Microplastics, Polycyclic Aromatic Hydrocarbons, and Heavy Metals in Milk: Analyses and Induced Health Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sampling and Sample Preparation
2.2.1. Isolation Procedure of Microplastics
2.2.2. Preparation of Milk Sample to Determine Polycyclic Aromatic Hydrocarbons by High-Performance Liquid Chromatography with Fluorescence Detection
2.2.3. Digestion of Milk Samples to Determine the Heavy Metals Concentration by Inductively Coupled Plasma Mass Spectrometry
2.3. Analytical Techniques
2.3.1. Optical Microscopy
2.3.2. Micro-Fourier Transform Infrared Spectroscopy
2.3.3. Scanning Electron Microscopy—Energy-Dispersive X-ray Spectroscopy
2.3.4. High-Performance Liquid Chromatography with Fluorescence Detection
2.3.5. Inductively Coupled Plasma Mass Spectrometry
2.4. Data Analysis
2.4.1. Pollution Load Index of Microplastics
2.4.2. Estimated Daily Intake for MPs
2.4.3. Estimated Daily Intake of Heavy Metals
2.4.4. Health Risk Index
2.4.5. Statistical Analysis
3. Results and Discussion
3.1. Isolation Procedure of Microplastics
3.2. Optical Microscopy
3.3. Micro-Fourier Transform Infrared Spectroscopy
