Evaluation of Different Lactic Acid Bacteria as Starter Cultures for Nono—A West African Fermented Dairy Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation, Characterization, and Identification of Indigenous Lactic Acid Bacteria in Nono
2.3. Nono Production
2.3.1. Standardization of Starter Culture Isolates
2.3.2. Fermentation Protocol
2.4. Physiochemical Analysis
Preliminary Sensory Property Screening
2.5. Evaluation of Effects of Best Starter Culture Consortium on Physical and Sensory Properties of Nono
2.6. Statistical Analyses
3. Results
3.1. Isolation, Characterization, and Identification of Indigenous Lactic Acid Bacteria in Nono
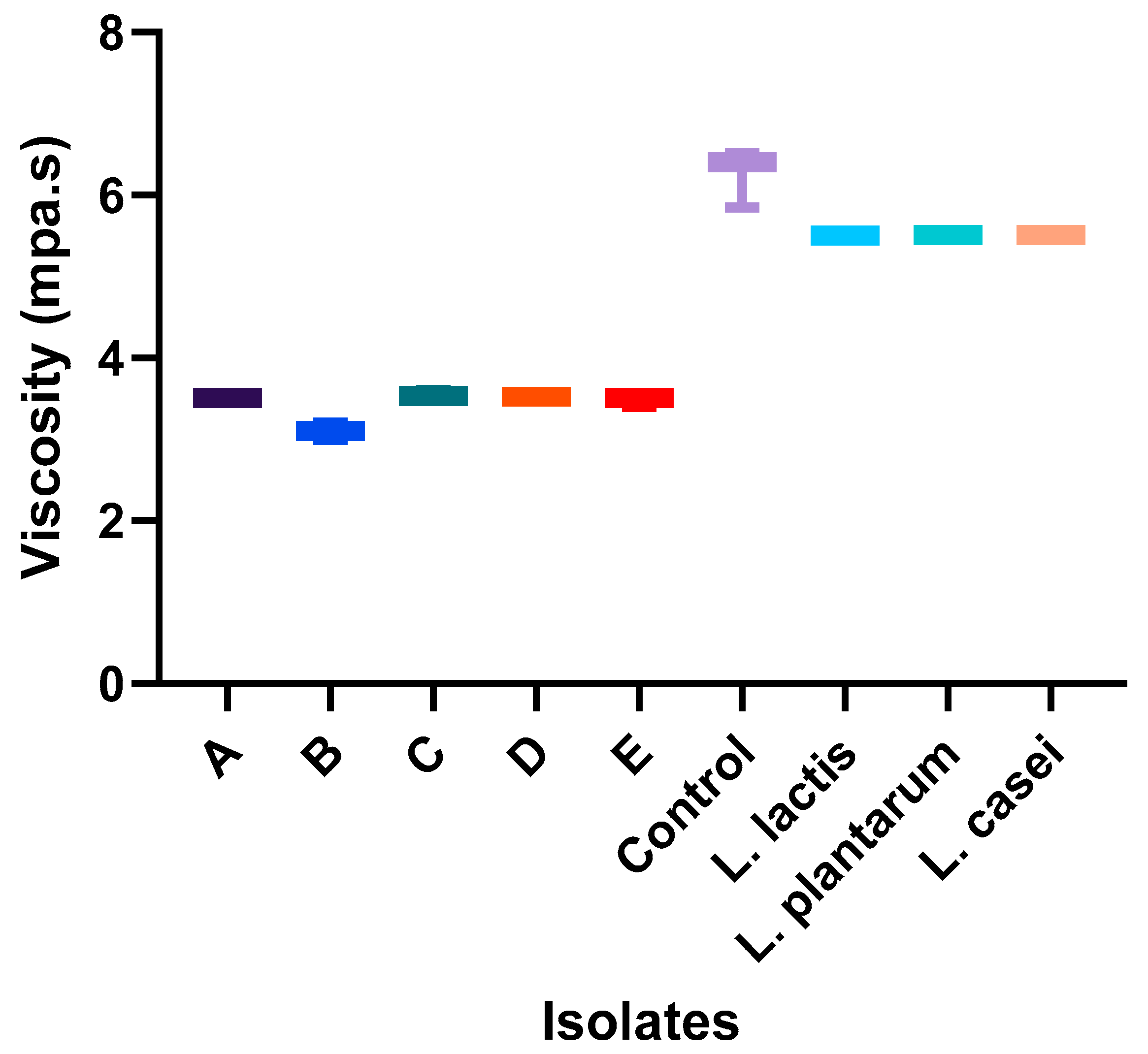
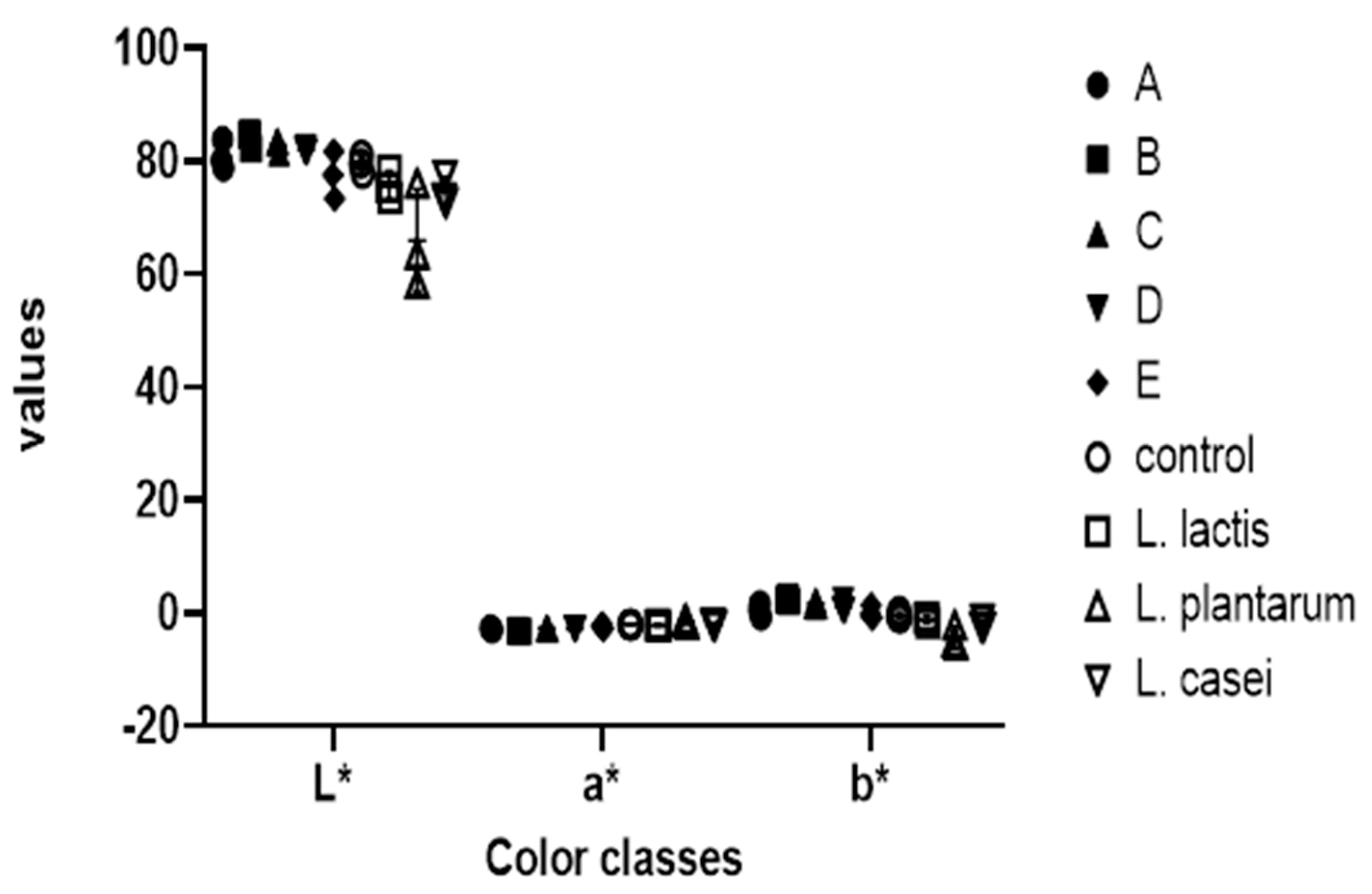
3.2. Assessment of Effects of Starter Culture Isolates on Physical and Sensory Properties of Nono
3.3. Evaluation of Effects of Best Starter Culture Consortium on Physical and Sensory Properties of Nono
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdulrahman, F.A.; Sanmi, E. Physicochemical Properties, Proximate Composition and Total Viable Counts of Staphylococcus aureus in ‘Nono‘ and Yoghurt Samples in Kaduna, Nigeria. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 15–20. [Google Scholar] [CrossRef]
- Omola, E.M.; Kawo, A.H.; Bukar, A. Evaluation of nutritional quality and mineral composition of fermented milk (nono) sold in Kano, north-western Nigeria. Bayero J. Pure Appl. Sci. 2021, 12, 81–88. [Google Scholar] [CrossRef]
- Omola, E.M.; Kawo, A.H.; Bukar, A. Microbiological Quality of Traditionally Fermented Fresh Cow Milk (Nono) Retailed in Selected Local Government Areas of Kano State, Nigeria. UMYU J. Microbiol. Res. 2019, 4, 45–52. [Google Scholar] [CrossRef]
- Atanda, O.O.; Ikenebomeh, M.J. Microbiology quality of nono. World J. Microbiol. 2006, 1, 89–91. [Google Scholar]
- Egwaikhide, P.A.; Malu, P.S.; Lawal, U.; Adelagun, R.O.; Andrew, C. Physicochemical and Microbiological analysis of Fermented cow milk (Nono) consumed within Kaduna town, North western, Nigeria. Food Sci. Qual. Manag. 2014, 29, 2225–2557. [Google Scholar]
