Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging
Abstract
1. Introduction
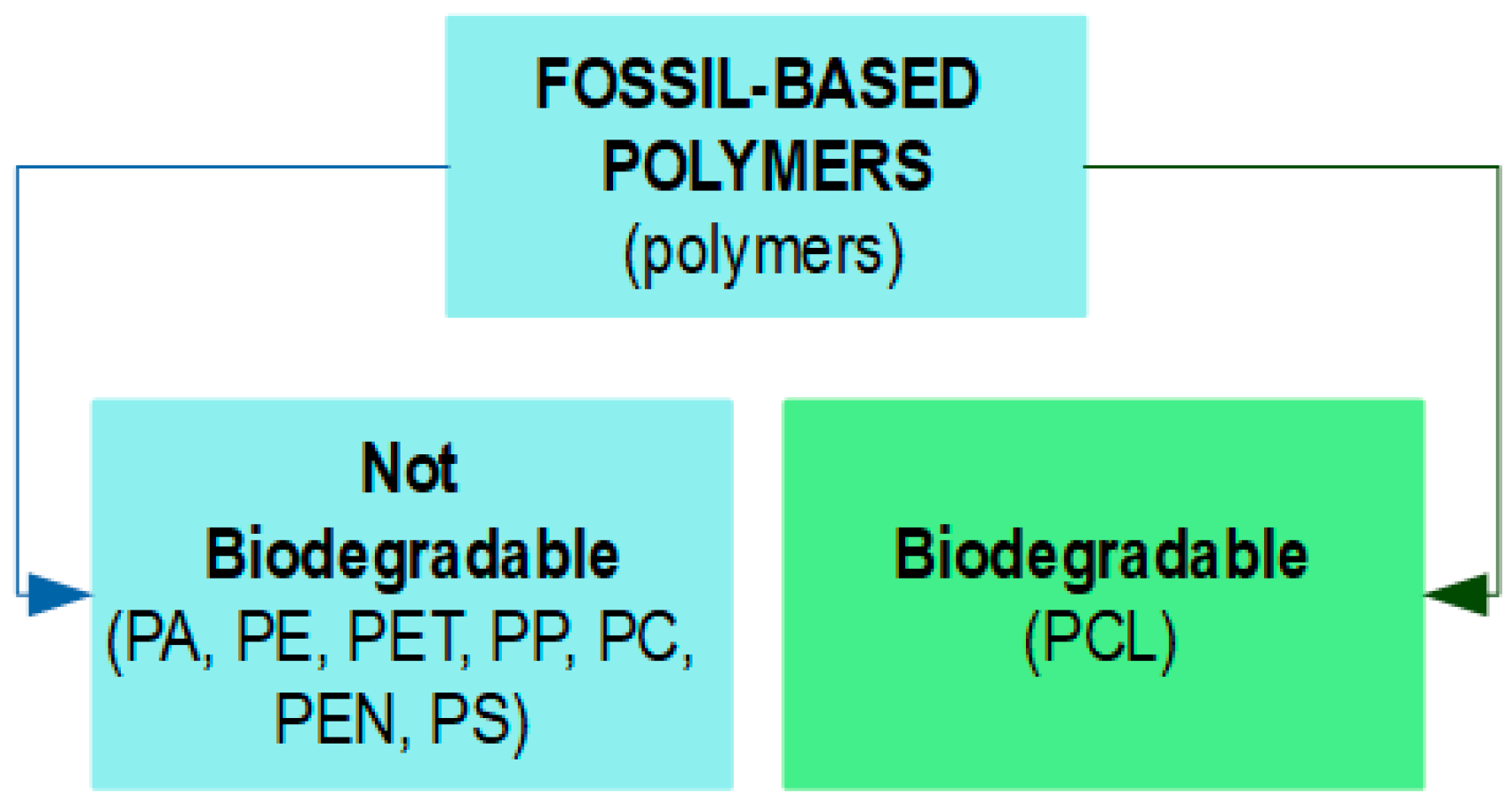
2. Bio-Based Biodegradable Polymers
2.1. Naturally Sourced Polymers
2.2. Biodegradable Polymers Synthesized from Biomass
3. Limitations of Biodegradable Biopolymers
4. Smart Packaging
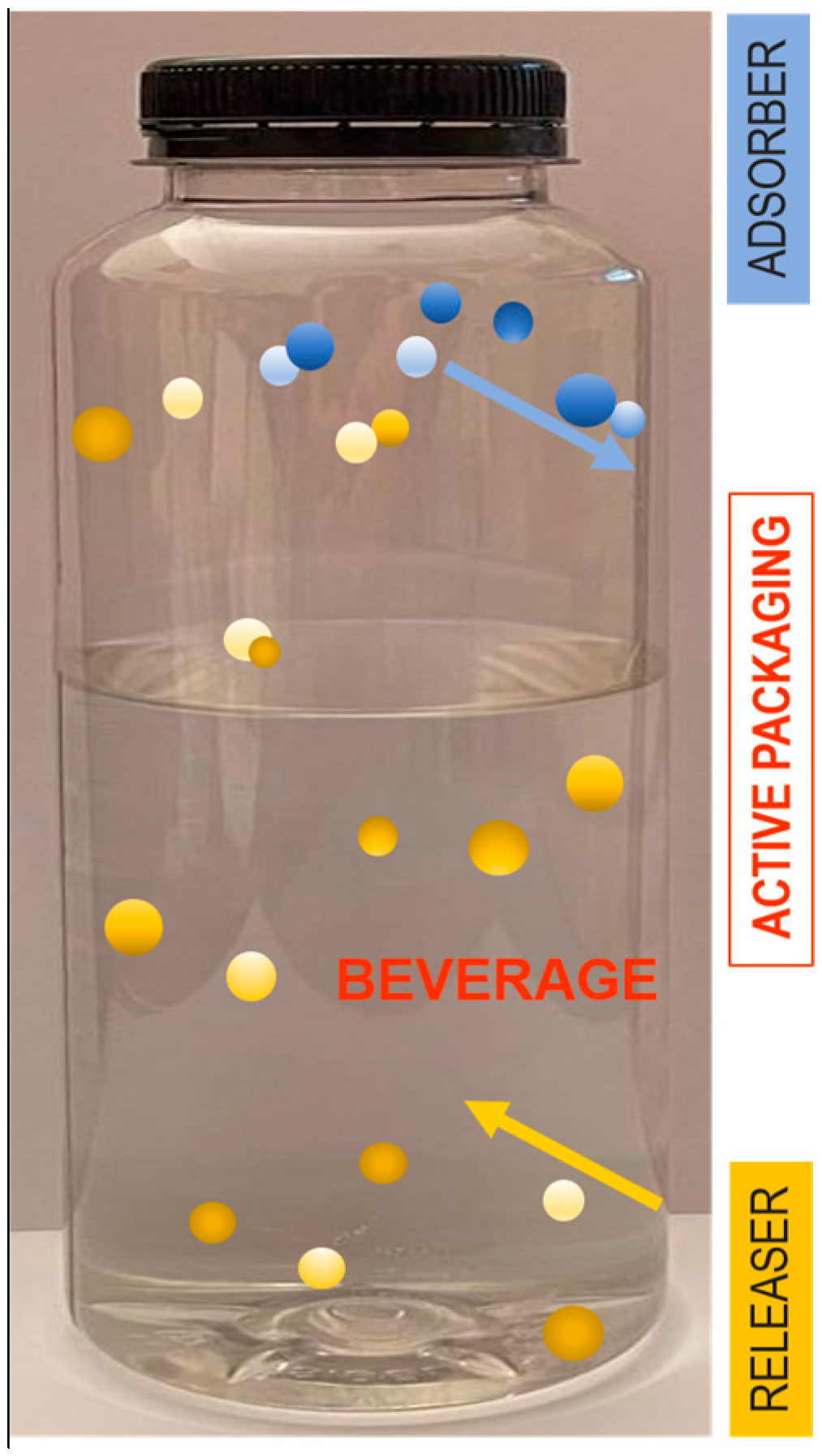
4.1. AP Releaser/Absorber Systems
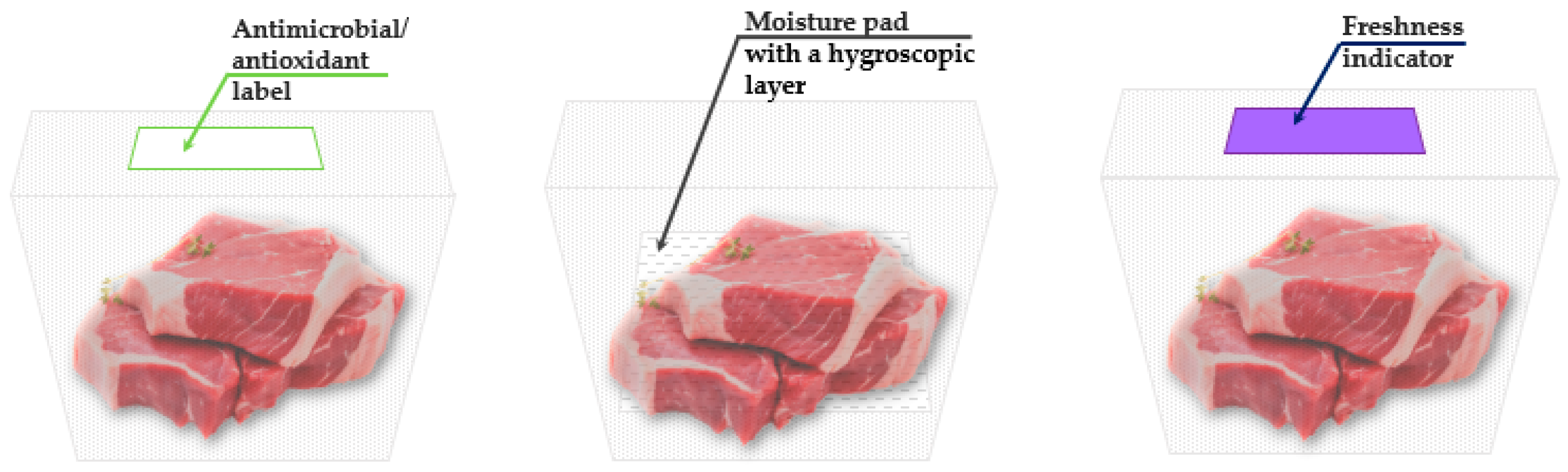
4.2. Intelligent Packaging
4.3. Consumer Views on Active Packaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, C.L.; Gao, L.; Dai, L.; Ji, N.; Qin, Y.; Shi, R.; Qiao, Y.; Xiong, L.; Sun, Q. Hydrophobic biopolymer-based films: Strategies, properties, and food applications. Food Eng. Rev. 2023, 15, 360–379. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Petka-Poniatowska, K. Antimicrobial Compounds in Food Packaging. Int. J. Mol. Sci. 2023, 24, 2457. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, T.; Rashvand, M.; Daramola, M.O.; Iwarere, S.A. A Review on Antimicrobial Packaging for Extending the Shelf Life of Food. Processes 2023, 11, 590. [Google Scholar] [CrossRef]
- Hong, S.J.; Riahi, Z.; Shin, G.H.; Kim, J.T. Pseudomonas aeruginosa-derived carbon dots doped with sulfur as active packaging materials for fresh food preservation. Food Biosci. 2024, 57, 103506. [Google Scholar] [CrossRef]
- Sarfraz, M.H.; Hayat, S.; Siddique, M.H.; Aslam, B.; Ashraf, A.; Saqalein, M.; Khurshid, M.; Sarfraz, M.F.; Afzal, M.; Muzammil, S. Chitosan based coatings and films: A perspective on antimicrobial, antioxidant, and intelligent food packaging. Prog. Org. Coat. 2024, 188, 108235. [Google Scholar] [CrossRef]
- Stoica, D.; Alexe, P.; Ivan, A.S.; Moraru, D.I.; Ungureanu, C.V.; Stanciu, S.; Stoica, M. Biopolymers: Global Carbon Footprint and Climate Change. In Biopolymers: Recent Updates, Challenges and Opportunities; Nadda, A.K., Sharma, S., Bhat, R., Eds.; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2022; pp. 35–54. [Google Scholar]
- Stoica, D.; Alexe, P.; Ivan, A.S.; Stanciu, S.; Tatu, D.M.; Stoica, M. Bioplastics from Biomass. In Biopolymers: Recent Updates, Challenges and Opportunities; Nadda, A.K., Sharma, S., Bhat, R., Eds.; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2022; pp. 353–372. [Google Scholar]
- Stoica, M. Biodegradable Nanomaterials for Drink Packaging. In Nanotechnology in the Beverage Industry: Fundamentals and Applications; Abdeltif, A., Ranjendran, S., Nguyen, T.A., Assadi, A., MahdySharoba, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 609–632. [Google Scholar]
- Yan, M.R.; Hsieh, S.; Ricacho, N. Innovative Food Packaging, Food Quality and Safety, and Consumer Perspectives. Processes 2022, 10, 747. [Google Scholar] [CrossRef]
- Vain, C. Sustainable Food Packaging. 2023. Available online: https://cpdonline.co.uk/knowledge-base/food-hygiene/sustainable-food-packaging/ (accessed on 10 August 2024).
