Physicochemical Characterization and Antioxidant Capacity of Açaí (Euterpe oleracea) in Ecuadorian Region
Abstract
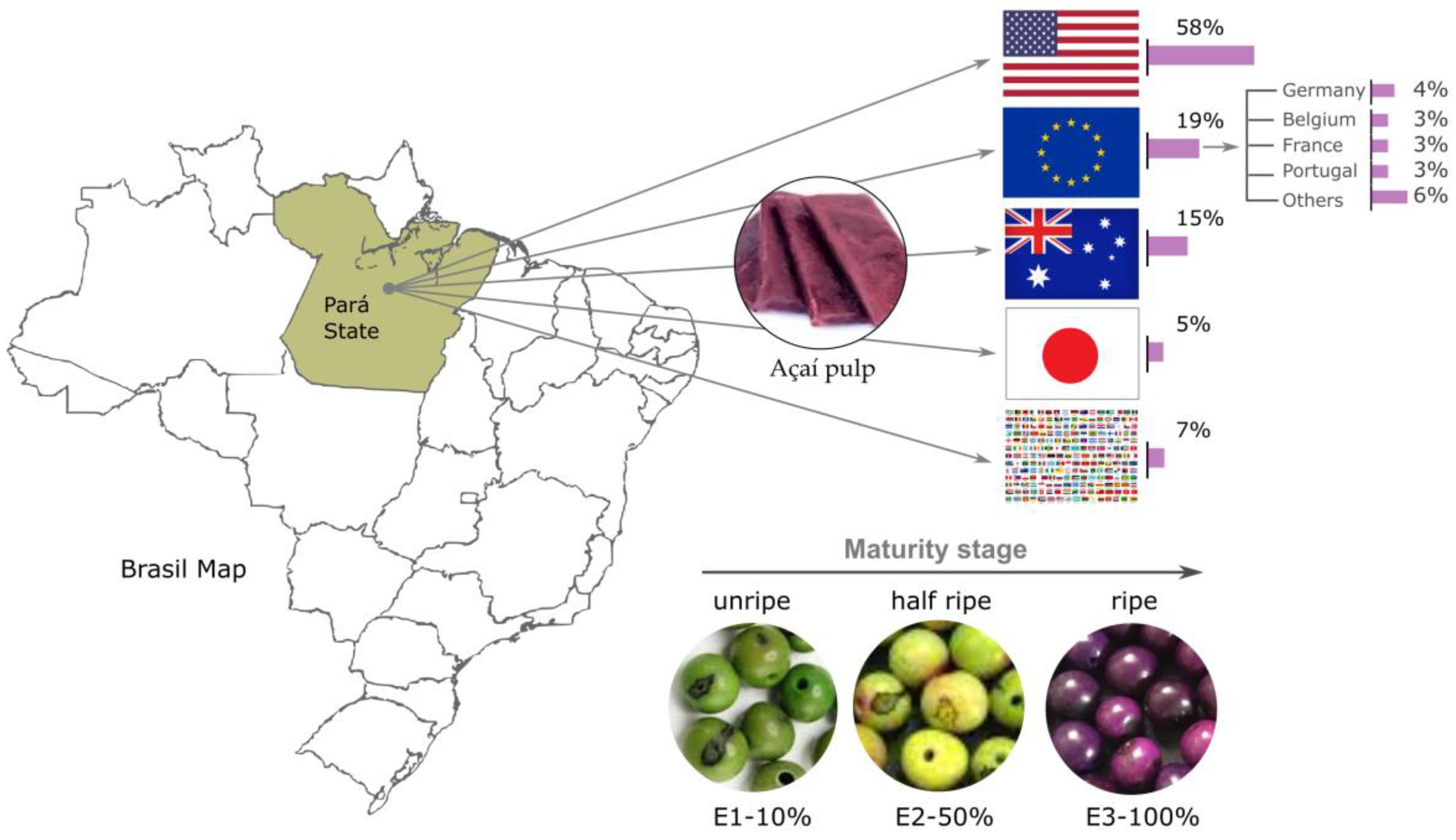
:1. Introduction
2. Materials and Methods
2.1. Material Plant
2.1.1. Chemical Reagents
2.1.2. Sample Preparation
2.2. Methodologies
2.2.1. Physicochemical Analysis
Determination of Total Soluble Solids (TSSs)
Titratable Acidity (TA) Determination
Maturity Index (MI)
2.3. Nutritional Quality Analysis
2.3.1. Ashes
2.3.2. Humidity
2.3.3. Protein
2.3.4. Fat
2.3.5. Total Fiber
2.4. Antioxidant Compound Extraction Process
2.4.1. Determination of Total Polyphenols
2.4.2. Determination of Total Flavonoids
2.4.3. Determination of Total Anthocyanins
2.5. Analysis of Antioxidant Capacity
2.5.1. Determination of Antioxidant Capacity by the 2,2-Azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS•+) Decolorization Method
2.5.2. Determination of Antioxidant Capacity by the Ferric Reducing Antioxidant Power (FRAP) Method
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of the Species Euterpe oleracea (Ecuador)
Soluble Solids (TSSs), Titratable Acidity (AT), and Maturity Index (MI)
3.2. Nutritional Quality
3.3. Quantification of Antioxidant Compounds
3.3.1. Total Polyphenols
3.3.2. Total Flavonoids (FT)
3.3.3. Total Anthocyanins (ACT)
3.4. Evaluation of Antioxidant Activity by the ABTS Method
3.5. Evaluation of Antioxidant Activity by the FRAP Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, J.; Matta, F.V.; Grace, M.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Phenolic content, anti-inflammatory properties, and dermal wound repair properties of industrially processed and non-processed acai from the Brazilian Amazon. Food Funct. 2020, 11, 4903–4914. [Google Scholar] [CrossRef] [PubMed]
- Rojano, B.A.; Vahos, I.C.Z.; Arbeláez, A.F.A.; Martínez, A.J.M.; Correa, F.B.C.; Carvajal, L.G. Polifenoles y Actividad Antioxidante del Fruto Liofilizado de Palma Naidi (Açai Colombiano) (Euterpe oleracea Mart). Rev. Fac. Nac. Agron. Medellín 2011, 64, 6213–6220. [Google Scholar]
- Paniagua-Zambrana, N.; Bussmann, R.W.; Macía, M.J. The socioeconomic context of the use of Euterpe precatoria Mart. and E. oleracea Mart in Bolivia and Peru. J. Ethnobiol. Ethnomed. 2017, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, R.; Aponte, R.; Lares, M. Bioactive Compounds of Acai (Euterpe oleracea) and the Effect of their Consumption on Oxidative Stress Markers. J. Nutr. Ther. 2021, 10, 1–9. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Andrade, N.P.; Monteros-Altamirano, A.; Tapia, C.; Lima, L.; Burbano, A.; Rivadeneira, J.; Avalos, N. Superfoods in the Production Systems of the Northern Amazon of Ecuador: A Case Study. February 2023. Available online: https://www.sidalc.net/search/Record/dig-iniap-41000-6013/Description (accessed on 12 August 2024).
- Silveira, J.T.D.; Rosa, A.P.C.D.; Morais, M.G.D.; Victoria, F.N.; Costa, J.A.V. An integrative review of Açaí (Euterpe oleracea and Euterpe precatoria): Traditional uses, phytochemical composition, market trends, and emerging applications. Food Res. Int. 2023, 173, 113304. [Google Scholar] [CrossRef]