- Godwin, A.O.; Emmanuel, T.A. Extent of microbial contamination of nono, fresh cow milk and yoghurt sold in Makurdi, Benue State, Nigeria. J. Microbiol. Biotechnol. Resour. 2013, 3, 6–14. [Google Scholar]
- Obi, C.N.; Ikenebomeh, M.J. Studies on the microbiological, nutritional quality of a Nigerian fermented milk products (nono). Int. J. Dairy Sci. 2007, 2, 95–99. [Google Scholar]
- Ogbonna, I.O. Microbiological Analysis and Safety Evaluation of Nono: A Fermented milk product consumed in most part of Northern Nigeria. Int. J. Dairy Sci. 2011, 6, 181–189. [Google Scholar]
- Uzeh, E.R.; Ohenhen, E.R.; Rojugbokan, K.A. Microbiological and Nutritional Qualities of Dairy Products: Nono and Wara. J. Nat. Sci. 2006, 4, 37–40. [Google Scholar]
- Nebedum, J.O.; Obiakor, T. The effects of different preservation methods on the quality of nunu, a locally fermented Nigerian dairy product. Afr. J. Biotechnol. 2007, 6, 454–458. [Google Scholar]
- Makut, M.D.; Nyam, M.A.; Amapu, T.Y.; Ahmed, A.M. Antibiogram of bacteria isolated from locally processed cow milk products sold in Keffi metropolis, Nasarawa state, Nigeria. J. Biol. Agric. Healthc. 2014, 4, 19–25. [Google Scholar]
- Adesokan, I.; Odetoyinbo, B.; Ekanola, Y.; Avanrenren, R.; Fakorede, S. Production of nigerian nono lactic starter cultures. Pak. J. Nutr. 2011, 10, 2013–2017. [Google Scholar] [CrossRef][Green Version]
- Okiki, P.A.; Adeniji, C.A.; Oyetunji, O.A.; Omosolape, A.Y.; Omolara, A.P. Assessment of the Physicochemical and Bacteriological Qualities of Nono—A Fermented Cow Milk. Potravin. Slovak J. Food Sci. 2018, 12, 26–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Obioha, P.I.; Anyogu, A.; Awamaria, B.; Ghoddusi, H.B.; Ouoba, L.I.I. Antimicrobial Resistance of Lactic Acid Bacteria from Nono, a Naturally Fermented Milk Product. Antibiotics 2023, 12, 843. [Google Scholar] [CrossRef]
- Obioha, P.I.; Ouoba, L.I.I.; Anyogu, A.; Awamaria, B.; Atchia, S.; Ojimelukwe, P.C.; Sutherland, J.P.; Ghoddusi, H.B. Identification and characterisation of the lactic acid bacteria associated with the traditional fermentation of dairy fermented product. Braz. J. Microbiol. 2021, 52, 869–881. [Google Scholar] [CrossRef]