- Zhang, Y.; Min, T.; Zhao, Y.; Cheng, C.; Yin, H.; Yue, J. The developments and trends of electrospinning active food packaging: A review and bibliometrics analysis. Food Control 2024, 160, 110291. [Google Scholar] [CrossRef]
- Bonnenfant, C.; Gontard, N.; Aouf, C. PHBV-based polymers as food packaging: Physical-chemical and structural stability under reuse conditions. Polymer 2023, 270, 125784. [Google Scholar] [CrossRef]
- Boone, L.; Préat, N.; Nhu, T.T.; Fiordelisi, F.; Guillard, V.; Blanckaert, M.; Dewulf, J. Environmental performance of plastic food packaging: Life cycle assessment extended with costs on marine ecosystem services. Sci. Total Environ. 2023, 894, 164781. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Kiani, F.; Hajjari, M.M.; Sharif, N.; Fazaeli, M.; Hosseini, S.M.H. Electrospun zein incorporating phycocyanin and Spirulina extract: Fabrication, characterization, and potential application. LWT 2023, 188, 115408. [Google Scholar] [CrossRef]
- Majumder, S.; Huang, S.; Zhou, J.; Wang, Y.; George, S. Tannic acid-loaded halloysite clay grafted with silver nanoparticles enhanced the mechanical and antimicrobial properties of soy protein isolate films for food-packaging applications. Food Packag. Shelf Life 2023, 39, 101142. [Google Scholar] [CrossRef]
- Mehrabian, M.; Kargari, A. Bio-based nonporous membranes: Evolution and benchmarking review. J. Ind. Eng. Chem. 2023, 124, 17–39. [Google Scholar] [CrossRef]
- Stoica, M.; Antohi, V.M.; Sorici, M.; Stoica, D. The financial impact of replacing plastic packaging by biodegradable biopolymers—A smart solution for the food industry. J. Clean. Prod. 2020, 277, 124013. [Google Scholar] [CrossRef]
- Stoica, M.; Dima, C.V.; Alexe, P. Eco-Friendly Nanocomposites from Bacterial Cellulose and Biopolyesters as a Sustainable Alternative for Food Plastic Packaging. In Food Packaging and Preservation: Techniques, Applications and Technology; Galaz, A.D., Bailey, D.S., Eds.; Nova Science Publishers: New York, NY, USA, 2018; pp. 113–127. [Google Scholar]
- Zhang, M.; Yang, B.; Yuan, Z.; Sheng, Q.; Jin, C.; Qi, J.; Yu, M.; Liu, Y.; Xiong, G. Preparation and performance testing of corn starch/pullulan/gallic acid multicomponent composite films for active food packaging. Food Chem. 2023, 19, 100782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Fan, L.; Li, J.; Zhong, S. Pickering emulsions stabilized by biopolymer-based nanoparticles or hybrid particles for the development of food packaging films: A review. Food Hydrocoll. 2024, 146, 109185. [Google Scholar] [CrossRef]
- Cammarelle, A.; Viscecchia, R.; Bimbo, F. Intention to Purchase Active and Intelligent Packaging to Reduce Household Food Waste: Evidence from Italian Consumers. Sustainability 2021, 13, 4486. [Google Scholar] [CrossRef]
- Plastics Use for Packaging Worldwide in 2019 with Projections to 2060. Available online: https://www.statista.com/statistics/1342657/plastic-packaging-consumption-worldwide-outlook/ (accessed on 10 August 2024).
- Bhaskar, R.; Zo, S.M.; Narayanan, K.B.; Purohit, S.D.; Gupta, M.K.; Han, S.S. Recent development of protein-based biopolymers in food packaging applications: A review. Polym. Test. 2023, 124, 108097. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.-W. Chitosan/gelatin-based multifunctional films integrated with sulfur-functionalized chitin for active packaging applications. Food Hydrocoll. 2024, 149, 109537. [Google Scholar] [CrossRef]
- Jiao, H.; Ali, S.S.; Alsharbaty, M.H.M.; Elsamahy, T.; Abdelkarim, E.; Schagerl, M.; Al-Tohamy, R.; Sun, J. A critical review on plastic waste life cycle assessment and management: Challenges, research gaps, and future perspectives. Ecotoxicol. Environ. Saf. 2024, 271, 115942. [Google Scholar] [CrossRef]
- ECHA. 2018. Available online: https://echa.europa.eu/ro/-/echa-to-consider-restrictions-on-the-use-of-oxo-plastics-and-microplasti-1 (accessed on 11 August 2024).
- Hadimani, S.; Supriya, D.; Roopa, K.; Soujanya, S.K.; Rakshata, V.; Netravati, A.; Akshayakumar, V.; De Britto, S.; Jogaiah, S. Biodegradable hybrid biopolymer film based on carboxy methyl cellulose and selenium nanoparticles with antifungal properties to enhance grapes shelf life. Int. J. Biol. Macromol. 2023, 237, 124076. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Assadpour, E.; Cong, X.; Jafari, S.M. Cross-linked biopolymeric films by citric acid for food packaging and preservation. Adv. Colloid Interface Sci. 2023, 314, 102886. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Agbesi, P.; Arafat, K.M.Y.; Urdaneta, F.; Dey, M.; Basak, M.; Hong, S.; Umeileka, C.; Argyropoulos, D. Bio-based smart packaging: Fundamentals and functions in sustainable food systems. Trends Food Sci. Technol. 2024, 145, 104369. [Google Scholar] [CrossRef]
- De Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Xue, W.H.; Zhu, J.X.; Sun, P.D.; Yang, F.M.; Wu, H.; Li, W.X. Permeability of biodegradable film comprising biopolymers derived from marine origin for food packaging application: A review. Trends Food Sci. Technol. 2023, 136, 295–307. [Google Scholar] [CrossRef]
- Kola, V.; Carvalho, I.S. Plant extracts as additives in biodegradable films and coatings in active food packaging. Food Biosci. 2023, 54, 102860. [Google Scholar] [CrossRef]
- Ray, S. Sensory Properties of Foods and Their Measurement Methods. In Techniques to Measure Food Safety and Quality; Khan, M.S., Shafiur Rahman, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 345–381. [Google Scholar]
- Khandeparkar, A.S.; Paul, R.; Sridhar, A.; Lakshmaiah, V.V.; Nagella, P. Eco-friendly innovations in food packaging: A sustainable revolution. Sustain. Chem. Pharm. 2024, 39, 101579. [Google Scholar] [CrossRef]
- Matthes, J.; Schmid, M. Biogenic raw materials from food waste and by-products for smart packaging applications. Curr. Opin. Green Sustain. Chem. 2024, 46, 100894. [Google Scholar] [CrossRef]
- Smart Packaging: Challenges, Opportunities, Types, and Benefits in 2024. Available online: https://www.designnbuy.com/blog/smart-packaging-challenges-opportunities-types-benefits/ (accessed on 11 August 2024).