- Silva, D.F.; da Silva, M.A.C.N.; Rodrigues, G.M.; Vidal, F.C.B.; Barbosa, M.D.C.L.; Brito, L.M.O.; Bezerra, G.F.d.B.; Filho, W.E.M.; Borges, K.R.A.; Rosa, I.G.; et al. Açaí (Euterpe oleracea Mart) Consumption and Prevention of Chronic Diseases: Is There an Association? A Preliminary Study. Sci. World J. 2020, 2020, 5782485. [Google Scholar] [CrossRef]
- Alves, L.M.S.; Oliveira, J.B.d.S.; Santana, C.L.V.; Dias, S.V.; Lobato, P.E.P.; Veiga, M.F.; Ramos, C.D.; Nogueira, V.M. The economic importance of Euterpe oleracea Mart. (açaí), for the Arióca Pruanã/Oeiras do Pará extraction reserve. Rev. Gestao Secr. 2024, 15, e3508. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- Cruz, J.E. Estudos em Agronegócio: Participação Brasileira nas Cadeias Produtivas no Brasil; VL 5; Editora Kelps: Goiânia, GO, USA, 2021. [Google Scholar]
- Garcia-Vallejo, M.C.; Poveda-Giraldo, J.A.; Alzate, C.A.C. Valorization Alternatives of Tropical Forest Fruits Based on the Açai (Euterpe oleracea) Processing in Small Communities. Foods 2023, 12, 2229. [Google Scholar] [CrossRef]
- Dias KK, B.; de Jesus, G.A.; da Costa AA, F.; Costa, F.F.; da Rocha Filho, G.N.; Oliveira, R.J.; Noronha, R.C.R.; Nascimento, L.A.S.D. Biological activities from açaí (Euterpe spp. Mart.) seeds and their pharmacological aspects: A systematic review and meta-analysis. Pharma Nutr. 2024, 29, 100405. [Google Scholar] [CrossRef]
- Da Silveira, T.F.F.; De Souza, T.C.L.; Carvalho, A.V.; Ribeiro, A.B.; Kuhnle, G.G.C.; Godoy, H.T. White açaí juice (Euterpe oleracea): Phenolic composition by LC-ESI-MS/MS, antioxidant capacity and inhibition effect on the formation of colorectal cancer related compounds. J. Funct. Foods 2017, 36, 215–223. [Google Scholar] [CrossRef]
- Martins, G.R.; Mattos, M.M.G.; Nascimento, F.M.; Brum, F.L.; Mohana-Borges, R.; Figueiredo, N.G.; Neto, D.F.M.; Domont, G.B.; Nogueira, F.C.S.; Campos, F.d.A.d.P.; et al. Phenolic Profile and Antioxidant Properties in Extracts of Developing Açaí (Euterpe oleracea Mart). Seeds. J. Agric. Food Chem. 2022, 70, 16218–16228. [Google Scholar] [CrossRef]
- Alessandra-Perini, J.; Perini, J.A.; Rodrigues-Baptista, K.C.; de Moura, R.S.; Junior, A.P.; dos Santos, T.A.; Souza, P.J.C.; Nasciutti, L.E.; Machado, D.E. Euterpe oleracea extract inhibits tumorigenesis effect of the chemical carcinogen DMBA in breast experimental cancer. BMC Complement. Altern. Med. 2018, 18, 116. [Google Scholar] [CrossRef]
- Earling, M.; Beadle, T.; Niemeyer, E.D. Açai Berry (Euterpe oleracea) Dietary Supplements: Variations in Anthocyanin and Flavonoid Concentrations, Phenolic Contents, and Antioxidant Properties. Plant Foods Hum. Nutr. 2019, 74, 421–429. [Google Scholar] [CrossRef]
- Lumieres—Repositorio institucional Universidad de América: Evaluación Técnico-Financiera para la Producción del Aceite de açaí Partiendo de los Residuos del Proceso de Despulpado para Green and Inclusive Group SAS. Available online: https://repository.uamerica.edu.co/handle/20.500.11839/8003 (accessed on 12 August 2024).