- Oladapo, O.; Zakariya, M.; Olaniyi, A.J.; Daodu, O.; Olorunshola, I.; Akpabio, U. Prevalence of Salmonella Species in Locally Fermented Milk (Nono) in Gambari Market, Ilorin East Local Government, Kwara State, Nigeria. Zagazig Vet. J. 2023, 51, 92–100. [Google Scholar] [CrossRef]
- Uzoaga, G.O.; Umeokonkwo, C.D.; Usman, A.B.; Kia, G.S.; Okolocha, E.C. Bacteriological quality of Nono, a milk product sold at retail outlets in Federal Capital Territory, Nigeria. J. Interv. Epidemiol. Public Health 2020, 3. [Google Scholar] [CrossRef]
- Oyedokun, N.O.; Olukotun, G.B.; Igwegbe, U.I.; Adamu, B.B.; Ideh, R.R.; Shekoni, O.C.; Igwegbe, A.O. Detection, Biochemical and Molecular Characterization of Clostridium sporogens in Nono: A Nigerian Traditionally Fermented Yoghurt Drink. Open J. Med. Microbiol. 2023, 13, 91–100. [Google Scholar] [CrossRef]
- Dafur, G.S.; Iheukwumere, C.C.; Azua, E.T. Physicochemical and Sensory Quality Assessment of ‘Nono’ Sold In Mangu Local Government Area of Plateau State, Nigeria. Int. J. Sci. Res. Publ. 2018, 8, 594–601. [Google Scholar] [CrossRef]
- Fagbemigun, O.; Cho, G.S.; Rösch, N.; Brinks, E.; Schrader, K.; Bockelmann, W.; Oguntoyinbo, F.A.; Franz, C.M.A.P. Isolation and characterization of potential starter cultures from the Nigerian fermented milk product nono. Microorganisms 2021, 9, 640. [Google Scholar] [CrossRef]
- Ikele, M.O.; Umeoduagu, N.D.; Nwakoby, N.E.; Ogbukagu, C.M. Efficacy of Probiotic Lactobacillus casei in Bio-control of Escherichia coli O157:H7 in Nono. Int. J. Innov. Res. Dev. 2020, 9, 135–138. [Google Scholar]
- Fabro, M.A.; Milanesio, H.V.; Robert, L.M.; Speranza, J.L.; Murphy, M.; Rodriguez, G.; Castenada, R. Technical Note: Determination of Acidity in Whole Raw Milk: Comparison of Results Obtained by Two Different Analytical Methods. Am. Dairy Sci. Assoc. 2006, 89, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, H.; Huppertz, T.; Kok, J.; Tarazanova, M. Altering textural properties of fermented milk by using surface-engineered Lactococcus lactis. Microb. Biotechnol. 2018, 11, 770–780. [Google Scholar] [CrossRef]
- Priyashantha, H.; Buldo, P.; Berg, T.; Gilleladen, C.; Ipsen, R. Understanding the fermentation factors affecting the separability of fermented milk: A model system study. Food Struct. 2021, 30, 100232. [Google Scholar] [CrossRef]
