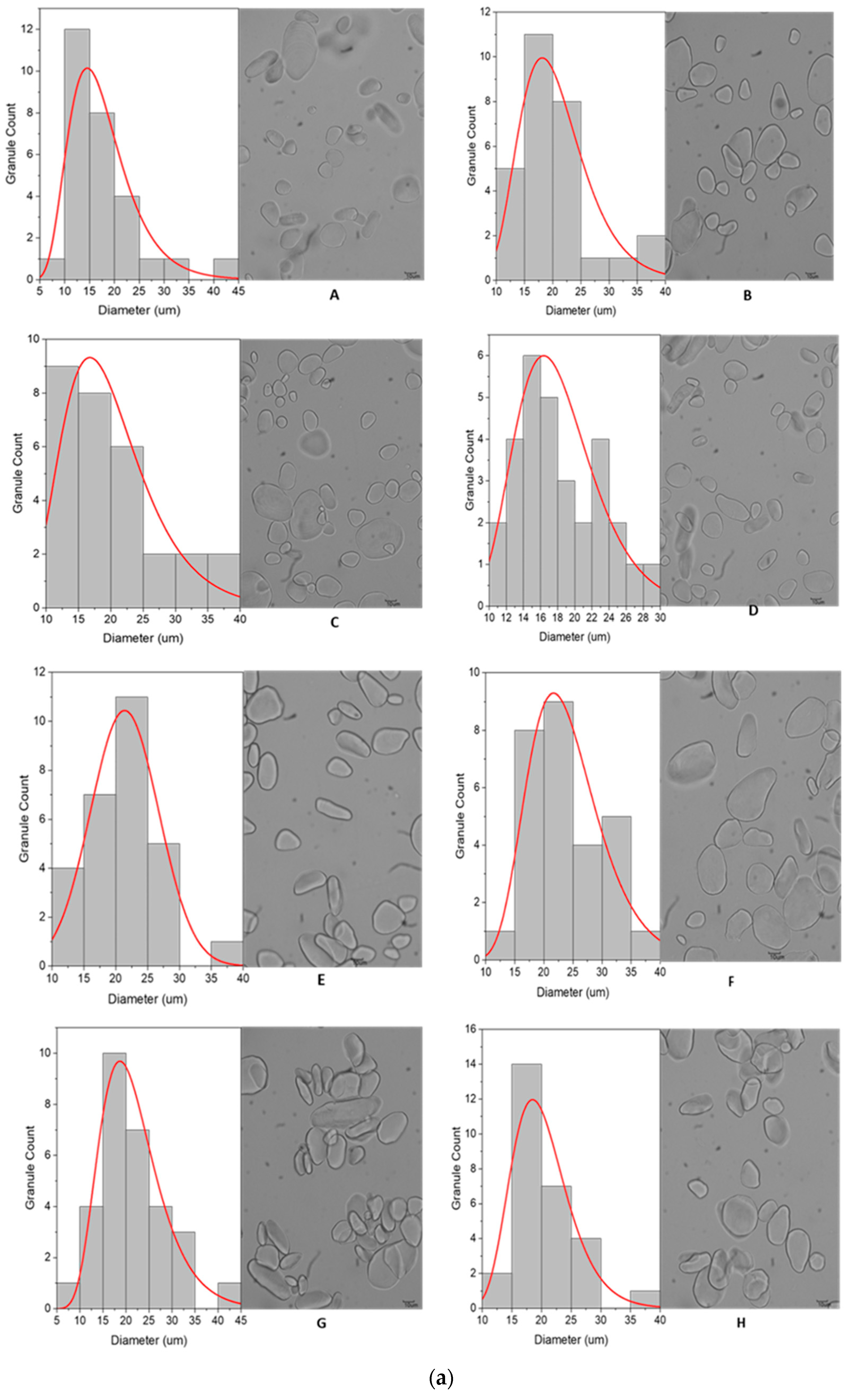
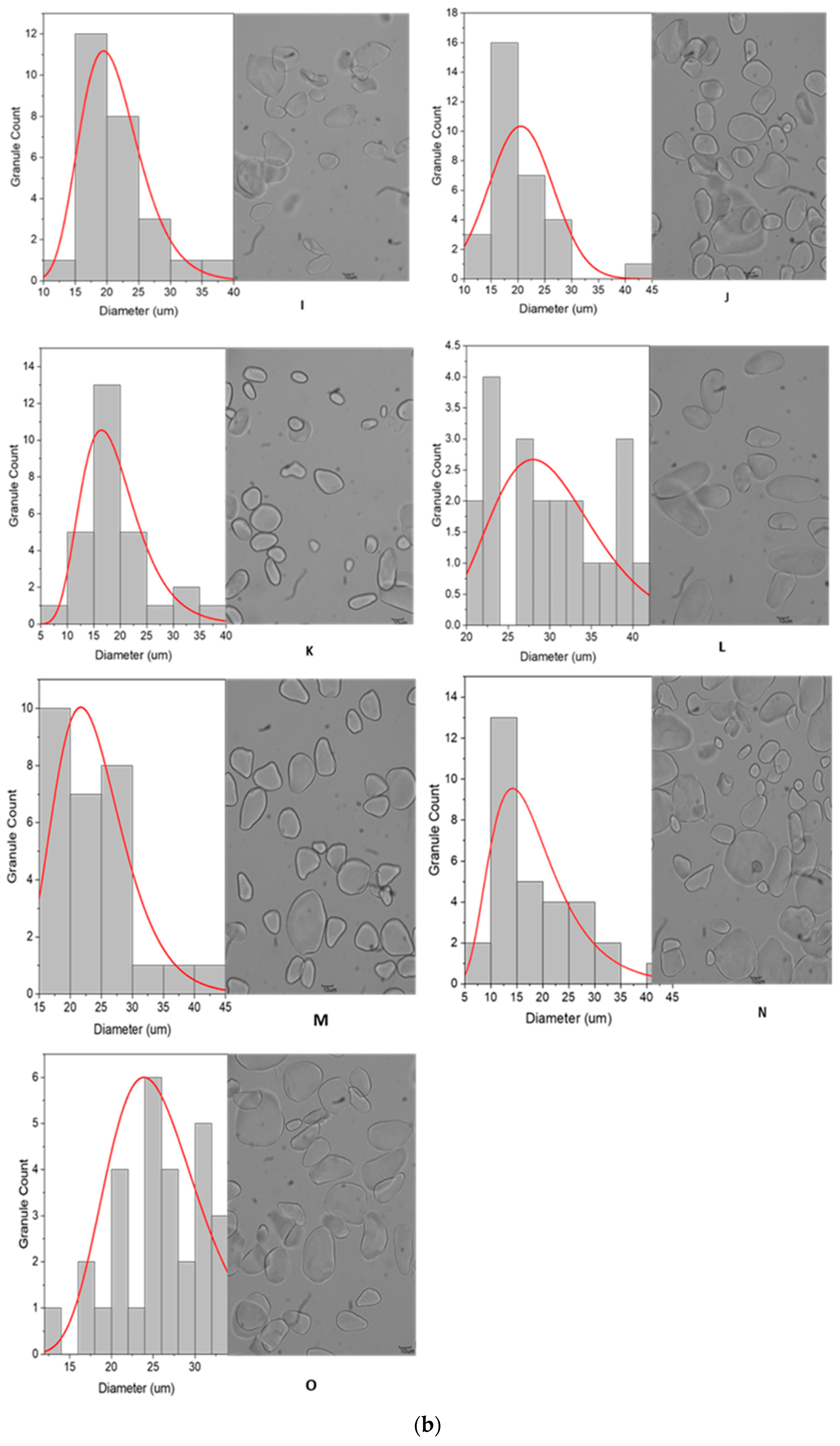
3.2.1. Granule Size and Distribution
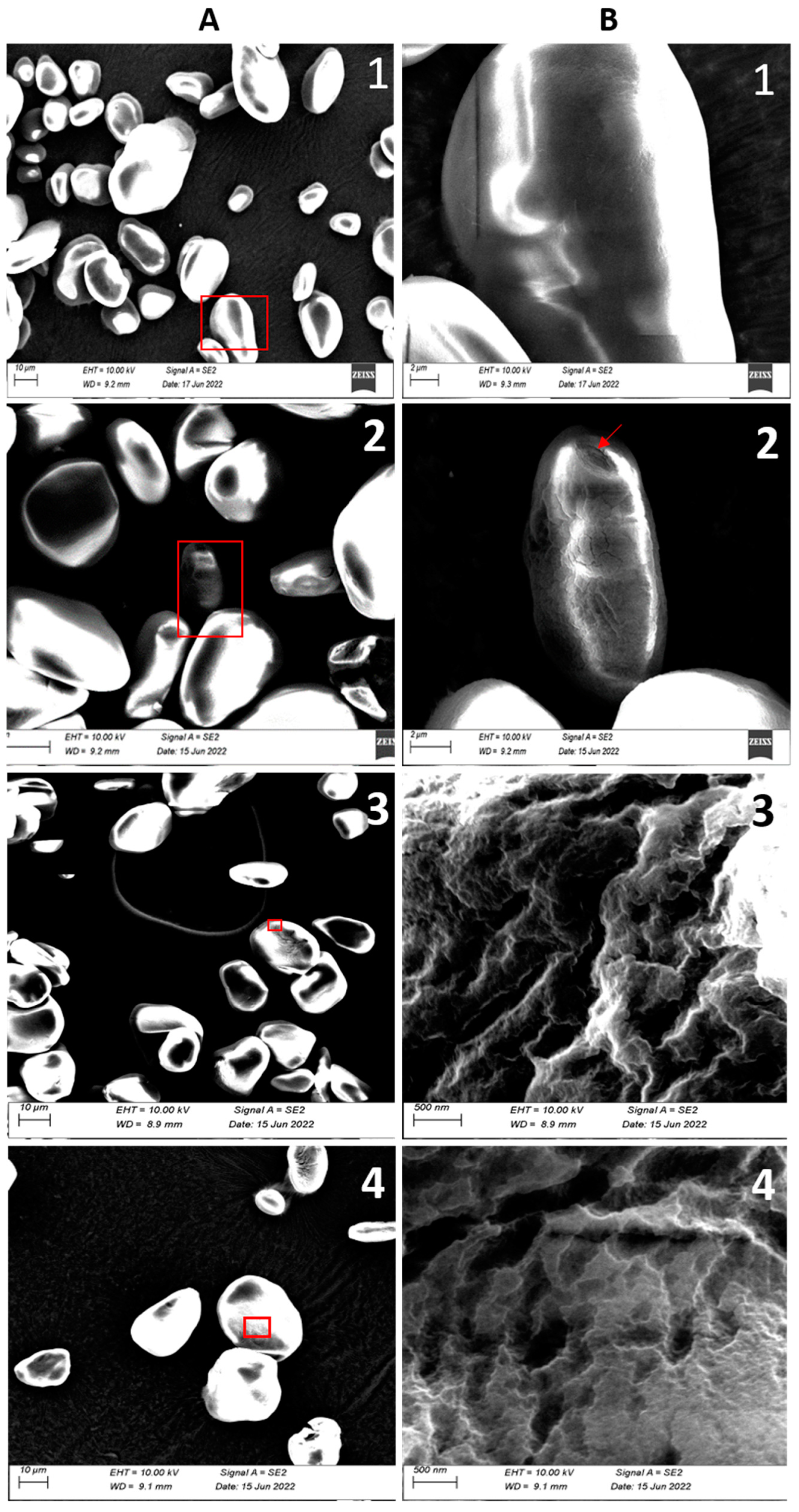
3.2.2. Granule Characteristics
3.3. Functional Properties
3.3.1. Clarity (Transmittance)
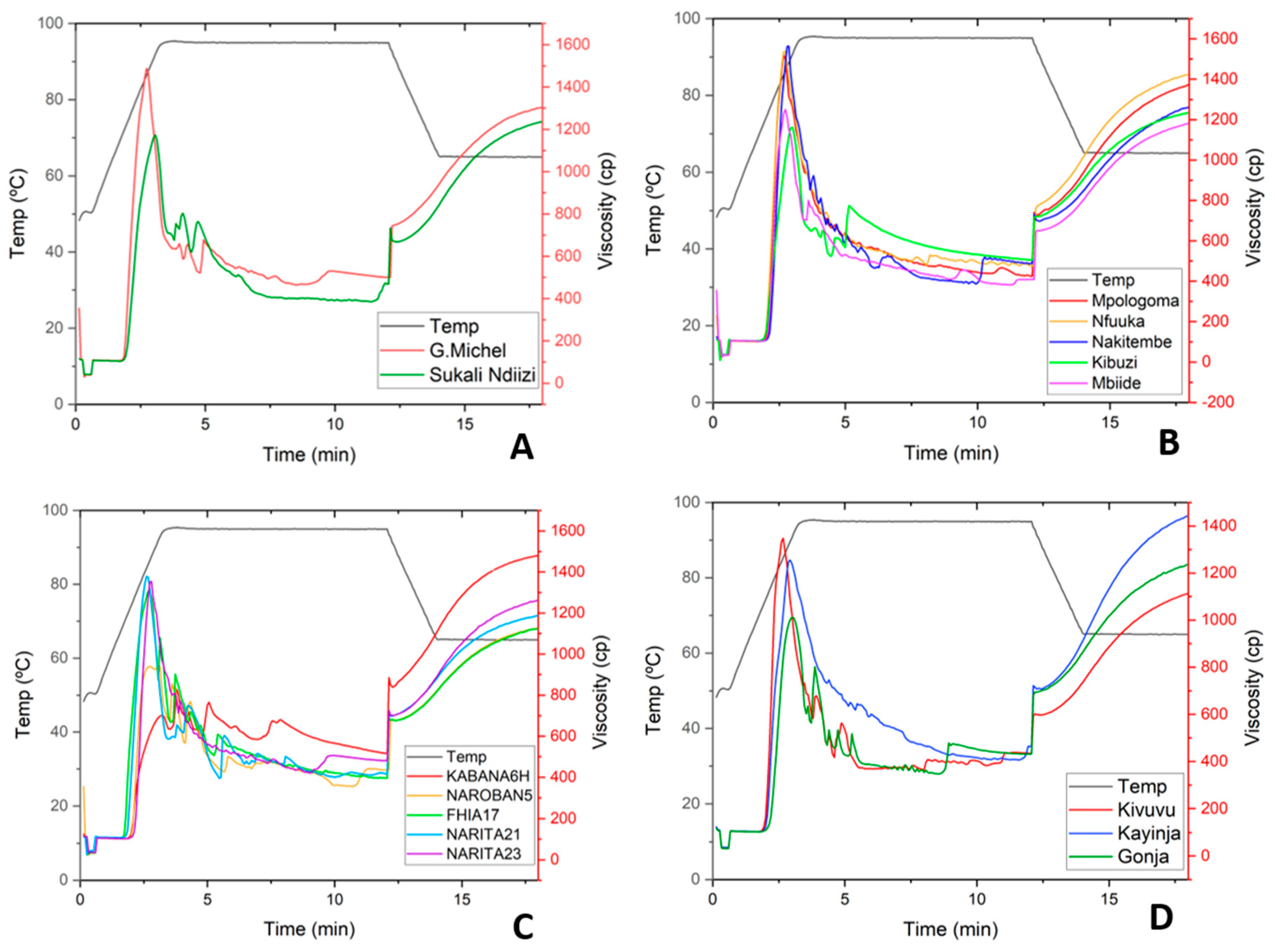
3.3.2. Pasting Properties of Banana RS
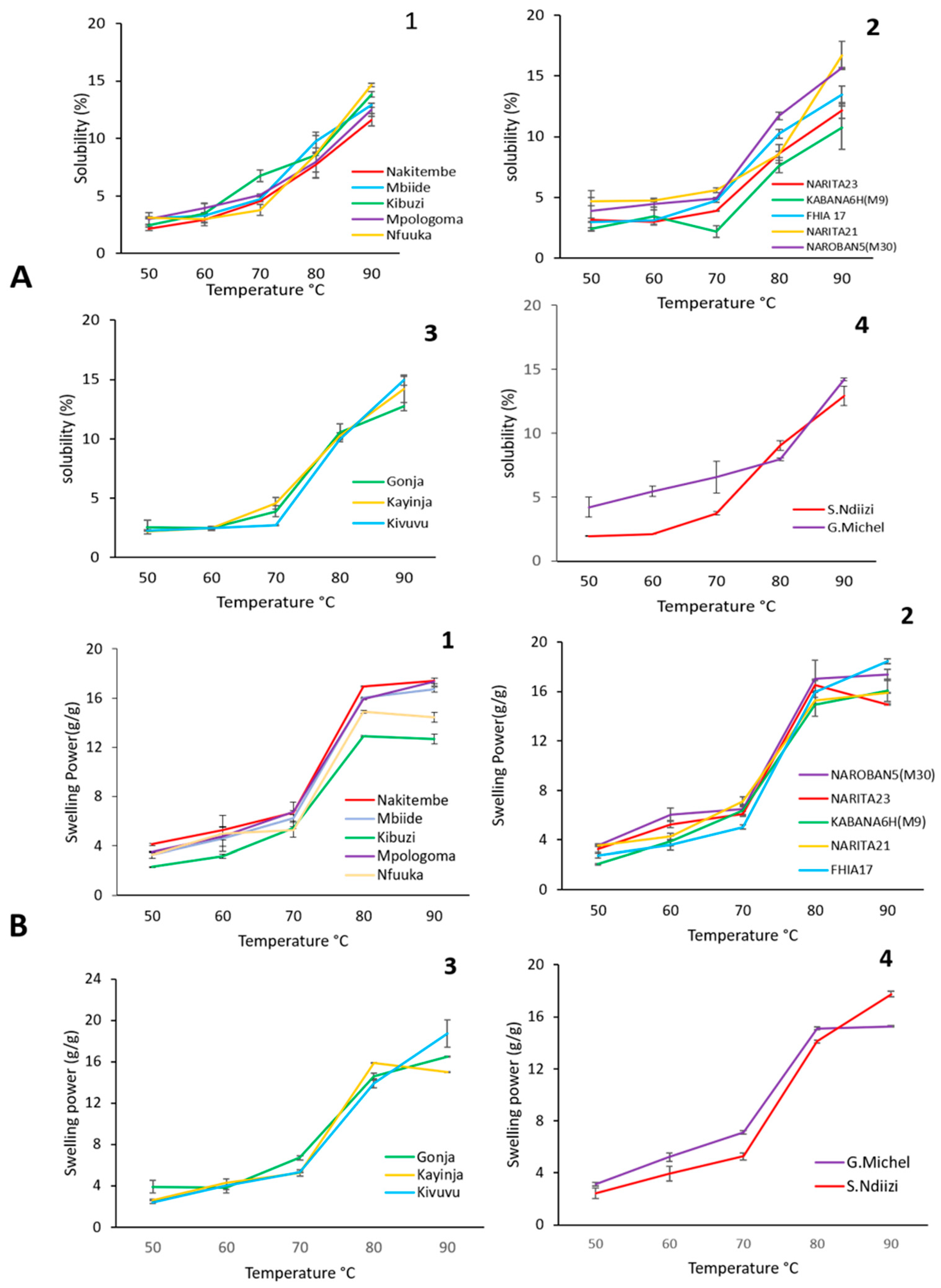
3.3.3. Swelling Power and Solubility
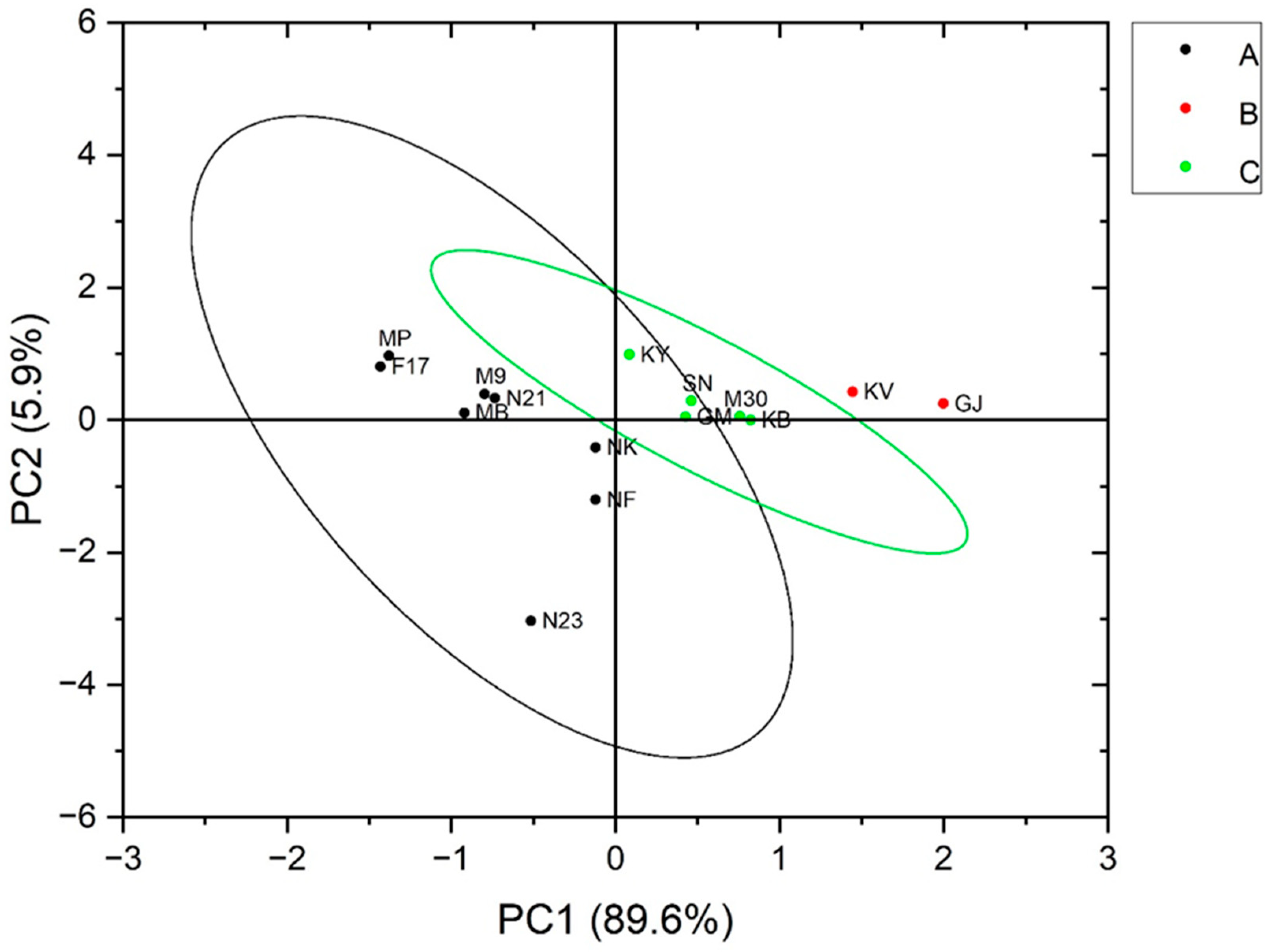
3.4. Molecular Organization of the Resistant Starch
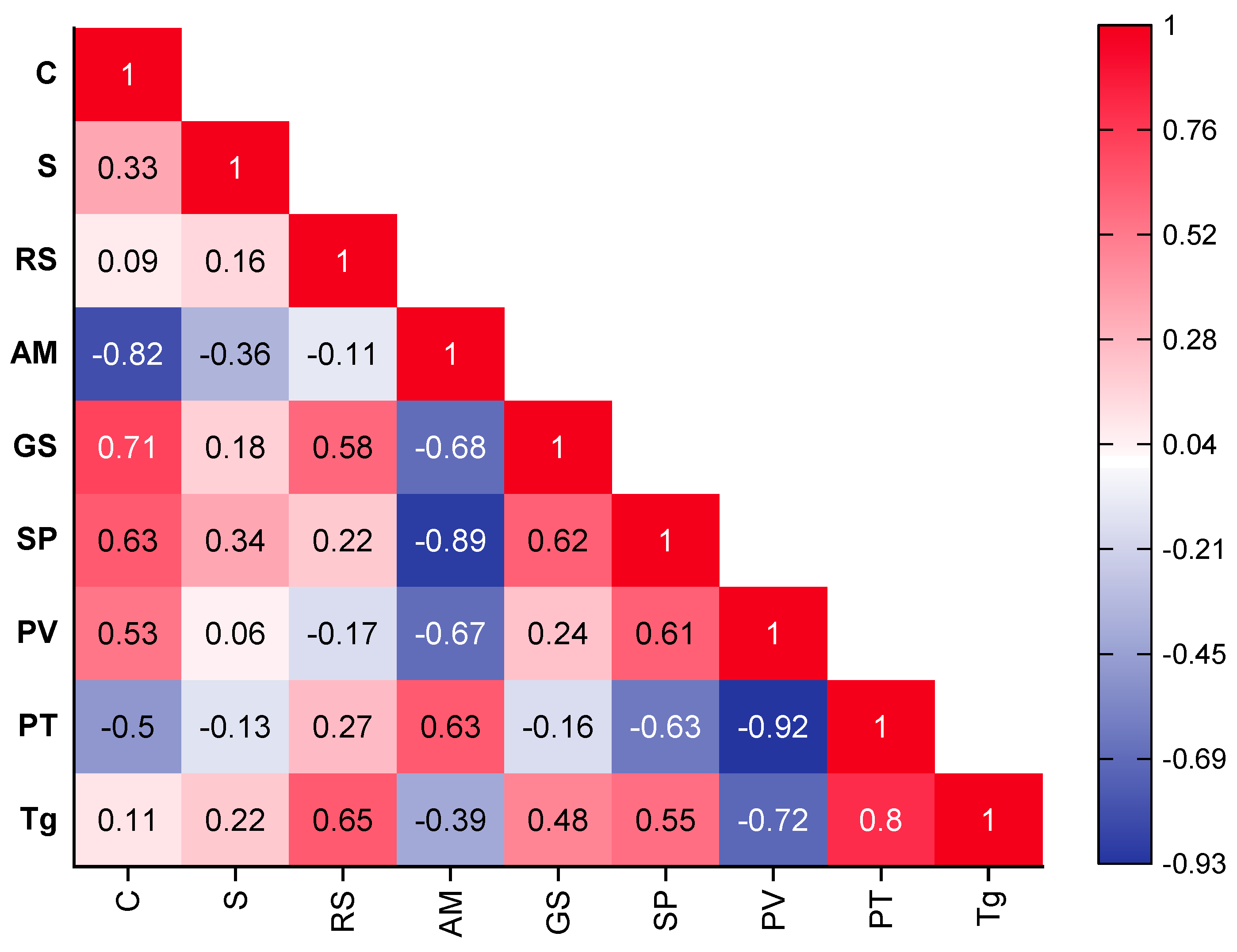
3.5. Relationships between the Characteristics of Resistant Starch
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Daniells, J. Bananas and Plantains—The Crops and Their Importance. In Encyclopedia of Food Science and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 372–378. [Google Scholar] [CrossRef]
- Paramasivam, S.K.; Saravanan, A.; Narayanan, S.; Shiva, K.N.; Ravi, I.; Mayilvaganan, M.; Pushpa, R.; Uma, S. Exploring Differences in the Physicochemical, Functional, Structural, and Pasting Properties of Banana Starches from Dessert, Cooking, and Plantain Cultivars (Musa spp.). Int. J. Biol. Macromol. 