- Toledo, M.V. Investigación Aplicada para el Estudio del Acaí como Cultivo Alternativo en Beneficio de las Comunidades Nativas de la Selva baja del Perú. Maestría’s Thesis, Universidad ESAN. Escuela de Administración de Negocios para Graduados, Repositorio Institucional Universidad ESAN, 2018. Available online: https://hdl.handle.net/20.500.12640/1412 (accessed on 12 August 2024).
- Quiroga, Y.M.C.; Gómez, M.S.H.; Lares, M. Componentes Bioactivos del Asai (Euterpe oleracea Mart. y Euterpe precatoria Mart.) y su efecto sobre la salud. Arch. Venez. Farmacol. Y Ter. 2017, 36, 58–66. [Google Scholar]
- Neida, S.; Elba, S. [Characterization of the acai or manaca (Euterpe oleracea Mart.): A fruit of the Amazon]. Arch. Latinoam. Nutr. 2007, 57, 94–98. Available online: https://pubmed.ncbi.nlm.nih.gov/17824205/ (accessed on 12 August 2024). [PubMed]
- De Almeida Magalhães TS, S.; de Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, Á.A. The use of Euterpe oleracea Mart. as a new perspective for disease treatment and prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Álvarez, J.A.; Ayuso, J.; Barbero, G.F. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 1: Pressurized Liquid Extraction. Agronomy 2020, 10, 183. [Google Scholar] [CrossRef]
- Araujo, N.M.P.; Berni, P.; Ramalho, L.; Toledo, N.; Porrelli, P.; de Toledo, A.A.; Junior, M.M. Potential of Bra-zilian berries in developing innovative, healthy, and sustainable food products. Sustain. Food Technol. 2024, 2, 506–530. [Google Scholar] [CrossRef]
- Ramos, S.L.F.; Lopes, M.T.G.; Lopes, R.; Dequigiovanni, G.; de Macêdo, J.L.V.; Sebbenn, A.M.; da Silva, E.B.; Garcia, J.N. Mating system analysis of Açaí-do-Amazonas (Euterpe precatoria Mart.) using molecular markers. Crop Breed. Appl. Biotechnol. 2019, 19, 126–130. [Google Scholar] [CrossRef]
- Instituto Interamericano de Cooperación para la Agricultura (IICA), I.I.; Mancomunidad del Norte del Ecuador (MNE), Quito-Ecuador Instituto Nacional de Investigaciones Agropecuarias (INIAP), Quito-EcuadorQ.; Ministerio de Agricultura y Ganadería (MAG), Quito-Ecuador. La agrobiodiversidad y Aspectos Nutricionales en Fincas Representativas del Proyecto “Sello de la Agricultura Familiar Campesina: Comercialización Asociativa e Inclusiva en la Frontera Norte del Ecuador”. Instituto Nacional de Investigaciones Agropecuarias (INIAP). 2022. Available online: https://repositorio.iica.int/handle/11324/21286 (accessed on 13 August 2024).
- Avila, M. Estudio de la oferta y demanda de mercado de asaí e innovación de productos y tecnología para su producción; BIF: Santa Cruz, Bolivia, 2023. [Google Scholar]
- Castro, M.X.B. Proyecto de factibilidad económico—Financiero para la fabricación y comercialización de productos cosméticos a base de la Baya Açaí. 2015. Available online: http://repositorio.ucsg.edu.ec/handle/3317/4877 (accessed on 13 August 2024).