- Li, H.; Gao, J.; Chen, W.; Qian, C.; Wang, Y.; Wang, J.; Chen, L. Lactic acid bacteria isolated from Kazakh traditional fermented milk products affect the fermentation characteristics and sensory qualities of yogurt. Food Sci. Nutr. 2022, 10, 1451–1460. [Google Scholar] [CrossRef]
- Stojanovska, S.; Gruevska, N.; Tomovska, J.; Tasevska, J.; Krstanovski, A.; Menkovska, M. Maillard Reaction and Lactose Structural Changes during Milk Processing. Chem. Res. J. 2017, 2, 139–145. [Google Scholar]
- Laverroux, S.; Picard, F.; Andueza, D.; Graulet, B. Vitamin B2 concentration in cow milk: Quantification by a new UHPLC method and prediction by visible and near-infrared spectral analysis. Food Chem. 2021, 342, 128310. [Google Scholar] [CrossRef]
- Milovanovic, B.I.D.; Miocinovic, J.V.D.; Lorenzo, J.M.; Barba, F.J.; Daniel, M.; Igor, T. What Is the Color of Milk and Dairy Products and how is it measured? Foods 2020, 9, 1629. [Google Scholar] [CrossRef]





| Isolate Groups | Colony Morphology | Biochemical Tests | Presumptive Organisms | 16s rDNA Identity | ||
|---|---|---|---|---|---|---|
| Gram stain | Catalase | Oxidase | ||||
| A | Glistening, punctiform whitish colonies with entire margins and smooth appearance | Positive rods | Negative | Negative | Lactobacillus sp. | L. fermentum |
| B | Punctiform milkish colonies with entire margins and smooth appearance. | Positive rods | Negative | Negative | Lactobacillus sp. | L. paracasei |
| C | Circular milkish colonies with glistening appearance | Positive cocco-bacilli | Negative | Negative | Lactobacillus sp. | L. fermentum |
| D | Circular whitish colonies with slimy appearance | Positive rods | Negative | Negative | Lactobacillus sp. | L. fermentum |
| E | Punctiform, milkish colonies with slimy appearance | Positive cocco-bacilli | Negative | Negative | Lactobacillus sp. | L. rhamnosus |
| Isolates | Sensory Property of Fermented Product |
|---|---|
| Lactobacillus fermentum 1 | Nono aroma, fresh milk taste, and white color |
| Lactobacillus paracasei | Nono taste only |
| Lactobacillus fermentum 2 | Fresh milk taste only |
| Lactobacillus fermentum 3 | Nono aroma, fresh milk taste, and white color |