2021, 191, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Gudimella, R.; Harikrishna, J.A.; Sin, L.W.; Khalid, N.; Keulemans, J. A Draft Musa balbisiana Genome Sequence for Molecular Genetics in Polyploid, Inter- and Intra-Specific Musa Hybrids. BMC Genom. 2013, 14, 683. [Google Scholar] [CrossRef] [PubMed]
- Kubiriba, J.; Ssali, R.T.; Barekye, A.; Akankwasa, K.; Tushemereirwe, W.K.; Batte, M.; Karamura, E.B.; Karamura, D. The Performance of East African Highland Bananas Released in Farmers Fields and the Need for Their Further Improvement. In Proceedings of the IX International Symposium on Banana: ISHS-ProMusa Symposium on Unravelling the Banana’s Genomic Potential, Brisbane, Australia, 17–22 August 2014; Van den Bergh, I., Smith, M., Daniells, J., Miller, R., Eds.; Acta Horticulturae. ISHS: Leuven, Belgium, 2014; Volume 1114, pp. 231–238, ISBN 978-94-62611-08-5. [Google Scholar]
- Miah, A.S.; Islam, S.; Abedin, N.; Islam, N.; Islam, F.; Tisa, K.J.; Saha, A.K.; Aziz, S. Physicochemical and Functional Properties of Banana Starch and Its Alternative Returns. Curr. Res. Nutr. Food Sci. 2023, 11, 866–879. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch—A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Bird, A.R.; Conlon, M.A.; Christophersen, C.T.; Topping, D.L. Resistant Starch, Large Bowel Fermentation and a Broader Perspective of Prebiotics and Probiotics. Benef. Microbes 2010, 1, 423–431. [Google Scholar] [CrossRef]
- Lehmann, U.; Jacobasch, G.; Schmiedl, D. Characterization of Resistant Starch Type III from Banana (Musa acuminata). J. Agric. Food Chem. 2002, 50, 5236–5240. [Google Scholar] [CrossRef]
- Henningsson, A.M.; Margareta, E.; Nyman, G.L.; Björck, I.M.E. Influences of Dietary Adaptation and Source of Resistant Starch on Short-Chain Fatty Acids in the Hindgut of Rats. Br. J. Nutr. 2003, 89, 319–328. [Google Scholar] [CrossRef]
- Martin, L.J.; Dumon, H.J.; Lecannu, G.; Champ, M.M. Potato and High-Amylose Maize Starches Are Not Equivalent Producers of Butyrate for the Colonic Mucosa. Br. J. Nutr. 2000, 84, 689–696. [Google Scholar] [CrossRef]