- Fuentes, P.; Ponce-Fuentes, E.; Muñoz-Murillo, P.; García-Mendoza, J. Yogur tipo II con adición de pulpa de acaí (Euterpe oleracea Mart) edulcorado con Stevia rebaudiana. Rev. Digit. Novasinergia 2023, 6, 36–49. [Google Scholar] [CrossRef]
- Samaniego, I.; Brito, B.; Viera, W.; Cabrera, A.; Llerena, W.; Kannangara, T.; Vilcacundo, R.; Angós, I.; Carrillo, W. Influence of the Maturity Stage on the Phytochemical Composition and the Antioxidant Activity of Four Andean Blackberry Cultivars (Rubus glaucus Benth) from Ecuador. Plants 2020, 9, 1027. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 22nd ed.Volume 1, Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 1 February 2024).
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of Analytical Methods for Determining Anthocyanins in Blood Orange Juices. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Babu, D.; Gurumurthy, P.; Borra, S.K.; Cherian, K.M. Antioxidant and free radical scavenging activity of triphala determined by using different in vitro models. JMPR 2013, 7, 2898–2905. [Google Scholar] [CrossRef]
- Llerena, W.; Samaniego, I.; Angós, I.; Brito, B.; Ortiz, B.; Carrillo, W. Biocompounds Content Prediction in Ecuadorian Fruits Using a Mathematical Model. Foods 2019, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; McRae, K.B.; Hamilton, L.C. Relationship between surface color and other maturity indices in wild lowbush blueberries. Can. J. Plant Sci. 1995, 75, 485–490. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. Soluble solids, acidity, phenolic content and antioxidant capacity of fruits and berries cultivated in Serbia. Fruits 2016, 71, 239–248. [Google Scholar] [CrossRef]
- Vasconcelos, M.D.S.; Mota, E.F.; Gomes-Rochette, N.F.; Nunes-Pinheiro, D.C.S.; Nabavi, S.M.; De Melo, D.F. Açai or Brazilian Berry (Euterpe oleracea). In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–133. [Google Scholar] [CrossRef]
- de L. Yamaguchi, K.K. Caracterização de Substâncias Fenólicas de Resíduos de Frutos Amazônicos e Avaliação Para o Uso Biotecnológico. Ago. 2015. Available online: https://tede.ufam.edu.br/handle/tede/4807 (accessed on 13 August 2024).
- Arias-Giraldo, S.; Ceballos-Peñaloza, A.; Gutiérrez-Mosquera, L. estudio de composición centesimal y propiedades termofisicas para la pulpa del acai. Vitae Supl. 2016, 23, S140–S144. Available online: https://www.proquest.com/docview/1783660990?sourcetype=Scholarly%20Journals (accessed on 10 August 2024).
- Quantificação do teor de Antocianinas Totais da Polpa de açaí de Diferentes Populações de Açaizeiro. Portal Embrapa. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/387591/quantificacao-do-teor-de-antocianinas-totais-da-polpa-de-acai-de-diferentes-populacoes-de-acaizeiro (accessed on 13 August 2024).
- Montenegro-Gómez, S.; Rosales-Escarria, M. Fruto de naidi (Euterpe olerácea) y su perspectiva en la seguridad alimentaria colombiana. Entramado 2015, 11, 200–207. [Google Scholar] [CrossRef]
- Gordon, A.; Gil Cruz, A.P.; Cabral, L.M.C.; de Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; Mattietto, R.d.A.; Friedrich, M.; da Matta, V.M.; Marx, F. Chemical characterization and evaluation of antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Bichara, C.M.G.; Rogez, H. Açai (Euterpe oleracea Martius). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–26, 27e. [Google Scholar] [CrossRef]
- Quiñones Ruiz, C.E. Determinación de polifenoles totales, antocianinas y capacidad antioxidante del ungurahui (Oenocarpus bataua Mart.), sinamillo (Oenocarpus mapora H. Karst.) y huasai (Euterpe oleracea Mart.). 2019. Available online: https://hdl.handle.net/20.500.14292/1601 (accessed on 13 August 2024).
- Correales Guevara, J.D. Revisión bibliográfica de los polifenoles del Asaí y su estabilidad química en los procesos de extracción de pulpa realizado en Colombia. 2022. Available online: https://repository.udca.edu.co/handle/11158/5004 (accessed on 13 August 2024).
- Caracterización del acai o manaca (Euterpe olerácea Mart.): Un fruto del Amazonas. Available online: http://www.alanrevista.org/ediciones/ediciones/2007/1/art-13/ (accessed on 13 August 2024).