- Dutta, D.; Sit, N. Comprehensive review on developments in starch-based films along with active ingredients for sustainable food packaging. Sustain. Chem. Pharm. 2024, 39, 101534. [Google Scholar] [CrossRef]
- Loučanová, E.; Parobek, J.; Nosáľová, M. The Perception of Intelligent Packaging Innovation: The Latest Process and Technological Progress. In Food Processing and Packaging Technologies—Recent Advances; Tumuluru, J.S., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Tregnago Cunha, K.C.; Mazieri, M.R. Intelligent packaging and value generating: Technological development opportunities based on Patent Analysis. World Pat. Inf. 2024, 76, 102258. [Google Scholar] [CrossRef]
- Venezia, V.; Prieto, C.; Verrillo, M.; Grumi, M.; Silvestri, B.; Vitiello, G.; Luciani, G.; Lagaron, J.M. Electrospun films incorporating humic substances of application interest in sustainable active food packaging. Int. J. Biol. Macromol. 2024, 263, 130210. [Google Scholar] [CrossRef]
- Aydın, A.; Yüceer, M.; Ulugergerli, E.U.; Caner, C. Improving food security as disaster relief using intermediate moisture foods and active packaging technologies. Appl. Food Res. 2024, 4, 100378. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Romanelli Vicente Bertolo, M.; Santos Fernandes, S.; Lemes, A.I.; da Cruz Silva, G.; Bogusz Junior, S.; Monteiro Cordeiro de Azeredo, M.; Capparelli Mattoso, L.H.; Buranelo Egea, M. Intelligent and active biodegradable biopolymeric films containing carotenoids. Food Chem. 2024, 434, 137454. [Google Scholar] [CrossRef]
- Fu, X.; Chang, X.; Xu, S.; Xu, H.; Ge, S.; Xie, Y.; Wang, R.; Xu, Y.; Luo, Z.; Shan, Y.; et al. Development of a chitosan/pectin-based multi-active food packaging with both UV and microbial defense functions for effectively preserving of strawberry. Int. J. Biol. Macromol. 2024, 254, 127968. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P. Role of oxygen absorbers in food as packaging material, their characterization and applications. J. Food Sci. Technol. 2024, 61, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Y.; Deng, Y. Latest Advances in Active Materials for Food Packaging and Their Application. Foods 2023, 12, 4055. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gomez, A.; Navarro-Martínez, A.; Martínez-Hernandez, G.B. Effects of essential oils released from active packaging on the antioxidant system and quality of lemons during cold storage and commercialization. Sci. Hortic. 2023, 312, 111855. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Julianti, E.; Safitri, A.; Jaafar, M. Properties of active packaging of PLA-PCL film integrated with chitosan as an antibacterial agent and syzygium cumini seed extract as an antioxidant agent. Heliyon 2024, 10, e23952. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Young, E.; Mirosa, M.; Bremer, P. A conceptual model for food industry views on the commercialisation of active and intelligent packaging. Packag. Technol. Sci. 2023, 36, 905–925. [Google Scholar] [CrossRef]
- GLOPACK. Granting Society with Low Environmental Impact Innovative Packaging. 2021. Available online: https://cordis.europa.eu/project/id/773375/reporting (accessed on 11 August 2024).
- Hirth, S.; Boons, F.; Doherty, B. Unpacking food to go: Packaging and food waste of on the go provisioning practices in the UK. Geoforum 2021, 126, 115–125. [Google Scholar] [CrossRef]
- O’Callaghan, K.A.M.; Kerry, J.P. Consumer attitudes towards the application of smart packaging technologies to cheese products. Food Packag. Shelf Life 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Young, E.M.M. A Systematic Review of Consumer Perceptions of Smart Packaging Technologies for Food. Front. Sustain. Food Syst. 2020, 4, 1–20. [Google Scholar] [CrossRef]
- Chu, Y.; Popovich, C.; Wang, Y. Heat sealable regenerated cellulose films enabled by zein coating for sustainable food packaging. Compos. C Open Access 2023, 12, 100390. [Google Scholar] [CrossRef]
- Mostafa, H.; Airouyuwaa, J.O.; Hamed, F.; Wang, Y.; Maqsood, S. Structural, mechanical, antioxidant and antibacterial properties of soy protein isolate (SPI)-based edible food packaging films as influenced by nanocellulose (NC) and green extracted phenolic compounds from date palm leaves. Food Packag. Shelf Life 2023, 38, 101124. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef]
- Singh, A.K.; Lee, M.; Jang, D.; Lee, Y.S. Non-conventional starch nanoparticles: Novel avenues towards improving sustainability of the food packaging sector. Trends Food Sci. Technol. 2024, 143, 104273. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, C.; Lv, S.; Ren, F.; Wang, J. Study on electrospinning of wheat gluten: A review. Food Res. Int. 2023, 169, 112851. [Google Scholar] [CrossRef]
- Babaremu, K.; Oladijo, O.P.; Akinlabi, E. Biopolymers: A suitable replacement for plastics in product packaging. Adv. Ind. Eng. Polym. Res. 2023, 6, 333–340. [Google Scholar] [CrossRef]
- Oluba, O.M.; Edeh, D.A.; Ojeaburu, S.I.; Bayo-Olorunmeke, O.A.; Josiah, S.J. Physicochemical and thermal characterization and antioxidant property of chicken feather keratin and ginger starch hybrid nanocomposite film. Carbohydr. Polym. 2023, 6, 100368. [Google Scholar] [CrossRef]
- Wang, C.; Gong, C.; Qin, Y.; Hu, Y.; Jiao, A.; Jin, Z.; Qiu, C.; Wang, J. Bioactive and functional biodegradable packaging films reinforced with nanoparticles. J. Food Eng. 2022, 312, 110752. [Google Scholar] [CrossRef]
- Pan, F.; Li, J.; Zhao, L.; Tuersuntuoheti, T.; Mehmood, A.; Zhou, N.; Hao, S.; Wang, C.; Guo, Y.; Lin, W. A molecular docking and molecular dynamics simulation study on the interaction between cyanidin-3-O-glucoside and major proteins in cow’s milk. J. Food Biochem. 2021, 45, e13570. [Google Scholar] [CrossRef] [PubMed]
- RCSB PDB 1F6S. Available online: https://www.rcsb.org/structure/1f6s (accessed on 6 September 2024).
- RCSB PDB 1BEB. Available online: https://www.rcsb.org/structure/1beb (accessed on 6 September 2024).
- RCSB PDB 1CAG. Available online: https://www.rcsb.org/structure/1CAG (accessed on 6 September 2024).
- Shayegan, M.; Javanshir Rezaei, N.; Lam, N.H.; Forde, N. Probing multiscale mechanics of collagen with optical tweezers. In Proceedings of the SPIE—The International Society for Optical Engineering, San Diego, CA, USA, 27 August 2013; Volume 8810, p. 88101P. [Google Scholar] [CrossRef]
- Enzymatic Modification of Native Chitin and Conversion to Specialty Chemical Products. Available online: https://www.researchgate.net/publication/338947208 (accessed on 11 August 2024).
- Tahoun, M.; Engeser, M.; Namasivayam, V.; Sander, P.M.; Müller, C.E. Chemistry and Analysis of Organic Compounds in Dinosaurs. Biology 2022, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- RCSB PDB 1GK4. Available online: https://www.rcsb.org/structure/1gk4 (accessed on 6 September 2024).
- Harter, C. Gluten and Wheat. In Gluten Sensitivity. Essentials; Springer: Wiesbaden, Germany, 2021. [Google Scholar] [CrossRef]
- Bold, J. Gluten and its main food sources and other components of grains that impact on health. In Gluten Related Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 33–48. [Google Scholar] [CrossRef]
- Yan, M.R.; Hsieh, S.; Ricacho, N. Developing Protein-Based Plastics. ACS Symp. Ser. 2014, 1178, 357–370. [Google Scholar]
- RCSB PDB 1FXZ. Available online: https://www.rcsb.org/structure/1fxz (accessed on 6 September 2024).
- Available online: https://www.shutterstock.com/ro/search/starch-molecule (accessed on 6 September 2024).
- Tivano, F.; Chiono, V. Zein as a Renewable Material for the Preparation of Green Nanoparticles for Drug Delivery. Front. Biomater. Sci. 2023, 2, 1156403. [Google Scholar] [CrossRef]
- Microbial Degradation of Cellulose (Enzymes, Steps, Mechanisms). Available online: https://microbenotes.com/microbial-degradation-of-cellulose/ (accessed on 11 August 2024).
- Wanchao, H.; Anne-Marie, C.; Dragos, C. Pectin in Metabolic Liver Disease. Nutrients 2023, 15, 157. [Google Scholar]
- Donati, I.; Christensen, B.E. Alginate-metal cation interactions: Macromolecular approach. Carbohydr. Polym. 2023, 321, 121280. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, S.Y.; Luo, Z.G.; Zong, M.H.; Li, X.X.; Lou, W.Y. Biotechnology and bioengineering of pullulanase: State of the art and perspectives. World J. Microbiol. Biotechnol. 2021, 37, 43. [Google Scholar] [CrossRef]
- Jiménez-Pérez, C.; Roldán-Hernández, L.; Cruz-Guerrero, A.; Trant, J.F.; Alatorre-Santamaría, S. Insights on the Interaction between Kefiran and Whey Proteins Using Computational Analyses. Chem. Proc. 2023, 14, 47. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S.; et al. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 1383–1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wusigale; Luo, Y. Colloidal nanoparticles prepared from zein and casein: Interactions, characterizations and emerging food applications. Food Sci. Hum. Wellness 2023, 12, 337–350. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vazvani, M.G.; Hassanisaadi, M.; Thakur, V.K.; Kennedy, J.F. Use of whey protein as a natural polymer for the encapsulation of plant biocontrol bacteria: A review. Int. J. Biol. Macromol. 2023, 234, 123708. [Google Scholar]
- Assanvo, E.F.; Nagaraj, S.; Boa, D.; Thanikaivelan, P. Hybrid collagen–cellulose–Fe₃O₄@TiO₂ magnetic bio-sponges derived from animal skin waste and Kenaf fibers for wastewater remediation. Sci. Rep. 2023, 13, 13365. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, K.; Ma, Y.; Yu, Z.; Chiou, B.; Jia, M.; Chen, M.; Zhong, F. Collagen films with improved wet state mechanical properties by mineralization. Food Hydrocoll. 2023, 139, 108579. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, K.X.; Teng, A.; Li, Y.; Wang, Y. A rethinking of collagen as tough biomaterials in meat packaging: Assembly from native to synthetic. Crit. Rev. Food Sci. Nutr. 2022, 23, 1–21. [Google Scholar] [CrossRef]
- Zheng, T.; Tang, P.; Yang, C.; Ran, R.; Li, G. Development of active packaging films based on collagen/gallic acid-grafted chitosan incorporating with ε-polylysine for pork preservation. Food Hydrocoll. 2023, 140, 108590. [Google Scholar] [CrossRef]
- Blázquez-Carmona, P.; Sanz-Herrera, J.A.; Mora-Macías, J.; Morgaz, J.; Domínguez, J.; Reina-Romo, E. Time-Dependent Collagen Fibered Structure in the Early Distraction Callus: Imaging Characterization and Mathematical Modeling. Ann. Biomed. Eng. 2022, 50, 1798–1809. [Google Scholar] [CrossRef]
- Chen, Q.; Pei, Y.; Tang, K.; Albu Kaya, M.G. Structure, extraction, processing, and applications of collagen as an ideal component for biomaterials—A review. Collagen Leather 2023, 5, 20. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.W.; He, L.; Liu, Y.W. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen Derived from Fish Industry Waste: Progresses and Challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; Sharma, V.; Gupta, R. Biobased Materials in Food Packaging. In Advanced Applications of Biobased Materials; Ahmed, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 147–172. [Google Scholar]
- Tapia-Blacido, D.R.; Garcia, A.L.; Beitum, L.R.; Zitei-Baptista, L.F.; Aguilar, P.F. Use of Biobased Materials from Agro-Industrial Residues in Food Packaging. In Advanced Applications of Biobased Materials; Ahmed, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 173–229. [Google Scholar]