| Lactobacillus rhamnosus | Nono appearance, taste, and aroma |
| Lactobacillus lactis | Yogurt aroma and taste |
| Lactobacillus plantarum | Fresh milk taste and yogurt aroma |
| Lactobacillus casei | Fresh milk taste and yogurt aroma |
| Samples | pH | Moisture Content (%) | Water Activity | Viscosity (mpa.s) | Total Titratable Acidity | Color | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| Fresh milk | 6.66 ± 0.02 b | 87.12 ± 0.01 a | 0.99 ± 0.00 a | 5.33 ± 0.29 a | 3.50 ± 0.20 a | 79.52 ± 1.58 a | −1.99 ± 0.05 a | −0.38 ± 0.63 a |
| Commercial nono | 6.56 ± 0.05 b | 87.51 ± 0.25 a | 0.99 ± 0.00 a | 165.3 ± 2.52 c | 2.93 ± 0.12 a | 85.59 ± 1.45 a | −3.19 ± 0.09 b | 4.09 ± 0.28 c |
| Starter culture mix | 4.49 ± 0.02 a | 87.30 ± 0.02 a | 1.00 ± 0.01 a | 113.7 ± 7.64 b | 5.50 ± 0.30 b | 83.45 ± 2.52 a | −3.02 ± 0.16 b | 2.73 ± 0.44 b |
| p-value | 0.002 | 0.144 | 0.066 | 0.003 | 0.003 | 0.104 | 0.007 | 0.0017 |
| Attributes | Mean ± S.D | p-Value |
|---|---|---|
| Appearance | 8.04 ± 0.70 | 0.584 |
| Taste | 8.37 ± 0.69 | 0.343 |
| Aroma | 7.49 ± 0.56 | 0.145 |
| Texture | 7.05 ± 0.89 | 0.015 |
| Overall acceptance | 7.65 ± 0.47 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikele, O.M.; Ogu, C.T.; Jiang, X.; Cavender, G.A. Evaluation of Different Lactic Acid Bacteria as Starter Cultures for Nono—A West African Fermented Dairy Product. Foods 2024, 13, 3030. https://doi.org/10.3390/foods13193030
Ikele OM, Ogu CT, Jiang X, Cavender GA. Evaluation of Different Lactic Acid Bacteria as Starter Cultures for Nono—A West African Fermented Dairy Product. Foods. 2024; 13(19):3030. https://doi.org/10.3390/foods13193030
Chicago/Turabian StyleIkele, Onyeka M., Chigoziri T. Ogu, Xiuping Jiang, and George A. Cavender. 2024. "Evaluation of Different Lactic Acid Bacteria as Starter Cultures for Nono—A West African Fermented Dairy Product" Foods 13, no. 19: 3030. https://doi.org/10.3390/foods13193030
APA StyleIkele, O. M., Ogu, C. T., Jiang, X., & Cavender, G. A. (2024). Evaluation of Different Lactic Acid Bacteria as Starter Cultures for Nono—A West African Fermented Dairy Product. Foods, 13(19), 3030. https://doi.org/10.3390/foods13193030