- Lisboa, C.R.; Oliveira, M.D.S.P.D.; Chisté, R.C.; Carvalho, A.V. Compostos bioativos e potencial antioxidante de diferentes acessos de Euterpe oleracea e Euterpe precatoria do banco ativo de germoplasma de açaí. Res. Soc. Dev. 2022, 11, e428111234824. [Google Scholar] [CrossRef]
- Teixeira, G.H.D.A.; Lopes, V.G.; Júnior, L.C.C.; Pessoa, J.D.C. Total Anthocyanin Content in Intact Açaí (Euterpe oleracea Mart.) and Juçara (Euterpe edulis Mart.) Fruit Predicted by Near Infrared Spectroscopy. HortScience 2015, 50, 1218–1223. [Google Scholar] [CrossRef]
- Agawa, S.; Sakakibara, H.; Iwata, R.; Shimoi, K.; Hergesheimer, A.; Kumazawa, S. Anthocyanins in Mesocarp/Epicarp and Endocarp of Fresh Acai (Euterpe oleracea Mart.) and their Antioxidant Activities and Bioavailability. Food Sci. Technol. Res. 2011, 17, 327–334. [Google Scholar] [CrossRef]
- Carvalho, A.V.; Da Silveira, T.F.F.; Mattietto, R.D.A.; De Oliveira, M.D.S.P.; Godoy, H.T. Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2017, 97, 1467–1474. [Google Scholar] [CrossRef]
- Fecka, I.; Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A. The effect of strawberry ripeness on the content of polyphenols, cinnamates, L-ascorbic and carboxylic acids. J. Food Compos. Anal. 2021, 95, 103669. [Google Scholar] [CrossRef]
- Kapoor, L.; Simkin, A.J.; Doss, C.G.P.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Dobros, N.; Zielińska, A.; Siudem, P.; Zawada, K.D.; Paradowska, K. Profile of Bioactive Components and Antioxidant Activity of Aronia melanocarpa Fruits at Various Stages of Their Growth, Using Chemometric Methods. Antioxidants 2024, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Bicudo, M.O.P.; Ribani, R.H.; Beta, T. Anthocyanins, Phenolic Acids and Antioxidant Properties of Juçara Fruits (Euterpe edulis M.) Along the On-tree Ripening Process. Plant Foods Hum. Nutr. 2014, 69, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Riedl, K.M.; Schwartz, S.J. Determination of Anthocyanins, Total Phenolic Content, and Antioxidant Activity in Andes Berry (Rubus glaucus Benth). J. Food Sci. 2009, 74, C227–C232. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.S.; Gomes, A.L.; da Silva, M.G.; Alves, A.B.; Agnol, W.H.D.; Ferrari, R.A.; Carvalho, P.R.N.; Pacheco, M.T.B. Antioxidant Capacity and Chemical Characterization of Açaí (Euterpe oleracea Mart.) Fruit Fractions. Food Sci. Technol. 2016, 4, 95–102. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Pérez-Jiménez, J.; Arranz, S.; Alves, R.E.; de Brito, E.S.; Oliveira, M.S.; Saura-Calixto, F. Açaí (Euterpe oleraceae) ‘BRS Pará’: A tropical fruit source of antioxidant dietary fiber and high antioxidant capacity oil. Food Res. Int. 2011, 44, 2100–2106. [Google Scholar] [CrossRef]
- Sánchez, L.C.A.; Real, C.P.V.; Pérez, Y.B. Variables determinantes de la madurez comercial en la mora de castilla (Rubus glaucus Benth). 2013. Available online: https://repository.ut.edu.co/entities/publication/be6784b2-8bf7-4dfb-ab7e-20dc553432a9 (accessed on 13 August 2024).
- Iwanami, H.; Moriya-Tanaka, Y.; Hanada, T.; Baba, T.; Sakamoto, D. Factors Explaining Variations in Soluble Solids Content of Apples during Ripening and Storage. Hort. J. 2024, 93, 135–142. [Google Scholar] [CrossRef]
- Rokaya, P.R.; Baral, D.R.; Gautam, D.M.; Shrestha, A.K.; Paudyal, K.P. Effect of Altitude and Maturity Stages on Quality Attributes of Mandarin (Citrus reticulata Blanco). Am. J. Plant Sci. 2016, 7, 958–966. [Google Scholar] [CrossRef]
- Aldhanhani, A.R.H.; Ahmed, Z.F.R.; Tzortzakis, N.; Singh, Z. Maturity stage at harvest influences antioxidant phytochemicals and antibacterial activity of jujube fruit (Ziziphus mauritiana Lamk and Ziziphus spina-christi L.). Ann. Agric. Sci. 2022, 67, 196–203. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, C.; Walker, R. The organic acids that are accumulated in the fles of fruits: Occurrence, metabolism and factors affecting their contents—A review. Rev. Chapingo. Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Fischer, G.; Parra-Coronado, A.; Balaguera-López, H.E. Altitude as a determinant of fruit quality with emphasis on the Andean tropics of Colombia. A review. Agron. Colomb. 2022, 40, 70. [Google Scholar] [CrossRef]
- Da Silva Campelo Borges, G.; Vieira, F.G.K.; Copetti, C.; Gonzaga, L.V.; Zambiazi, R.C.; Filho, J.M.; Fett, R. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Res. Int. 2011, 44, 2128–2133. [Google Scholar] [CrossRef]
- Criado-Navarro, I.; López-Bascón, M.A.; Priego-Capote, F. Evaluating the Variability in the Phenolic Concentration of Extra Virgin Olive Oil According to the Commission Regulation (EU) 432/2012 Health Claim. J. Agric. Food Chem. 2020, 68, 9070–9080. [Google Scholar] [CrossRef] [PubMed]
- Gündeşli, M.A.; Uğur, R.; Yaman, M. The Effects of Altitude on Fruit Characteristics, Nutrient Chemicals, and Biochemical Properties of Walnut Fruits (Juglans regia L.). Horticulturae 2023, 9, 1086. [Google Scholar] [CrossRef]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of ’Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Journal Home:: International Journal of Nutrition and Food Sciences:: Science Publishing Group. Available online: https://www.sciencepublishinggroup.com/journal/ijnfs (accessed on 13 August 2024).