- Rather, A.; Akhter, N.; Ashraf, Q.S.; Mir, H.A.; Makroo, S.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Dede, S.; Sadak, O.; Didin, M.; Gunasekaran, S. Antimicrobial food packaging application of angelica root (Angelica sylvestris) oil-loaded electrospun biofibers. Food Packag. Shelf Life 2023, 35, 101035. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Viscusi, G.; Vittoria, V. Physical and Barrier Properties of Chemically Modified Pectin with Polycaprolactone through an Environmentally Friendly Process. Colloid Polym. Sci. 2021, 299, 429–437. [Google Scholar] [CrossRef]
- İnan-Çınkır, N.; Ağcam, E.; Altay, F.; Akyıldız, A. Emulsion electrospinning of zein or gelatin-pectin with carotenoid from watermelon. Food Chem. Adv. 2023, 3, 100346. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; de la Caba, K.; Benjakul, S.; Prodpran, T. Fish gelatin films laminated with emulsified gelatin film or poly(lactic) acid film: Properties and their use as bags for storage of fried salmon skin. Food Hydrocoll. 2021, 111, 106199. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.W.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO₂ for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Frias, J.M.C. Extraction, quantification, characterization, and application in food packaging of chitin and chitosan from mushrooms: A review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef] [PubMed]
- Donkor, L.; Kontoh, G.; Yaya, A.; Bediako, J.K.; Apalangya, V. Bio-based and sustainable food packaging systems: Relevance, challenges, and prospects. Appl. Food Res. 2023, 3, 100356. [Google Scholar] [CrossRef]
- Hernández-Téllez, C.N.; Luque-Alcaraz, A.G.; Núñez-Mexía, S.A.; Cortez-Rocha, M.O.; Lizardi-Mendoza, J.; Rosas-Burgos, E.C.; Rosas-Durazo, A.D.J.; Parra-Vergara, N.V.; Plascencia-Jatomea, M. Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus. Polymers 2022, 14, 2774. [Google Scholar] [CrossRef]
- Hisham, F.; Akmal, M.H.M.; Ahmad, F.; Ahmad, K.; Samat, N.; Shams, A. Biopolymer Chitosan: Potential Sources, Extraction Methods, and Emerging Applications. Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- Khatami, N.; Guerrero, P.; Martín, P.; Quintela, E.; Ramos, V.; Saa, L.; Cortajarena, A.L.; de la Caba, K.; Camarero-Espinosa, S.; Abarrategi, A. Valorization of Biological Waste from Insect-Based Food Industry: Assessment of Chitin and Chitosan Potential. Carbohydr. Polym. 2024, 324, 121529. [Google Scholar] [CrossRef]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent Developments in Edible Films and Coatings for Fruits and Vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Józwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Caruso, M.R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes/Keratin Composites for Wool Treatment. Appl. Clay Sci. 2023, 238, 106930. [Google Scholar]
- Mora-Maldonado, E.; Estrada-Monje, A.; Guzmán, R.Z.; Baldenegro-Pérez, L.; Rodríguez Sánchez, I.; Zaragoza-Contreras, E.A. Effect of a Keratin Coupling Agent on the Mechanical Properties of a Bovine Hair-Thermoplastic Starch Composite. Mater. Chem. Phys. 2023, 308, 128266. [Google Scholar]
- Polesca, C.; Ghatta, A.A.; Passos, H.; Coutinho, J.A.P.; Hallett, J.P.; Freire, M.G. Sustainable Keratin Recovery Process Using a Bio-Based Ionic Liquid Aqueous Solution and Its Techno-Economic Assessment. Green Chem. 2023, 25, 3995. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Chowdhury, S.; Sanpui, P. Extraction of Keratin from Keratinous Wastes: Current Status and Future Directions. J. Mater. Cycles Waste Manag. 2023, 25, 1–16. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Ma, K.; Ullah, R.; Ali, E.A.; Qayum, A.; Zahoor, U.; Uddin, N.; Zhu, D. Laccase and Dye-Decolorizing Peroxidase-Modified Lignin Incorporated with Keratin-Based Biodegradable Film: An Elucidation of Structural Characterization, Antibacterial, and Antioxidant Properties. Food Chem. 2023, 20, 101035. [Google Scholar]
- Wang, L.; Shang, Y.; Zhang, J.; Yuan, J.; Shen, J. Historical Perspective and Recent Advances in Keratin for Biomedical Applications. Adv. Colloid Interface Sci. 2023, 321, 103012. [Google Scholar] [CrossRef] [PubMed]
- Linares-Castañeda, A.; Franco-Hernández, M.O.; Gómez, Y.; Gómez, Y.L.M.; Corzo-Rios, L.J. Physical Properties of Zein-Alginate-Glycerol Edible Films and Their Application in the Preservation of Chili Peppers (Capsicum annuum L.). Food Sci. Biotechnol. 2023, 33, 889–902. [Google Scholar] [CrossRef]
- Rezaei, M.; Pirsa, S.; Chavoshizadeh, S. Photocatalytic/Antimicrobial Active Film Based on Wheat Gluten/ZnO Nanoparticles. J. Inorg. Organomet. Polym. 2020, 30, 2654–2665. [Google Scholar] [CrossRef]
- Sharma, T.; Kaur, G.; Singh, A.; Kaur, P.; Dar, B.N.; Kaur, A. An Emerging Sustainable Approach for Development and Characterization of Gluten-Based Nanocomposite Films Reinforced with Starch Nanocrystals in Conjunction with Chitosan. Sustain. Chem. Pharm. 2023, 36, 101338. [Google Scholar] [CrossRef]
- Adilah, Z.A.M.; Jamilah, B.; Hanani, Z.A.N. Storage Stability of Mayonnaise Packaged in Soy Protein Isolate Films Incorporated with Mango Kernel Extract. Food Packag. Shelf Life 2023, 40, 101216. [Google Scholar] [CrossRef]
- Dong, Y.; Lan, T.; Wang, L.; Wang, X.; Xu, Z.; Jiang, L.; Zhang, Y.; Sui, X. Development of Composite Electrospun Films Utilizing Soy Protein Amyloid Fibrils and Pullulan for Food Packaging Applications. Food Chem. 2023, 20, 100995. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Shahabi, N.; Fallah, A.A.; Mahmoudi, E.; Al-Musawi, M.H.; Ghorbani, M. Soy Protein Isolate/Kappa-Carrageenan/Cellulose Nanofibrils Composite Film Incorporated with Zein Essential Oil-Loaded MOFs for Food Packaging. Int. J. Biol. Macromol. 2023, 250, 126176. [Google Scholar]
- Shahabi, N.; Soleimani, S.; Ghorbani, M. Investigating Functional Properties of Halloysite Nanotubes and Propolis Used in Reinforced Composite Film Based on Soy Protein/Basil Seed Gum for Food Packaging Application. Int. J. Biol. Macromol. 2023, 231, 123350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Shen, Z.; Hongtao, Z.; Xiaobei, Z. Arginine-Carboxylated Pullulan, a Potential Antibacterial Material for Food Packaging. Biomater. Adv. 2023, 154, 213584. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; McClements, D.J.; Martinez, M.M.; Hadidi, M. Electrospun Plant Protein-Based Nanofibers in Food Packaging. Food Chem. 2024, 432, 137236. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Guo, J.; Liu, Y.; Xue, X.; Wang, X.; Wei, L.; Mao, L.; Zhang, Z.; Zhuo, Y.; Li, S.; et al. Fabrication of Novel Electrospun Zein/Polyethylene Oxide Film Incorporating Nisin for Antimicrobial Packaging. LWT 2023, 185, 115176. [Google Scholar] [CrossRef]
- Amini, E.; Valls, C.; Roncero, M.B. Promising Nanocomposites for Food Packaging Based on Cellulose–PCL Films Reinforced by Using ZnO Nanoparticles in an Ionic Liquid. Ind. Crops Prod. 2023, 193, 116246. [Google Scholar] [CrossRef]
- Huang, K.; Maltais, A.; Wang, Y. Enhancing Water Resistance of Regenerated Cellulose Films with Organosilanes and Cellulose Nanocrystals for Food Packaging. Carbohydr. Polym. 2023, 6, 100391. [Google Scholar] [CrossRef]
- Jang, E.J.; Padhan, B.; Patel, M.; Pandey, J.K.; Xu, B.; Patel, K. Antibacterial and Biodegradable Food Packaging Film from Bacterial Cellulose. Food Control 2023, 153, 109902. [Google Scholar] [CrossRef]
- Mejía-Jaramillo, A.M.; Gomez-Hoyos, C.; Gutierrez, A.I.C.; Correa-Hincapie, N.; Gallego, R.Z.; Triana-Chavez, O. Tackling the Cytotoxicity and Genotoxicity of Cellulose Nanofibers from the Banana Rachis: A New Food Packaging Alternative. Heliyon 2023, 9, 21560. [Google Scholar] [CrossRef]
- Oliviero, M.; Lamberti, E.; Cafiero, L.; Pace, B.; Cefola, M.; Gorrasi, G.; Sambandam, A.; Sorrentino, A. Biodegradable Cellulose Acetate/Layered Double-Hydroxide Composite Film for Active Packaging of Fresh Food. Mater. Chem. Phys. 2023, 310, 128469. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.R.L.; Basumatary, I.B.; Nayak, A.; Dutta, D.; Konwar, J.; Purkayastha, M.D.; Mukherjee, A. Recent Progress in Pectin Extraction and Their Applications in Developing Films and Coatings for Sustainable Food Packaging: A Review. Int. J. Biol. Macromol. 2023, 239, 124281. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, L.; Biswas, D.; Chandel, V.; Rhim, J.W. Recent Progress in Pectin Extraction, Characterization, and Pectin-Based Films for Active Food Packaging Applications: A Review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef] [PubMed]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zuo, J.; Liu, Y.; Zheng, B.; Dai, X.; Bai, Z.; Liu, Y.; Yao, J. Alginate-Based Films Integrated with Nitrogen-Functionalized Carbon Dots and Layered Clay for Active Food Packaging Applications. Int. J. Biol. Macromol. 2023, 253, 126653. [Google Scholar] [CrossRef]
- Nath, P.C.; Sharma, R.; Debnath, S.; Sharma, M.; Inbaraj, B.S.; Dikkala, P.K.; Nayak, P.K.; Sridhar, K. Recent Trends in Polysaccharide-Based Biodegradable Polymers for Smart Food Packaging Industry. Int. J. Biol. Macromol. 2023, 253, 127524. [Google Scholar] [CrossRef]
- Ureña, M.; Carullo, D.; Thanh, T.; Phùng, T.; Fournier, P.; Farris, S.; Lagorce, A.; Karbowiak, T. Effect of Polymer Structure on the Functional Properties of Alginate for Film or Coating Applications. Food Hydrocoll. 2024, 149, 109557. [Google Scholar] [CrossRef]
- Yan, P.; Lan, W.; Xie, J. Modification on Sodium Alginate for Food Preservation: A Review. Trends Food Sci. Technol. 2024, 143, 104217. [Google Scholar] [CrossRef]
- Ertan, K.; Celebioglu, A.; Chowdhury, R.; Sumnu, G.; Sahin, S.; Altier, C.; Uyar, T. Carvacrol/Cyclodextrin Inclusion Complex Loaded Gelatin/Pullulan Nanofibers for Active Food Packaging Applications. Food Hydrocoll. 2023, 142, 108864. [Google Scholar] [CrossRef]
- De Souza, C.K.; Ghosh, T.; Lukhmana, N.; Tahiliani, S.; Priyadarshi, R.; Purohit, S.D.; Han, S.S. Pullulan as a Sustainable Biopolymer for Versatile Applications: A Review. Mater. Today Commun. 2023, 36, 106477. [Google Scholar] [CrossRef]
- Mugnaini, G.; Bonini, M.; Gentile, L.; Panza, O.; Del Nobile, M.A.; Conte, A.; Esposito, R.; D’Errico, G.; Moccia, F.; Panzella, L. Effect of Design and Molecular Interactions on the Food Preserving Properties of Alginate/Pullulan Edible Films Loaded with Grape Pomace Extract. J. Food Eng. 2024, 361, 111716. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rostamabadi, H.; Habibi, N.; Falsafi, S.R. Pullulan Nanocomposites: Effect of Nanoparticles and Essential Oil Reinforcement on Its Performance and Food Packaging Applications. Food Humanit. 2023, 1, 887–894. [Google Scholar] [CrossRef]
- Dedhia, N.; Marathe, S.J.; Singhal, R.S. Food Polysaccharides: A Review on Emerging Microbial Sources, Bioactivities, Nanoformulations, and Safety Considerations. Carbohydr. Polym. 2022, 287, 119355. [Google Scholar] [CrossRef] [PubMed]
- Gentry, B.; Cazón, P.; O’Brien, K. A Comprehensive Review of the Production, Beneficial Properties, and Applications of Kefiran, the Kefir Grain Exopolysaccharide. Int. Dairy J. 2023, 144, 105691. [Google Scholar] [CrossRef]
- Prasad, S.; Purohit, S.R. Microbial Exopolysaccharide: Sources, Stress Conditions, Properties and Application in Food and Environment: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 242, 124925. [Google Scholar] [CrossRef]
- Touranlou, A.; Noori, S.M.A.; Salari, A.; Afshari, A.; Hashemi, M. Application of Kefir for Reduction of Contaminants in the Food Industry: A Systematic Review. Int. Dairy J. 2023, 146, 105748. [Google Scholar] [CrossRef]
- PHA: A Biopolymer Whose Time Has Finally Come. Available online: https://cen.acs.org/business/biobased-chemicals/PHA-biopolymer-whose-time-finally/97/i35 (accessed on 11 August 2024).