- Fung, K.Y.C.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A Review of the Potential Mechanisms for the Lowering of Colorectal Oncogenesis by Butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef]
- Malcomson, F.C.; Willis, N.D.; Mathers, J.C. Is Resistant Starch Protective against Colorectal Cancer via Modulation of the WNT Signalling Pathway? Proc. Nutr. Soc. 2015, 74, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.D.; Mauro, M.O.; Pesarini, J.R.; Ogo, F.M.; Oliveira, R.J. Resistant Starch: A Functional Food That Prevents DNA Damage and Chemical Carcinogenesis. Genet. Mol. Res. 2015, 14, 1679–1691. [Google Scholar] [CrossRef]
- Nugent, A.P. Health Properties of Resistant Starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Östman, E.M.; Holst, J.J.; Björck, I.M.E. Including Indigestible Carbohydrates in the Evening Meal of Healthy Subjects Improves Glucose Tolerance, Lowers Inflammatory Markers, and Increases Satiety after a Subsequent Standardized Breakfast. J. Nutr. 2008, 138, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Kwok, K.; Li, Y.; Fu, L. In Vitro Study of Possible Role of Dietary Fiber in Lowering Postprandial Serum Glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef]
- Amini, A.; Khalili, L.; Keshtiban, A.K.; Homayouni, A. Resistant Starch as a Bioactive Compound in Colorectal Cancer Prevention. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion, 1st ed.; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780128023716. [Google Scholar]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmed, Z. Nutraceutical Aspects of β-Glucan with Application in Food Products. In Nutraceuticals; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Hurkman, W.J.; McCue, K.F.; Altenbach, S.B.; Korn, A.; Tanaka, C.K.; Kothari, K.M.; Johnson, E.L.; Bechtel, D.B.; Wilson, J.D.; Anderson, O.D.; et al. Effect of Temperature on Expression of Genes Encoding Enzymes for Starch Biosynthesis in Developing Wheat Endosperm. Plant Sci. 2003, 164, 873–881. [Google Scholar] [CrossRef]
- Ji, Y.; Pollak, L.M.; Duvick, S.; Seetharaman, K.; Dixon, P.M.; White, P.J. Gelatinization Properties of Starches from Three Successive Generations of Six Exotic Corn Lines Grown in Two Locations. Cereal Chem. 2004, 81, 59–64. [Google Scholar] [CrossRef]
- Pollak, L.M.; Scott, M.P.; Duvick, S.A. Resistant Starch and Starch Thermal Characteristics in Exotic Corn Lines Grown in Temperate and Tropical Environments. Cereal Chem. 2011, 88, 435–440. [Google Scholar] [CrossRef]
- Hussain, T.; Tausend, P.; Graham, G.; Ho, J. Registration of IBM2 SYN10 Doubled Haploid Mapping Population of Maize. J. Plant Regist. 2007, 1, 81. [Google Scholar] [CrossRef]
- Vatanasuchart, N.; Niyomwit, B.; Wongkrajang, K. Resistant Starch Content, in Vitro Starch Digestibility and Physico-Chemical Properties of Flour and Starch from Thai Bananas. Maejo Int. J. Sci. Technol. 2012, 6, 259–271. [Google Scholar]
- Karamura, D.A. Numerical Taxonomic Studies of the East African Highland Bananas (Musa AAA-East Africa) in Uganda. International Network for the Improvement of Banana and Plantain, Conference Proceedings. 1999. Available online: https://cgspace.cgiar.org/items/b4535d2a-4460-4ec3-867c-49cf487ae48d (accessed on 15 August 2024).