- Millena, C.; Baloloy, K.A.; Doma, N.; Hernan, P. Effect of Maturity on Physicochemical and Fatty Acid Profile of Philippine Pili (Canarium ovatum). Engl. Philipp. J. Sci. 2022, 152, 159–171. [Google Scholar] [CrossRef]
- Goulao, L.; Oliveira, C. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Andrade, M.T.; Neto, D.F.M.; Nascimento, J.R.S.; Soares, E.L.; Coutinho, C.; Velásquez, E.; Domont, G.B.; Nogueira, F.C.S.; Campos, F.A.P. Proteome Dynamics of the Developing Açaí Berry Pericarp (Euterpe oleracea Mart.). J. Proteome Res. 2020, 19, 437–445. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent advances in natural polyphenol research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Elmastaş, M.; Demir, A.; Genç, N.; Dölek, Ü.; Güneş, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef]
- Morais, R.A.; Teixeira, G.L.; Ferreira, S.R.S.; Cifuentes, A.; Block, J.M. Nutritional Composition and Bioactive Compounds of Native Brazilian Fruits of the Arecaceae Family and Its Potential Applications for Health Promotion. Nutrients 2022, 14, 4009. [Google Scholar] [CrossRef]
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The influence of ripeness on the phenolic content, antioxidant and antimicrobial activities of pumpkins (Cucurbita moschata Duchesne). Molecules 2021, 26, 3623. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.R.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolic acids, vitamin C and antioxidant activity of pomegranate juice (cv. Wonderful). Sci. Hortic. 2014, 179, 36–45. [Google Scholar] [CrossRef]
- Attanayake, R.; Eeswaran, R.; Rajapaksha, R.; Weerakkody, P.; Bandaranayake, P.C.G. Biochemical Composition and Expression of Anthocyanin Biosynthetic Genes of a Yellow Peeled and Pinkish Ariled Pomegranate (Punica granatum L.) Cultivar are Differentially Regulated in Response to Agro-Climatic Conditions. J. Agric. Food Chem. 2018, 66, 8761–8771. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern chile. J. Soil. Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. IJMS 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, G.; Zhang, Q.; Wang, Y.; Dia, V.P.; Meng, X. Ripening affects the physicochemical properties, phytochemicals and antioxidant capacities of two blueberry cultivars. Postharvest Biol. Technol. 2020, 162, 111097. [Google Scholar] [CrossRef]
- Avula, B.; Katragunta, K.; Osman, A.G.; Ali, Z.; Adams, S.J.; Chittiboyina, A.G.; Khan, I.A. Advances in the Chemistry, Analysis and Adulteration of Anthocyanin Rich-Berries and Fruits: 2000–2022. Molecules 2023, 28, 560. [Google Scholar] [CrossRef] [PubMed]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Cardenas-Toro, F.P.; Osorio-Tobón, J.F.; Barbero, G.F.; Meireles, M.A.D.A. Obtaining anthocyanin-rich extracts from frozen açai (Euterpe oleracea Mart.) pulp using pressurized liquid extraction. Food Sci. Technol. 2017, 37, 48–54. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Solak, A.; Dyankova, S.; Doneva, M.; Pavlova, M. Edible pH sensitive polysaccharide-anthocyanin complex films for meat freshness monitoring. BIO Web Conf. 2023, 58, 01007. [Google Scholar] [CrossRef]
- Karaaslan, M.; Yılmaz, F.M.; Karaaslan, A.; Vardin, H. Synthesis and accumulation of anthocyanins in sour cherries during ripening in accordance with antioxidant capacity development and chalcone synthase expression. Eur. Food Res. Technol. 2016, 242, 189–198. [Google Scholar] [CrossRef]