- Imágenes Libres de Regalías de Polylactic Acid. Available online: https://www.shutterstock.com/es/search/polylactic-acid (accessed on 14 August 2024).
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.-J.W.; Krooneman, J. Polyhydroxyalkanoates (PHAs) Synthesis and Degradation by Microbes and Applications Towards a Circular Economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef]
- Yousefi, A.M.; Wnek, G.E. Poly(hydroxyalkanoates): Emerging Biopolymers in Biomedical Fields and Packaging Industries for a Circular Economy. Biomed. Mater. Devices 2024, 2, 1–26. [Google Scholar] [CrossRef]
- Eraslan, K.; Aversa, C.; Nofar, M.; Barletta, M.; Gisario, A.; Salehiyan, R.; Alkan Goksu, Y. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH): Synthesis, Properties, and Applications—A Review. Eur. Polym. J. 2022, 167, 111044. [Google Scholar] [CrossRef]
- Stoica, M.; Stoica, D. Nanofillers for Food Packaging: Antimicrobial Potential of Metal-Based Nanoparticles. Curr. Nanotoxic. Prev. 2020, 1, 1–23. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Podzorova, M.V.; Varyan, I.A.; Tcherdyntsev, V.V.; Zadorozhnyy, M.Y.; Medvedeva, E.V. Promising Agromaterials Based on Biodegradable Polymers: Polylactide and Poly-3-Hydroxybutyrate. Polymers 2023, 15, 1029. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhao, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of Whey Protein-Based Edible Films and Coatings in Food Industries: An Updated Overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Rosseto, M.; Rigueto, C.V.T.; Alessandretti, I.; de Oliveira, R.; Wohlmuth, D.A.R.; Loss, R.A.; Dettmer, A.; Dos Santos Richards, N.S.P. Whey-Based Polymeric Films for Food Packaging Applications: A Review of Recent Trends. J. Sci. Food Agric. 2022, 103, 3217–3229. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Physiochemical, Antioxidant, and Food Simulant Release Properties of Collagen-Carboxymethyl Cellulose Films Enriched with Berberis lyceum Root Extract. J. Food Process. Preserv. 2022, 46, 16485. [Google Scholar] [CrossRef]
- Da Costa, G.F.; Grisi, C.V.B.; de Albuquerque Meireles, B.R.L.; de Sousa, S.; de Magalhães Cordeiro, A.M.T. Collagen Films, Cassava Starch, and Their Blends: Physical–Chemical, Thermal, and Microstructure Properties. Packag. Technol. Sci. 2022, 35, 229–240. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent Advances on Chitosan-Based Films for Sustainable Food Packaging Applications. Food Packag. Shelf Life. 2020, 26, 100551. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan-Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Song, T.; Qian, S.; Lan, T.; Wu, Y.; Liu, J.; Zhang, H. Recent Advances in Bio-Based Smart Active Packaging Materials. Foods 2022, 11, 2228. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible Films from Chitosan-Gelatin: Physical Properties and Food Packaging Application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Yao, X.; Qin, Y.; Zhang, M.; Zhang, J.; Qian, C.; Liu, J. Development of Active and Smart Packaging Films Based on Starch, Polyvinyl Alcohol and Betacyanins from Different Plant Sources. Int. J. Biol. Macromol. 2021, 183, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, R.; Shi, J.; Zhang, R.; Tang, H.; Xie, C.; Wang, F.; Han, J.; Jiang, L. Chitosan/Esterified Chitin Nanofibers Nanocomposite Films Incorporated with Rose Essential Oil: Structure, Physicochemical Characterization, Antioxidant and Antibacterial Properties. Food Chem. 2023, 18, 100714. [Google Scholar] [CrossRef]
- Dong, M.; Tian, L.; Li, J.; Jia, J.; Dong, Y.; Tu, Y.; Liu, X.; Tan, C.; Duan, X. Improving Physicochemical Properties of Edible Wheat Gluten Protein Films with Proteins, Polysaccharides, and Organic Acid. LWT 2022, 154, 112868. [Google Scholar] [CrossRef]
- Yong, Y.; Wang, S.; Li, L.; Li, R.; Ahmad, H.N.; Munawar, N.; Zhu, J. A Curcumin-Crosslinked Bilayer Film of Soy Protein Isolate and Chitosan with Enhanced Antibacterial Property for Beef Preservation and Freshness Monitoring. Int. J. Biol. Macromol. 2023, 247, 125778. [Google Scholar] [CrossRef] [PubMed]
- Chetia, P.; Bharadwaj, C.; Purbey, R.; Bora, D.; Yadav, A.; Lal, M.; Rajulu, A.V.; Sadiku, E.R.; Selvam, S.P.; Jarugala, J. Influence of Silylated Nano Cellulose Reinforcement on the Mechanical, Water Resistance, Thermal, Morphological and Antibacterial Properties of Soy Protein Isolate (SPI)-Based Composite Films. Int. J. Biol. Macromol. 2023, 242, 124861. [Google Scholar] [CrossRef]
- Shan, G.; Xu, Z.; Jiang, L.; Zhang, Y.; Sui, X. Fabrication and Characterization of Glycerin-Plasticized Soy Protein Amyloid Fibril Scaffolds by Unidirectional Freeze Casting Method. Food Hydrocoll. 2024, 147, 109400. [Google Scholar] [CrossRef]
- Zhao, W.; Li, C.; Ma, W.; He, R.; Rong, Y.; Sarker, S.; Liu, Q.; Tian, F. A Novel Active Packaging Film Containing Citronella Oil: Preparation, Characterization, Antimicrobial Activity and Application in Grape Preservation. Food Packag. Shelf Life 2023, 40, 101168. [Google Scholar] [CrossRef]
- Sarak, S.; Pisitaro, W.; Rammak, T.; Kaewtatip, K. Characterization of Starch Film Incorporating Hom Nil Rice Extract for Food Packaging Purposes. Int. J. Biol. Macromol. 2024, 254, 127820. [Google Scholar] [CrossRef]
- Almeida, T.; Karamysheva, A.; Valente, B.F.A.; Silva, J.M.; Braz, M.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Biobased Ternary Films of Thermoplastic Starch, Bacterial Nanocellulose and Gallic Acid for Active Food Packaging. Food Hydrocoll. 2023, 144, 108934. [Google Scholar] [CrossRef]
- Shan, P.; Wang, K.; Sun, F.; Li, Y.; Sun, L.; Li, H.; Peng, L. Humidity-Adjustable Functional Gelatin Hydrogel/Ethyl Cellulose Bilayer Films for Active Food Packaging Application. Food Chem. 2024, 439, 138202. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; de Sousa, T.L.; Bertolo, M.R.V.; Junior, S.B.; Mattoso, L.H.C.; Pimentel, T.C.; Egea, M.B. Next-Generation Food Packaging: Edible Bioactive Films with Alginate, Mangaba Pulp (Hancornia speciosa), and Saccharomyces boulardii. Food Biosci. 2023, 54, 102799. [Google Scholar] [CrossRef]
- Tong, W.Y.; Rafiee, A.R.A.; Leong, C.R.; Tan, W.N.; Dailin, D.J.; Almarhoon, Z.M.; Shelkh, M.; Nawaz, A.; Chuah, L.F. Development of Sodium Alginate-Pectin Biodegradable Active Food Packaging Film Containing Cinnamic Acid. Chemosphere 2023, 336, 139212. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Santos, M.M.; Antonio, F.; Antunes, F.; Arruda, G.L.; Shibukawa, V.P.; Prado, C.A.; Ortiz-Silos, N.; Castro-Alonso, M.J.; Marcelino, P.R.F.; Santos, J.C. Production and Applications of Pullulan from Lignocellulosic Biomass: Challenges and Perspectives. Bioresour. Technol. 2023, 385, 129460. [Google Scholar] [CrossRef] [PubMed]
- Ruiyan, N.I.; Cheng, M.; Meng, J.; Hu, W.; Ke, Q.; Zhao, Y. Edible Pullulan Enhanced Water-Soluble Keratin with Improved Sizing Performance for Sustainable Textile Industry. Int. J. Biol. Macromol. 2023, 238, 124066. [Google Scholar]
- Wei, Z.; Huang, L.; Feng, X.; Cui, F.; Wu, R.; Kong, Q.; Sun, K.; Gao, J.; Guo, J. Development of Functional, Sustainable Pullulan-Sodium Alginate-Based Films by Incorporating Essential Oil Microemulsion for Chilled Pork Preservation. Int. J. Biol. Macromol. 2023, 253, 127257. [Google Scholar] [CrossRef]
- De Luca, S.; Milanese, D.; Gallichi-Nottiani, D.; Cavazza, A.; Sciancalepore, C. Poly(lactic acid) and Its Blends for Packaging Application: A Review. Clean Technol. 2023, 5, 1304–1343. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Jin, H.; Feng, B.; Jiang, G. Konjac Glucomannan/Polyvinyl Alcohol/Citric Acid–Based Active Food-Packaging Films Containing Polygonatum sibiricum Polysaccharide. Food Chem. Adv. 2024, 4, 100660. [Google Scholar] [CrossRef]
- Ribeiro, I.S.; Maciel, G.M.; Gonçalves Bortolini, D.; de Andrade Arruda Fernandes, I.; Volpato Maroldi, V.; Pedro, A.C.; Thaís Vieira Rubio, F.; Windson Isidoro Haminiuk, C. Sustainable Innovations in Edible Films and Coatings: An Overview. Trends Food Sci. Technol. 2024, 143, 104272. [Google Scholar] [CrossRef]
- Higuchi, M.T.; Aguiar, A.C.d.; Leles, N.R.; Ribeiro, L.T.M.; Bosso, B.E.C.; Yamashita, F.; Youssef, K.; Roberto, S.R. Active Packaging Systems to Extend the Shelf Life of ‘Italia’ Table Grapes. Horticulturae 2024, 10, 214. [Google Scholar] [CrossRef]
- Stuparu-Cretu, M.; Braniste, G.; Necula, G.-A.; Stanciu, S.; Stoica, D.; Stoica, M. Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health. Foods 2023, 12, 1882. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Ravishankar, C.N. Oxygen Scavenger Packaging for Seafood Preservation. Fish Technol. 2020, 57, 147–155. [Google Scholar]
- EFSA. Scientific Opinion on the Safety Evaluation of the Active Substances, Sodium Borohydride and Palladium Acetate for Use in Active Food Contact Materials. EFSA J. 2012, 10, 2642 . [Google Scholar] [CrossRef][Green Version]
- EFSA. Scientific Opinion on the Safety Evaluation of the Active Substance, Acrylic Acid, Sodium Salt, Co-Polymer with Acrylic Acid, Methyl Ester, Methacrylic Acid, 2-Hydroxypropyl Ester, and Acrylic Acid Cross-Linked for Use in Active Food Contact Materials. EFSA J. 2013, 11, 3154. [Google Scholar] [CrossRef][Green Version]