- Evans, T.C.; Gavrilovich, E.; Mihai, R.C.; Isbasescu, I.E.L. Method of Isolating a Banana Starch from Green Bananas Cross. Pat. Appl. Publ. Madson 2015, 2, 354. [Google Scholar] [CrossRef]
- Wang, J.; Huang, H.H.; Chen, P.S. Structural and Physicochemical Properties of Banana Resistant Starch from Four Cultivars. Int. J. Food Prop. 2017, 20, 1338–1347. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Marta, H.; Cahyana, Y.; Arifin, H.R.; Khairani, L. Comparing the Effect of Four Different Thermal Modifications on Physicochemical and Pasting Properties of Breadfruit (Artocarpus altilis) Starch. Int. Food Res. J. 2019, 26, 269–276. [Google Scholar]
- Craig, S.A.S.; Maningat, C.C.; Seib, P.A.; Hoseney, R.C. Starch Paste Clarity. Cereal Chem. 1989, 66, 173–182. [Google Scholar]
- Pozo, C.; Rodríguez-llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N. Study of the Structural Order of Native Starch Granules Using Combined FTIR and XRD Analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Utrilla-Coello, R.G.; Rodríguez-Huezo, M.E.; Carrillo-Navas, H.; Hernández-Jaimes, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. In Vitro Digestibility, Physicochemical, Thermal and Rheological Properties of Banana Starches. Carbohydr. Polym. 2014, 101, 154–162. [Google Scholar] [CrossRef]
- Vatanasuchart, N.; Niyomwit, B.; Wongkrajang, K. Resistant Starch Contents and the in Vitro Starch Digestibility of Thai Starchy Foods. Kasetsart J.-Nat. Sci. 2009, 43, 178–186. [Google Scholar]
- Eggleston, G.; Heverlee, R.; Akoni, S. Phvsicochemical Studies on Starches Isolated. Starch/Starke 1992, 44, 121–128. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Aparicio, M.A.; Bello, L.A.; Monroy, J.A. Changes of Banana Starch by Chemical and Physical Modification. Carbohydr. Polym. 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Eerlingen, R.C.; Delcour, J.A. Formation, Analysis, Structure and Properties of Type III Enzyme Resistant Starch. J. Cereal Sci. 1995, 22, 129–138. [Google Scholar] [CrossRef]
- Soares, C.A.; Peroni-Okita, F.H.G.; Cardoso, M.B.; Shitakubo, R.; Lajolo, F.M.; Cordenunsi, B.R. Plantain and Banana Starches: Granule Structural Characteristics Explain the Differences in Their Starch Degradation Patterns. J. Agric. Food Chem. 2011, 59, 6672–6681. [Google Scholar] [CrossRef]
- Ssonko, U.L.; Muranga, F.I. Partial Characterization of Starches from Major Banana (Matooke) Cultivars Grown in Uganda. Food Sci. Nutr. 2017, 5, 1145–1153. [Google Scholar] [CrossRef]
- Yadav, R.B.; Kumar, N.; Yadav, B.S. Characterization of Banana, Potato, and Rice Starch Blends for Their Physicochemical and Pasting Properties. Cogent Food Agric. 2016, 2, 1127873. [Google Scholar] [CrossRef]
- Cordoba, L.d.P.; da Silva, R.G.; Gomes, D.d.S.; Schnitzler, E.; Waszczynskyj, N. Brazilian Green Banana: A Thermal, Structural and Rheological Investigation of Resistant Starch from Different Cultivars. J. Therm. Anal. Calorim. 2018, 134, 2065–2073. [Google Scholar] [CrossRef]
- Fontes, S.D.M.; Cavalcanti, M.T.; Candeia, R.A.; Almeida, E.L. Characterization and Study of Functional Properties of Banana Starch Green Variety of Mysore (Musa AAB—Mysore). Food Sci. Technol. 2017, 37, 224–231. [Google Scholar] [CrossRef]
- Patil, S.; Sonawane, S.K.; Mali, M.; Mhaske, S.T.; Arya, S.S. Pasting, Viscoelastic and Rheological Characterization of Gluten Free (Cereals, Legume and Underutilized) Flours with Reference to Wheat Flour. J. Food Sci. Technol. 2020, 57, 2960–2966. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Sánchez-Hernández, L.; Paredes-López, O. Isolation and Partial Characterization of Banana Starches. J. Agric. Food Chem. 1999, 47, 854–857. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical Properties, Modifications and Applications of Starches from Different Botanical Sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Hoover, R. The Impact of Heat-Moisture Treatment on Molecular Structures and Properties of Starches Isolated from Different Botanical Sources. Crit. Rev. Food Sci. Nutr. 2010, 50, 835–847. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and Chemical Properties of Corn, Cassava, and Potato Starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Kong, L.; Lee, C.; Kim, S.H.; Ziegler, G.R. Characterization of Starch Polymorphic Structures Using Vibrational Sum Frequency Generation Spectroscopy. J. Phys. Chem. B 2014, 118, 1775–1783. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the External Region of the Starch Granule as Determined by Infrared Spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiao, L.; Kong, Y.; Wang, H.; Yang, B. Characterization of the Starch Molecular Structure of Wheat Varying in the Content of Resistant Starch. Food Chem. X 2024, 21, 101103. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Q.; Feng, N.; Wang, J.R.; Wang, S.J.; He, Z.H. Characterization of A- and B-Type Starch Granules in Chinese Wheat Cultivars. J. Integr. Agric. 2016, 15, 2203–2214. [Google Scholar] [CrossRef]
| Cultivar/Variety | Genotype | Class (Utilisation) | |
|---|---|---|---|
| 1 | Mpologoma | AAA-EA | Cooking |
| 2 | Nfuuka | AAA-EA | Cooking |
| 3 | Kibuzi | AAA-EA | Cooking |
| 4 | Nakitembe | AAA-EA | Cooking |
| 5 | Mbidde | AAA-EA | Brewing |
| 6 | Kayinja | ABB | Brewing |
| 7 | Sukali Ndiizi | AAB | Dessert |
| 8 | Gros Michel (Bogoya) | AAA | Dessert |
| 9 | Gonja | AAB | Plantain |
| 10 | Kivuvu | ABB | Plantain |
| 11 | NARITA21 | AAA | Hybrids |
| 12 | NARITA23 | AAA | Hybrids |
| 13 | KABANA 6H (M9) | AAA | Hybrids |
| 14 | NAROBAN5 (M30) | AAA | Hybrids |
| 15 | FHIA17 | AAAA | Hybrids |
| Sample | Genotype | RS Content (g/100 g) * | Amylose (%) † | Granule Size (µm) |
|---|---|---|---|---|
| Kayinja | ABB | 79.27 ± 3.2 a | 22.4 ± 0.9 bc | 23.80 ± 6.3 |
| Kivuvu | ABB | 69.93 ± 2.8 ab | 13.34 ± 0.8 defg | 30.04 ± 6.5 |
| Gonja | AAB | 33.34 ± 2.9 gh | 17.7 ± 0.1 bcd | 18.93 ± 6.0 |
| NARITA23 | AAA | 35.77 ± 0.3 fgh | 15.2 ± 0.7 defg | 20.54 ± 5.9 |
| NARITA21 | AAA | 35.945 ± 0.4 fgh | 14.5 ± 1.1 defg | 21.12 ± 5.3 |
| KABANA6H | AAA | 44.33 ± 2.9 defg | 12.9 ± 0.1 defg | 21.63 ± 7.0 |
| NAROBAN5 | AAA | 55.295 ± 1.1 cd | 9.59 ± 2.9 g | 20.22 ± 5.1 |
| FHIA17 | AAAA | 45.48 ± 1.2 def | 9.23 ± 0.4 g | 24.09 ± 6.4 |
| Mbiide | AAA-EA | 65.91 ± 2.2 bc | 14.3 ± 2.9 defg | 21.39 ± 5.3 |
| Nfuuka | AAA-EA | 53.97 ± 3.3 d | 14.0 ± 0.5 defg | 18.18 ± 4.9 |
| Nakitembe | AAA-EA | 51.785 ± 0.9 d | 13.98 ± 2.1 defg | 20.13 ± 7.5 |
| Mpologoma | AAA-EA | 50.75 ± 0.5 de | 16.3 ± 0.3 cde | 20.69 ± 6.5 |
| Kibuzi | AAA-EA | 30.23 ± 3.0 h | 10.03 ± 0.2 fg | 17.59 ± 7.0 |
| Sukali Ndiizi | AAB | 29.3 ± 7.9 h | 12.9 ± 1.3 defg | 25.71 ± 5.2 |
| Gros Michel | AAA | 49.315 ± 0.1 de | 11.2 ± 0.1 efg | 18.45 ± 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajubi, A.; Baingana, R.; Matovu, M.; Katwaza, R.; Kubiriba, J.; Namanya, P. Variation and Abundance of Resistant Starch in Selected Banana Cultivars in Uganda. Foods 2024, 13, 2998. https://doi.org/10.3390/foods13182998
Kajubi A, Baingana R, Matovu M, Katwaza R, Kubiriba J, Namanya P. Variation and Abundance of Resistant Starch in Selected Banana Cultivars in Uganda. Foods. 2024; 13(18):2998. https://doi.org/10.3390/foods13182998
Chicago/Turabian StyleKajubi, Ali, Rhona Baingana, Moses Matovu, Ronald Katwaza, Jerome Kubiriba, and Priver Namanya. 2024. "Variation and Abundance of Resistant Starch in Selected Banana Cultivars in Uganda" Foods 13, no. 18: 2998. https://doi.org/10.3390/foods13182998
APA StyleKajubi, A., Baingana, R., Matovu, M., Katwaza, R., Kubiriba, J., & Namanya, P. (2024). Variation and Abundance of Resistant Starch in Selected Banana Cultivars in Uganda. Foods, 13(18), 2998. https://doi.org/10.3390/foods13182998