- Abaza, L.; Youssef, N.B.; Manai, H.; Haddada, F.M.; Methenni, K.; Zarrouk, M. Chétoui olive leaf extracts: Influence of the solvent type on phenolics and antioxidant activities. Grasas Aceites 2011, 62, 96–104. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2018, 244, 705–719. [Google Scholar] [CrossRef]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Total Phenolic Content and Total Flavonoid Content in Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Odeh, I.; Bisher, A.; Abbadi, J.; Qabbajeh, M. Effect of Geographical Region and Harvesting Date on Antioxidant Activity, Phenolic and Flavonoid Content of Olive Leaves. J. Food Nutr. Res. 2014, 2, 925–930. [Google Scholar] [CrossRef]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Guevara-Terán, M.; Padilla-Arias, K.; Beltrán-Novoa, A.; González-Paramás, A.M.; Giampieri, F.; Battino, M.; Vásquez-Castillo, W.; Fernandez-Soto, P.; Tejera, E.; Alvarez-Suarez, J.M. Influence of Altitudes and Development Stages on the Chemical Composition, Antioxidant, and Antimicrobial Capacity of the Wild Andean Blueberry (Vaccinium floribundum Kunth). Molecules 2022, 27, 7525. [Google Scholar] [CrossRef]
- Prada-Muñoz, J.; Coy-Barrera, E. Targeted Anthocyanin Profiling of Fruits from Three Southern Highbush Blueberry Cultivars Propagated in Colombia. Molecules 2024, 29, 691. [Google Scholar] [CrossRef]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root Growth Adaptation to Climate Change in Crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef]
- Bodin, J.E.; Thorstensen, T.; Alsheikh, M.; Basic, D.; Edvardsen, R.B.; Dalen, K.T.; das Neves, C.G.; Duale, N.; Eklo, O.M.; Ergon, Å.G.; et al. Genome Editing in Food and Feed Production—Implications for Risk Assessment. Scientific Opinion of the Scientific Steering Committee of the Norwegian Scientific Committee for Food and Environment. 2021. 291p. Available online: https://ntnuopen.ntnu.no/ntnu-xmlui/handle/11250/2991423 (accessed on 14 August 2024).
- La Spada, P.; Dominguez, E.; Continella, A.; Heredia, A.; Gentile, A. Factors influencing fruit cracking: An environmental and agronomic perspective. Front. Plant Sci. 2024, 15, 1343452. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Sucumbíos (Gonzalo Pizarro) | Orellana (Loreto) |
|---|---|---|
| Temperature (mean and gradient) | 29.5–21.1 | 29.1–21.5 |
| Average annual rainfall (mm) | 3029.9–4816.4/average of 3923 | 2029.3–3029.9 (2529) |
| Relative humidity | 74–84 (average of 79) | 75–84 (79.5) |
| Hot days above 30 °C (4-year average) | 145 | 116 |
| Warm days around 20 °C (4-year average) | 358 | 331 |
| Average height (masl) | 1102 m https://goo.su/nKdh accessed on 18 August 2024 | 403 m https://goo.su/nX3b accessed on 18 August 2024 |
| Average evaporation (mm) | 400–850 (average of 625) | 400–1000 (average of 700) |
| City/Province | Maturity Stage | Soluble Solids (°Brix) | Titratable Acidity (% Citric Acid) | Maturity Index (TSS/TA) | |||
|---|---|---|---|---|---|---|---|
| Gonzalo Pizarro/Sucumbíos | Unripe | 0.45 ± 0.07 | Cf | 0.44 ± 0.003 | Ab | 1.01 ± 0.15 | Cd |
| Half ripe | 1.75 ± 0.07 | Bc | 0.44 ± 0.002 | Ab | 3.94 ± 0.17 | Bc | |
| Ripe | 3.6 ± 0.14 | Aa | 0.26 ± 0.011 | Bc | 13.97 ± 0.07 | Ab | |
| Loreto/Orellana | Unripe | 0.65 ± 0.07 | Ce | 0.54 ± 0.003 | Aa | 1.2 ± 0.12 | Cd |
| Half ripe | 1.45 ± 0.07 | Bd | 0.45 ± 0.004 | Bb | 3.24 ± 0.13 | Bc | |
| Ripe | 2.75 ± 0.07 | Ab | 0.22 ± 0.003 | Cd | 12.61 ± 0.50 | Aa | |