- EFSA. Scientific Opinion on the Safety Assessment of the Active Substances Iron, Iron Oxides, Sodium Chloride, and Calcium Hydroxide for Use in Food Contact Materials. EFSA J. 2013, 11, 3387 . [Google Scholar] [CrossRef][Green Version]
- EFSA. Scientific Opinion on the Safety Evaluation of the Active Substances, Sodium Carbonate Peroxyhydrate Coated with Sodium Carbonate and Sodium Silicate, Bentonite, Sodium Chloride, Sodium Carbonate for Use in Active Food Contact Materials. EFSA J. 2013, 11, 3153 . [Google Scholar] [CrossRef]
- Rozo, D.F.; Alvarado, J.F.; Chaparro, L.M.; Medina, J.A.; Salcedo, F. Modeling Oxidation Kinetics of Linseed Oil in Oxygen Scavenger Nanocapsules to Be Potentially Used in Active Food Packaging. Food Packag. Shelf Life 2024, 42, 101256. [Google Scholar] [CrossRef]
- Rüegg, N.; Röcker, B.; Yildirim, S. Application of Palladium-Based Oxygen Scavenger to Extend the Mould Free Shelf Life of Bakery Products. Food Packag. Shelf Life 2022, 31, 100771. [Google Scholar] [CrossRef]
- Hu, X.; Lu, C.; Tang, H.; Pouri, H.; Joulin, E.; Zhang, J. Active Food Packaging Made of Biopolymer-Based Composites. Materials 2023, 16, 279. [Google Scholar] [CrossRef]
- Active Packaging: What It Is and Why It’s Important. Available online: https://felixinstruments.com/blog/active-packaging-what-it-is-and-why-its-important/ (accessed on 11 August 2024).
- Cheng, C.; Liang, X.; Wei, W.; Zhang, N.; Yao, G.; Yan, R. Enhanced Shelf Life Quality of Peaches (Prunus persica L.) Using Ethylene Manipulating Active Packaging in E-Commerce Logistics. Sci. Hortic. 2024, 326, 112701. [Google Scholar] [CrossRef]
- Kumar, P.; Tripathi, S.; Ramakanth, D.; Gaikwad, K.K. Novel Ethylene Scavenger Based on Sillimanite and Bentonite Clay for Packaging Applications: A Sustainable Alternative for Preservation of Fresh Produce. Sustain. Chem. Pharm. 2024, 38, 101516. [Google Scholar] [CrossRef]
- Lee, D.S.; Wang, H.J.; Jaisan, C.; An, D.S. Active Food Packaging to Control Carbon Dioxide. Packag. Tech. Sci. 2022, 35, 213–227. [Google Scholar] [CrossRef]
- Mariah, M.A.A.; Vonnie, J.M.; Erna, K.H.; Nur’Aqilah, N.M.; Huda, N.; Abdul Wahab, R.; Rovina, K. The Emergence and Impact of Ethylene Scavengers Techniques in Delaying the Ripening of Fruits and Vegetables. Membranes 2022, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.D.; Chaiwong, S.; Weltzien, C.; Mahajan, P.V. A Model Integrating Fruit Physiology, Perforation, and Scavenger for Prediction of Ethylene Accumulation in Fruit Package. Postharvest Biol. Technol. 2024, 209, 112734. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Safety Evaluation of the Active Substance, Open-Cell Expanded Polystyrene Manufactured with Talc and Alkyl (C8-C22) Sulphonic Acid (Salts) for Use in Active Food Contact Materials. EFSA J. 2012, 10, 2467. [Google Scholar] [CrossRef][Green Version]
- EFSA. Scientific Opinion on the Safety Evaluation of the Active Substances Sodium Carboxy Methyl Cellulose, Bentonite, Aluminium Potassium Sulphate for Use in Active Food Contact Materials. EFSA J. 2012, 10, 2904. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Safety Evaluation of the Active Substances Citric Acid (E330) and Sodium Hydrogen Carbonate (E500ii), Used as Carbon Dioxide Generators, Together with Liquid Absorbers Cellulose and Polyacrylic Acid Sodium Salt Crosslinked, in AC. EFSA J. 2013, 11, 3152. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the Safety Evaluation of the Active Substances Iron, Sodium Chloride, Water, Silica Gel, Activated Carbon, Monosodium Glutamate, Potassium Acid Tartrate, Powdered Cellulose, Malic Acid, Chabazite, Hydroxypropyl Cellulose, Potassium C. EFSA J. 2013, 11, 3155. [Google Scholar] [CrossRef]
- EFSA. Safety Assessment of the Active Substances Carboxymethylcellulose, Acetylated Distarch Phosphate, Bentonite, Boric Acid, and Aluminium Sulfate, for Use in Active Food Contact Materials. EFSA J. 2018, 16, 5121. [Google Scholar] [CrossRef]
- EFSA. Safety Assessment of the Active Substance Polyacrylic Acid, Sodium Salt, Cross-Linked, for Use in Active Food Contact Materials. EFSA J. 2018, 16, 5548. [Google Scholar] [CrossRef]
- Selvaraj Arokiyaraj, A.; Yuvaraj Dinakarkumar, B.; Shin, H. A Comprehensive Overview on the Preservation Techniques and Packaging of Processed Meat Products: Emphasis on Natural Derivatives. J. King Saud Univ. Sci. 2024, 36, 103032. [Google Scholar] [CrossRef]
- Stoica, M.; Stoean, S.; Alexe, P. Overview of Biological Hazards Associated with the Consumption of Meat Products. J. Agroaliment. Process. Technol. 2014, 20, 192–197. [Google Scholar]
- Stoica, M. Overview of Sodium Nitrite—As a Multifunctional Meat-Curing Ingredient. Ann. Univ. Dunarea Jos Galati, Fascicle VI–Food Technol. 2019, 43, 155–167. [Google Scholar] [CrossRef]
- Stoica, M.; Antohi, V.M.; Alexe, P.; Ivan, A.S.; Stanciu, S.; Stoica, D.; Zlati, M.L.; Stuparu-Cretu, M. New Strategies for the Total/Partial Replacement of Conventional Sodium Nitrite in Meat Products: A Review. Food Bioprocess Technol. 2022, 15, 514–538. [Google Scholar] [CrossRef]
- Smaoui, S.; Chérif, I.; Ben Hlima, H.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc Oxide Nanoparticles in Meat Packaging: A Systematic Review of Recent Literature. Food Packag. Shelf Life 2023, 36, 101045. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Wang, L.; Scullen, J.; Sommers, C.H. Antimicrobial Films and Coatings for Inactivation of Listeria innocua on Ready-to-Eat Deli Turkey Meat. Food Control 2014, 40, 64–70. [Google Scholar] [CrossRef]
- Pan, I.F.; Granda, X.C.; Mate, J.L. Antimicrobial Efficiency of Edible Coatings on the Preservation of Chicken Breast Fillets. Food Control 2014, 36, 69–75. [Google Scholar]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef]
- Rahman, M.P.; Mujeeb, V.M.A.; Muraleedharan, K. Flexible Chitosan-Nano ZnO Antimicrobial Pouches as a New Material for Extending the Shelf Life of Raw Meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca Starch Active Nanocomposite Films and Their Antimicrobial Effectiveness on Ready-to-Eat Chicken Meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite Films Based on CMC, Okra Mucilage and ZnO Nanoparticles: Physico-Mechanical and Antibacterial Properties. Carbohydr. Polym. 2018, 181, 351–357. [Google Scholar] [CrossRef]
- Zhao, Y.; Teixeira, J.S.; Saldaña, M.D.A.; Gänzle, M.G. Antimicrobial Activity of Bioactive Starch Packaging Films Against Listeria monocytogenes and Reconstituted Meat Microbiota on Ham. Int. J. Food Microbiol. 2019, 305, 108253. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ahmadi, P.; Ehsani, A. Development of an Active Packaging System Containing Zinc Oxide Nanoparticles for the Extension of Chicken Fillet Shelf Life. Food Sci. Nutr. 2020, 8, 5461–5473. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-Friendly Active Packaging Consisting of Nanostructured Biopolymer Matrix Reinforced with TiO2 and Essential Oil: Application for Preservation of Refrigerated Meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional Betanin Nanoliposomes-Incorporated Gelatin/Chitosan Nanofiber/ZnO Nanoparticles Nanocomposite Film for Fresh Beef Preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.M.; Rhim, J.W. Carboxymethyl Cellulose-Based Multifunctional Film Combined with Zinc Oxide Nanoparticles and Grape Seed Extract for the Preservation of High-Fat Meat Products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Kong, J.; Ge, X.; Sun, Y.; Mao, M.; Yu, H.; Chu, R.; Wang, Y. Multi-Functional pH-Sensitive Active and Intelligent Packaging Based on Highly Cross-Linked Zein for the Monitoring of Pork Freshness. Food Chem. 2023, 404, 134754. [Google Scholar] [CrossRef]
- Li, R.; Zhuang, D.; Feng, H.; Wang, S.; Zhu, J. Novel “All-in-One” Multifunctional Gelatin-Based Film for Beef Freshness Maintaining and Monitoring. Food Chem. 2023, 418, 136003. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, Y.; Zhang, X.; Tao, K.; Li, G. High-Strength Collagen/Delphinidin Film Incorporated with Vaccinium oxycoccus Pigment for Active and Intelligent Food Packaging. Collagen Leather 2023, 5, 11. [Google Scholar] [CrossRef]
- Elhadef, K.; Chaari, M.; Akermi, S.; Ennouri, K.; Ben Hlima, H.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Sarkar, T.; Shariati, M.A.; et al. Gelatin-Sodium Alginate Packaging Film with Date Pits Extract: An Eco-Friendly Packaging for Extending Raw Minced Beef Shelf Life. Meat Sci. 2024, 207, 109371. [Google Scholar] [CrossRef]
- Hong, S.J.; Riahi, Z.; Shin, G.H.; Kim, J.T. Development of Innovative Active Packaging Films Using Gelatin/Pullulan-Based Composites Incorporated with Cinnamon Essential Oil-Loaded Metal-Organic Frameworks for Meat Preservation. Int. J. Biol. Macromol. 2024, 267 Pt 2, 131606. [Google Scholar] [CrossRef]
- Mondejar-Lopez, M.; Castillo, R.; López Jiménez, A.J.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. Polysaccharide Film Containing Cinnamaldehyde-Chitosan Nanoparticles, a New Eco-Packaging Material Effective in Meat Preservation. Food Chem. 2024, 437, 137710. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Kaur, G.; Shah, I.A.; Majid, D.; Makroo, H.A.; Dar, B.N. Sustainable Gelatin-Based Packaging with Nanoemulsified Chilli Seed Oil for Enhancing Poultry Meat Preservation: An Eco-Friendly Approach. Food Chem. Adv. 2024, 5, 100761. [Google Scholar] [CrossRef]
- Sasidharan, S.; Tey, L.-H.; Djearamane, S.; Mahmud Ab Rashid, N.K.; Rajeshwari, P.A.; Rajendran, V.; Syed, A.; Wong, L.S.; Santhanakrishnan, V.K.; Asirvadam, V.S.; et al. Innovative Use of Chitosan/ZnO NPs Bio-Nanocomposites for Sustainable Antimicrobial Food Packaging of Poultry Meat. Food Packag. Shelf Life 2024, 43, 101298. [Google Scholar] [CrossRef]
- Sul, Y.; Khan, A.; Rhim, J.-W. Effects of Coffee Bean Types on the Characteristics of Carbon Dots and Their Use for Manufacturing Cellulose Nanofibers-Based Films for Active Packaging of Meat. Food Packag. Shelf Life 2024, 43, 101282. [Google Scholar] [CrossRef]
- Active Packaging Market Size, Share, and Trends 2024 to 2034. Available online: https://www.precedenceresearch.com/active-packaging-market (accessed on 14 August 2024).
- Azeredo, H.M.C.; Correa, D.S. Smart choices: Mechanisms of intelligent food packaging. Curr. Res. Food Sci. 2021, 4, 932–936. [Google Scholar] [CrossRef]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent packaging for real-time monitoring of food quality: Current and future developments. Appl. Sci. 2021, 11, 3532. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Yildiz, Z.; Yildiz, P.; Strachowski, P.; Forough, M.; Esmaeili, Y.; Naebe, M.; Abdollahi, M. Advanced technologies in biodegradable packaging using intelligent sensing to fight food waste. Int. J. Biol. Macromol. 2024, 261, 129647. [Google Scholar] [CrossRef]
- Senapati, M.; Sahu, P.P. Meat quality assessment using Au patch electrode Ag-SnO2/SiO2/Si MIS capacitive gas sensor at room temperature. Food Chem. 2020, 324, 126893. [Google Scholar] [CrossRef]
- Bhatlawande, A.R.; Ghatge, P.U.; Shinde, G.U.; Anushree, R.K.; Patil, S.D. Unlocking the future of smart food packaging: Biosensors, IoT, and nanomaterials. Food Sci. Biotechnol. 2024, 33, 1075–1091. [Google Scholar] [CrossRef]
- Dalapati, R.; Hunter, M.; Zang, L. A dual fluorometric and colorimetric sulfide sensor based on coordinating self-assembled nanorods: Applicable for monitoring meat spoilage. Chemosensors 2022, 10, 500. [Google Scholar] [CrossRef]
- Ding, T.; Li, Y. Biogenic amines are important indices for characterizing the freshness and hygienic quality of aquatic products: A review. LWT 2024, 194, 115793. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Jain, P.; Kumar, L.; Singh, S.; Gaikwad, K.K. Catechu (Senegalia catechu) based oxygen scavenger for active food packaging: A sustainable alternative. Sustain. Chem. Pharm. 2024, 37, 101350. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Sun, X.; Wei, W.; Mu, X.; Ahmad, W.; Hassan, M.M.; Ouyang, Q.; Chen, Q. Fabricating a nano-bionic sensor for rapid detection of H2S during pork spoilage using Ru NPs modulated catalytic hydrogenation conversion. Meat Sci. 2021, 177, 108507. [Google Scholar] [CrossRef]
- Mansourbahmani, S.; Ghareyazie, B.; Zarinnia, V.; Kalatejari, S.; Mohammadi, R.S. Study on the efficiency of ethylene scavengers on the maintenance of postharvest quality of tomato fruit. Food Meas. 2018, 12, 691–701. [Google Scholar] [CrossRef]
- Taechutrakul, S.; Piroonpan, T.; Pasanphan, W. Active film strips to extend the shelf life of fruits: Multibranched PLA-gallic acid as an antioxidant/oxygen scavenger in a case study of bananas (Musa AAA group). J. Food Eng. 2024, 364, 111794. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Chen, H.; Bhandari, B. Freshness monitoring technology of fish products in intelligent packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 1279–1292. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Wilson, C.T.; Harte, J.; Almenar, E. Effects of Sachet Presence on Consumer Product Perception and Active Packaging Acceptability—A Study of Fresh-Cut Cantaloupe. LWT 2018, 92, 531–539. [Google Scholar] [CrossRef]
- Du, H.; Sun, X.; Chong, X.; Yang, M.; Zhu, Z.; Wen, Y. A Review on Smart Active Packaging Systems for Food Preservation: Applications and Future Trends. Trends Food Sci. Technol. 2023, 141, 104200. [Google Scholar] [CrossRef]

| Name | Structure | Refs. | |
|---|---|---|---|
| Caseins |  |  | [63] |
| α-casein | β-casein | ||
| Whey proteins |  |  | [64,65] |
| α-lactalbumin | β-lactoglobulin | ||
| Collagen and gelatin |  |  | [66,67] |
| collagen | denaturated gelatin | ||
| Chitin and chitosan |  | [68] | |
| Keratin |  | [69,70] | |
| Gluten |  | [71,72] | |
| Soy proteins | 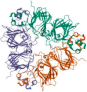 | [73,74] | |
| Starch |  | [75] | |
| Zein |  | [76] | |
| Cellulose |  | [77] | |
| Pectin |  | [78] | |
| Alginate | 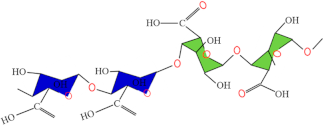 | [79,80] | |
| Pullulan | 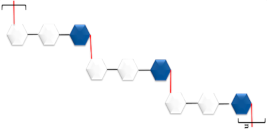 | [81] | |
| Kefiran | 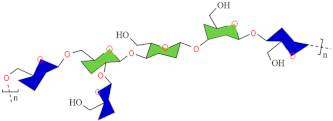 | [82] | |
| Name | Structure | Refs. | |
|---|---|---|---|
| PHAs |  | 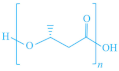 | [151] |
| PHA | P(HB) | ||
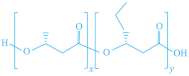 | 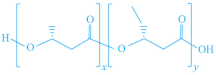 | ||
| P(HBH) | P(HBcoHV) | ||
| PLA | 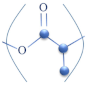 | [152] | |
| Biodegradable Biopolymers | Limitations | Solutions |
|---|---|---|
| Caseins | Extremely sensitive to moisture, which severely affects their mechanical characteristics [23] | Cross-linking treatment with divalent cations, which leads to a more stable structure [83] |
| Whey proteins | Poor tensile strength and moisture resistance due to high amounts of hydrophilic amino acids in the chain of milk proteins [57,83] | Incorporation into the matrix: glycerol, unmodified Na+-montmorillonite, other biopolymers (zein, sodium caseinate, nanocelluloses), EOs, or various methods of cross-linking [23,83,159,160,161] |
| Collagen | Poor wet mechanical properties due to its poor moisture resistance and low thermal stability [57,89,96] | Use of plasticizers (glycerol), suitable cross-linking treatments, blending with other biopolymers (chitosan), and the addition of active compounds [96,162,163,164,165,166] |
| Gelatin | Poor mechanical properties and strong sensitivity to moisture, tending to swell and dissolve when in contact with food with great humidity levels [57,88,102,105,106] | Cross-linking or combining with other biopolymers (carboxymethyl cellulose, chitosan, soy protein isolate, starch) [88,167,168] |
| Chitin | Insoluble in some common solvents and has poor biodegradability due to its high crystallinity and high content of acetamido groups [107] | Deacetylation under alkali conditions to produce chitosan [110,111,112] |
| Chitosan | Poor mechanical properties, barrier performance, and water resistance characteristics due to the presence of many hydrophilic groups in its structure [110,169] | Blending with other biopolymers [110,169] |
| Keratin | Hydrophobic compound, not suitable as a packaging material in its pure form [57,106] | Proteolytic cleavage by enzymes [61,115,116,119] |
| Gluten | Pronounced solubility in water, high water attraction, rigid structure, and opacity [23,43,122,170]. Additionally, human intolerance to gluten is one of the biggest disadvantages of gluten-based edible films [112,120] | Incorporation into the matrix: pectin, carboxymethyl cellulose, or other proteins [170] |
| Soy proteins | High water-solubility, poor mechanical properties, low tensile strength, low thermal stability, reduced transparency, and low heat resistance [15,23,57,126,128,171] | Combining with other biopolymers (chitosan, gelatin, nanocellulose, etc.), plasticizers (glycerin), lipids, and plant extracts [1,126,172,173,174] |
| Starch | Inferior water resistance and mechanical qualities [108,127,147] | Adding plasticizer (glycerol, sorbitol, sugars), blending with bioactive compounds and other biopolymers (gelatin, pectin, pullulan), and reinforcing with bacterial nanocellulose, metal-oxides, and nanoclay [28,59,108,140,175,176] |
| Zein | Poor mechanical and thermal qualities, and low water resistance, making it unsuitable for use as food packaging films in its pure form [43,57,128,129] | Blending with other biopolymers (chitosan, pullulan, gelatin, carrageenan, cellulose, alginate, PHAs, soy protein, whey protein), adding plasticizers (glycerol, polyethylene glycol, sorbitol), or incorporating NPs [43,120,128] |
| Cellulose | Sensitive to water, with reduced mechanical strength and limited barrier characteristics [131] | Incorporation of resins, wax, and reinforcing agents (clay, metal-based NPs, nanocellulose); coating with surfactants; blending with other biopolymers (gelatin, zein); and chemical modifications (acylation, esterification, grafting, and silylation) [55,131,177] |