| City/Province | Maturity Stage | Ashes (g·100 g−1) | Protein (g·100 g−1) | Fat (g·100 g−1) | Fiber (g·100 g−1) | Total Carbohydrates (g·100 g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonzalo Pizarro/Sucumbíos | Unripe | 0.15 ± 0.01 | Ac | 4.38 ± 0.58 | Ab | 0.83 ± 0.04 | Ae | 46.46 ± 0.26 | Ae | 48.18 ± 0.53 | Ab |
| Half ripe | 0.18 ± 0.01 | Aa | 4.61 ± 0.19 | Aab | 9.38 ± 0.33 | Ab | 54.07 ± 0.03 | Aa | 30.92 ± 0.23 | Ae | |
| Ripe | 0.12 ± 0.01 | Ab | 4.95 ± 0.09 | Aab | 12.15 ± 0.11 | Aa | 51.55 ± 0.05 | Ac | 31.57 ± 0.01 | Ae | |
| Loreto/Orellana | Unripe | 0.14 ± 0.01 | Bbc | 4.43 ± 0.17 | Bab | 1.27 ± 0.04 | Be | 40.51 ± 0.47 | Bf | 53.30 ± 0.27 | Ba |
| Half ripe | 0.15 ± 0.01 | Bc | 4.78 ± 0.27 | Bab | 4.03 ± 0.10 | Bd | 47.51 ± 0.24 | Bd | 42.95 ± 0.57 | Bc | |
| Ripe | 0.13 ± 0.01 | Bbc | 5.36 ± 0.55 | Ba | 8.74 ± 0.18 | Bc | 53.10 ± 0.15 | Bb | 33.59 ± 0.17 | Bd | |
| City/Province | Coating Color | Total Polyphenols | Total Flavonoids | Total Anthocyanins | |||
|---|---|---|---|---|---|---|---|
| (PT) | (FT) | (ACT) | |||||
| (mg Gallic Acid·g−1) | (mg (+)-Catechin·g−1) | (mg Cyanidin-3-O Glu·g−1) | |||||
| Gonzalo Pizarro/Sucumbíos | Unripe | 74.14 ± 3.40 | Ac | 42.32 ± 1.29 | Ab | 2.86 ± 0.25 | Ce |
| Half ripe | 45.87 ± 1.72 | Be | 36.47 ± 0.76 | Bbc | 22.25 ± 0.42 | Bc | |
| Ripe | 33.20 ± 2.18 | Cf | 33.03 ± 0.39 | Ccd | 90.16 ± 1.53 | Ab | |
| Loreto/Orellana | Unripe | 121.81 ± 2.55 | Aa | 60.14 ± 2.28 | Aa | 4.28 ± 0.78 | Ce |
| Half ripe | 91.45 ± 1.06 | Bb | 38.82 ± 4.12 | Bbc | 19.85 ± 0.33 | Bd | |
| Ripe | 51.50 ± 2.17 | Cd | 28.84 ± 3.19 | Cd | 99.59 ± 0.65 | Aa |
| City/Province | Coating Color | Antioxidant Activity | Antioxidant Activity | ||
|---|---|---|---|---|---|
| (FRAP) | (ABTS) | ||||
| (µmol trolox·g−1) | (µmol trolox·g−1) | ||||
| Gonzalo Pizarro/Sucumbíos | Unripe | 880.58 ± 19.69 | Ab | 1336.96 ± 89.25 | Ab |
| Half ripe | 544.10 ± 7.32 | Bc | 835.76 ± 6.96 | Bd | |
| Ripe | 315.43 ± 4.96 | Ce | 402.41 ± 10.38 | Ce | |
| Loreto/Orellana | Unripe | 1033.87 ± 19.98 | Aa | 1474.97 ± 35.54 | Aa |
| Half ripe | 881.42 ± 10.26 | Bb | 1048.63 ± 23.40 | Bc | |
| Ripe | 430.94 ± 9.23 | Cd | 463.22 ± 24.92 | Ce |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flor-Unda, O.; Guanochanga, F.; Samaniego, I.; Arias, V.; Ortiz, B.; Rosales, C.; Palacios-Cabrera, H. Physicochemical Characterization and Antioxidant Capacity of Açaí (Euterpe oleracea) in Ecuadorian Region. Foods 2024, 13, 3046. https://doi.org/10.3390/foods13193046
Flor-Unda O, Guanochanga F, Samaniego I, Arias V, Ortiz B, Rosales C, Palacios-Cabrera H. Physicochemical Characterization and Antioxidant Capacity of Açaí (Euterpe oleracea) in Ecuadorian Region. Foods. 2024; 13(19):3046. https://doi.org/10.3390/foods13193046
Chicago/Turabian StyleFlor-Unda, Omar, Fernanda Guanochanga, Iván Samaniego, Verónica Arias, Bladimir Ortiz, Carmen Rosales, and Hector Palacios-Cabrera. 2024. "Physicochemical Characterization and Antioxidant Capacity of Açaí (Euterpe oleracea) in Ecuadorian Region" Foods 13, no. 19: 3046. https://doi.org/10.3390/foods13193046
APA StyleFlor-Unda, O., Guanochanga, F., Samaniego, I., Arias, V., Ortiz, B., Rosales, C., & Palacios-Cabrera, H. (2024). Physicochemical Characterization and Antioxidant Capacity of Açaí (Euterpe oleracea) in Ecuadorian Region. Foods, 13(19), 3046. https://doi.org/10.3390/foods13193046