| Pectin | Brittle and more hydrophilic, with poor mechanical properties [135,137] | Adding plasticizers (glycerol, sorbitol, sucrose, polyethylene glycol, mannitol), embedding pectin with other polysaccharides (agar, carrageenan, pullulan, chitosan), proteins (gelatin), or synthetic biopolymers (PLA) [135,137] |
| Alginate | Strong hydrophilicity, limited antimicrobial and antioxidant characteristics and UV-light barrier, and instability under heat treatment [139,151] | Blending with other biopolymers (chitin, chitosan, carboxymethyl cellulose, fish scale gelatin, pectin), embedding NPs embedding (nanosilver, montmorillonite, TiO2), incorporating plant extracts, yeasts, or bioactive compounds (carotenoids, vitamin C, phenolic substances), and chemical modifications (amidation, esterification, sulfation, oxidation, and reductive amination) [139,142,178,179] |
| Pullulan | High hydrophilicity, poor mechanical properties, and limited antioxidant and antibacterial potential [19,124,127,147] | Blending with other biopolymers (alginate, starch, chitosan, zein), embedding of organic/inorganic NPs (e.g., ZnO), or chemical modifications (esterification, oxidation, etherification, sulfation, and amination) [19,127,145,146,180,181,182] |
| Kefiran | Poor mechanical characteristics [148] | Combining with other biopolymers (carboxymethyl cellulose, starch, chitosan, whey proteins), adding plasticizers (glucose, sucrose, glycerol, lipids), or incorporating reinforcing agents (montmorillonite, nanocellulose, CuO, TiO2, ZnO) [148] |
| PHAs | Inferior thermal and mechanical stability, poor moisture and gas barrier properties, higher aroma permeability, and high cost [8,155,156] | Addition of NPs (nanocellulose, nanoclays, nanosilver, and metal-oxides in nanoforms) [8] |
| PLA | Brittleness, low gas and vapor barrier properties, low flexibility, thermal instability, and a slow biodegradation rate that can take up to 3–5 years [8,156] | Blending with other biopolymers: poly(butylene succinate), poly(butylene succinate-co-butylene adipate), poly(butylene adipate-co-butylene terephthalate), and PHAs [183] |
| Engineered Films or Coatings | Applications | Refs. |
|---|---|---|
| PLA/chitosan | Ready-to-eat deli turkey meat | [213] |
| Whey protein isolate/oregano/clove essential oil | Chicken breast fillets | [214] |
| Zein/lysozyme/EDTA | Ground beef patties | [215] |
| Chitosan/ZnO | Raw meat | [216] |
| Tapioca starch/grape pomace | Ready-to-eat chicken deli meat | [217] |
| Okra mucilage/ZnO | Chicken breast meat | [218] |
| Starch/gallic acid/chitosan/carvacrol | Ham product | [219] |
| Cellulose/ZnO/gelatin | Chicken fillets | [220] |
| Cellulose/wheyprotein/TiO2/rosemary essential oil | Lamb meat | [221] |
| Gelatin/chitosan/ZnO | Meat beef | [222] |
| Pullulan/chitosan/ZnO | Pork belly | [223] |
| Zein/tea tree essential oil/blueberry anthocyanin | Pork products | [224] |
| Gelatin/alizarin/oregano essential oil | Beef freshness | [225] |
| Collagen/delphinidin | Casings in the meat industry | [226] |
| Collagen/chitosan/gallic acid | Pork | [91] |
| Gelatin/alginate | Raw minced beef meat | [227] |
| Gelatin/pullulan/cinnamon essential oil | Meat | [228] |
| Chitosan/cinnamaldehyde | Handmade meat patties | [229] |
| Gelatin/chilli seed oil | Fresh chicken breast cubes | [230] |
| Chitosan/ZnO | Refrigerated poultry meat | [231] |
| Cellulose/carbon dots | Minced pork | [232] |
| Parameters | Brief Introduction | Detectors/Controllers |
|---|---|---|
| pH | During food storage, both aerobic and anaerobic microorganisms can proliferate, producing organic acids (lactic acid, acetic acid), which lower the pH of food. Additionally, CO2, a byproduct of microbial growth, can dissolve in food products, forming carbonic acid that further reduces the pH. | Synthetic pH-sensitive dyes (e.g., bromocresol green, methyl red) Natural pH-sensitive dyes (e.g., anthocyanins, betalains, carotenoids, chlorophyll, curcumin) embedded into biodegradable films (e.g., cellulose, chitin, chitosan, gelatin, and starch), which offer additional antibacterial and antioxidant benefits. |
| O2 | O2 in the package headspace can initiate undesirable chemical reactions in numerous foods, especially fresh and highly perishable foods. This leads to quality deterioration through color changes, off-flavors, microbial growth, and nutrient loss. | Smart technologies include O2 scavengers (to maintain low O2 levels inside the package), O2 luminescence-based indicators, and colorimetric redox sensors that display color changes to signal when O2 levels exceed safe limits. In high-moisture environments typical of food packaging, redox dyes may leach from the water-insoluble polymer matrix, raising health concerns. Alginate’s cation-binding properties have been used to mitigate dye leaching. |
| CO2 | High levels of CO2, produced during the respiration of fresh produce or through modified atmosphere packaging, can adversely affect food quality and packaging integrity. | Luminescent dyes (e.g., 8-hydroxypyrene-1,3,5-trisulfonic acid in polymeric films) offer high sensitivity but they do not come in contact with the foods, being unsuitable for consumer use. Safer alternatives include colorimetric indicators that detect pH changes caused by CO2 hydrolysis, although they offer lower sensitivity (e.g., L-lysine, poly L-lysine, anthocyanins). |
| N2 | Animal-derived foods are highly susceptible to the growth of pathogenic microorganisms, which can lead to the formation of biogenic amines, posing a potential safety risk to human health. The total volatile basic nitrogen level (TVB-N) is commonly used as an indicator of spoilage and the production of harmful biogenic amines. | A rapid wireless sensor based on a hydrogel-coated pH-electrode, sensitive to volatile amine levels, provides a highly sensitive response to spoilage. However, integration into packaging systems remains challenging. The sensor changes color or sends an alert when amines are detected. |
| C2H4 | Ethylene, a volatile plant hormone, released by fruits and vegetables during ripening, can accelerate the ripening process in both climacteric and non-climacteric produce. This leads to reduced shelf life and affects post-harvest storage and marketing. | Smart technologies incorporate C2H4 scavengers (to maintain low C2H4 levels inside the package) and C2H4 nanotechnology sensors, which can alert consumers to the ripening stage of fruits and vegetables, facilitating timely consumption. |
| H2S | Hydrogen sulfide (H2S), a volatile produced during enzymatic hydrolysis of sulfur-containing amino acids (e.g., cysteine, homocysteine, and methionine), is a reliable marker for assessing meat freshness. | Colorimetric sensors based on gellan gum-capped silver nanoparticles Nano-bionic sensor using ruthenium nanoparticles Chemical sensor based on supramolecular nanorods synthesized via copper ions |
| Humidity | Excessive humidity in packaged foods can promote bacterial and fungal growth, degrading nutritional and sensory qualities. Conversely, low humidity levels may cause food dehydration and reduce shelf life. | Wireless humidity sensors, consisting of a planar inductor and capacitor on a paper substrate, can be easily integrated into packaging. However, substrate moisture absorption can alter capacitance. Other options include RFID-coupled humidity sensors and photonic crystal-based humidity sensors. |
| Temperature | Temperature fluctuations can significantly affect food stability, particularly in refrigerated and frozen products. Temperature abuse can degrade food texture and promote the growth of psychrotrophic bacteria. | Devices in direct contact with food, such as thermochromic inks or sensors, alert consumers and supply chain stakeholders when products are exposed to unfavorable temperatures, helping prevent the sale or consumption of spoiled items. Smart packaging that combines temperature and humidity data with the expected shelf life of the product can more accurately predict expiration dates. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, M.; Bichescu, C.I.; Crețu, C.-M.; Dragomir, M.; Ivan, A.S.; Podaru, G.M.; Stoica, D.; Stuparu-Crețu, M. Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods 2024, 13, 3027. https://doi.org/10.3390/foods13193027
Stoica M, Bichescu CI, Crețu C-M, Dragomir M, Ivan AS, Podaru GM, Stoica D, Stuparu-Crețu M. Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods. 2024; 13(19):3027. https://doi.org/10.3390/foods13193027
Chicago/Turabian StyleStoica, Maricica, Cezar Ionuț Bichescu, Carmen-Mihaela Crețu, Maricela Dragomir, Angela Stela Ivan, Geanina Marcela Podaru, Dimitrie Stoica, and Mariana Stuparu-Crețu. 2024. "Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging" Foods 13, no. 19: 3027. https://doi.org/10.3390/foods13193027
APA StyleStoica, M., Bichescu, C. I., Crețu, C.-M., Dragomir, M., Ivan, A. S., Podaru, G. M., Stoica, D., & Stuparu-Crețu, M. (2024). Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods, 13(19), 3027. https://doi.org/10.3390/foods13193027