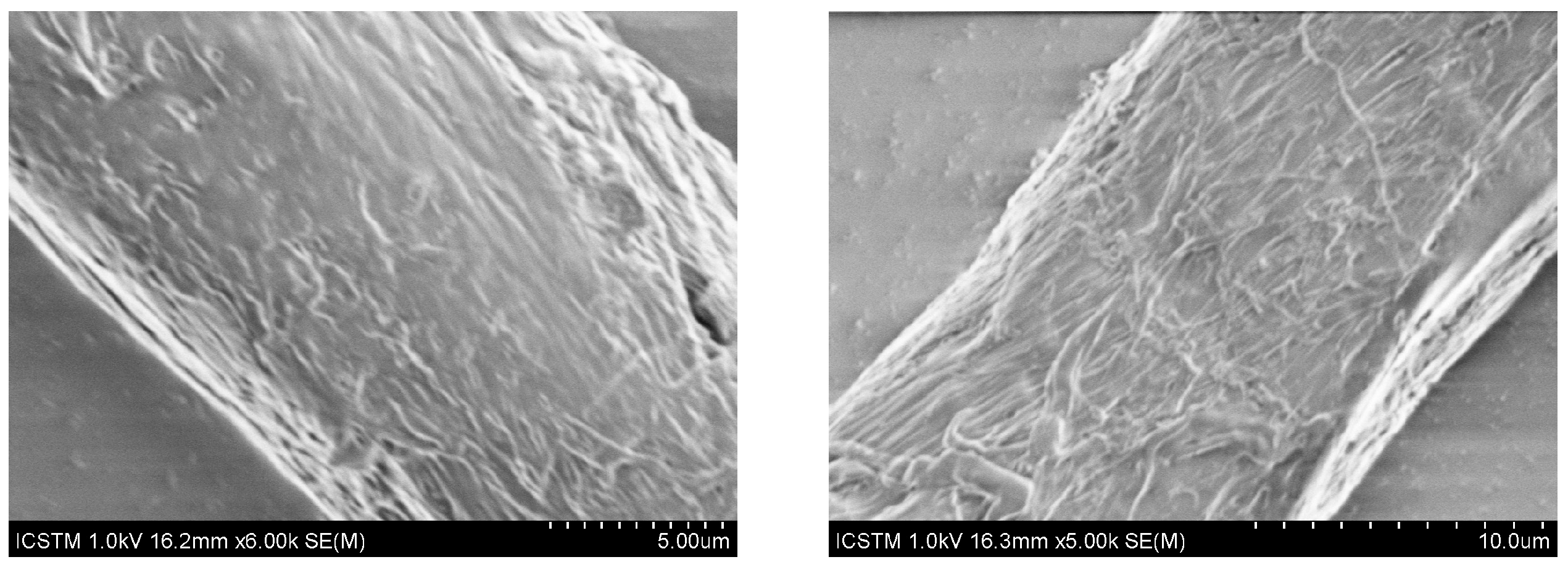
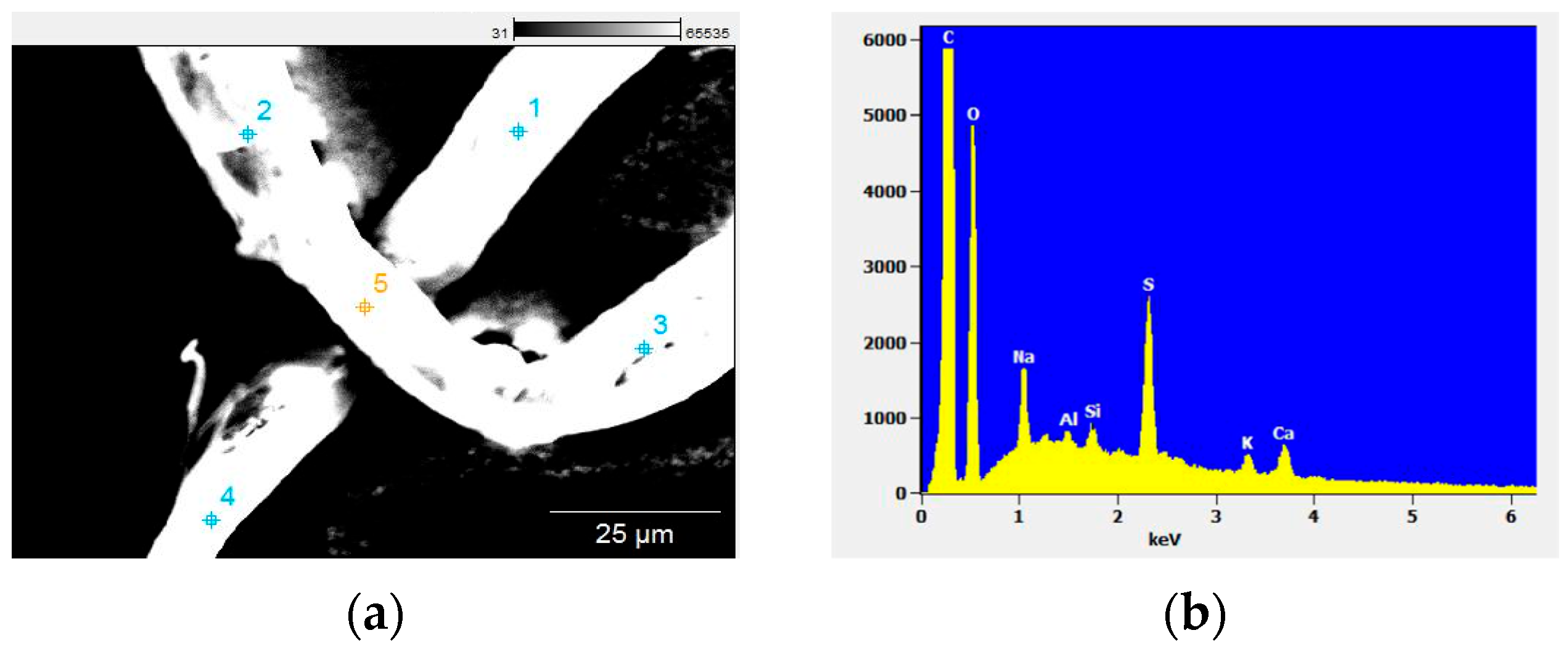
3.4. Scanning Electron Microscopy—Energy-Dispersive X-ray Spectroscopy
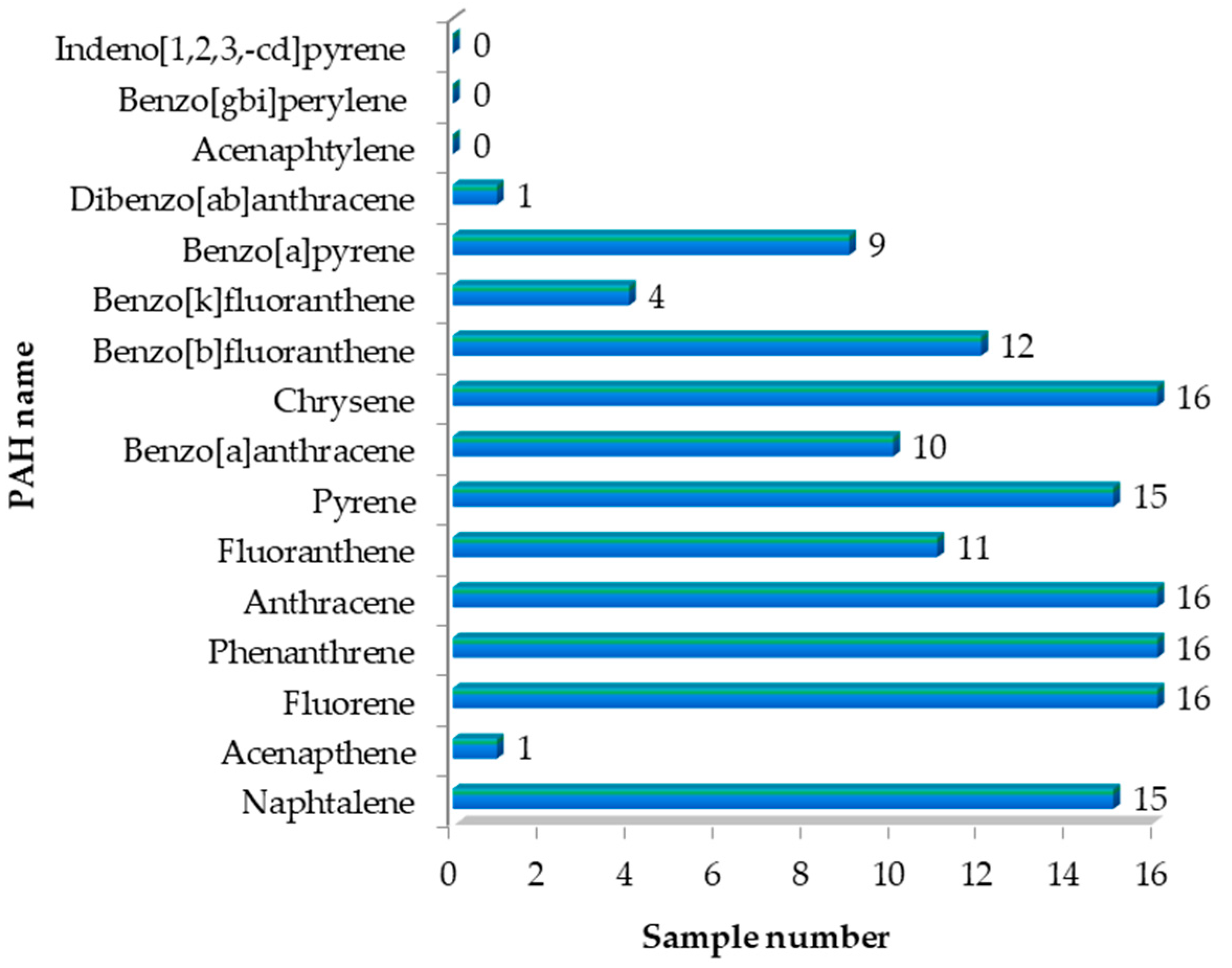
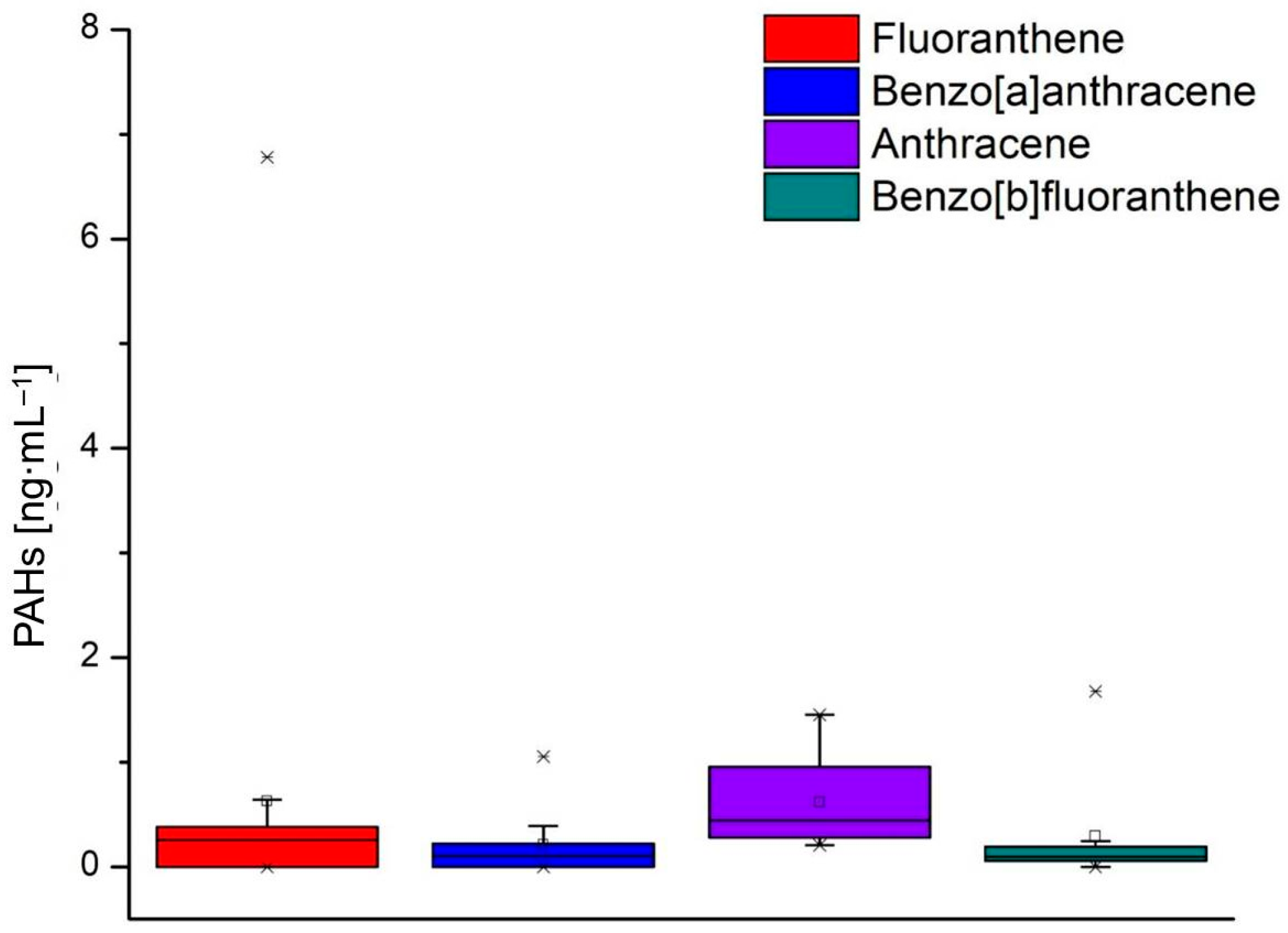
3.5. High-Performance Liquid Chromatography with Fluorescence Detection
3.6. Inductively Coupled Plasma Mass Spectrometry
3.7. Data Analysis
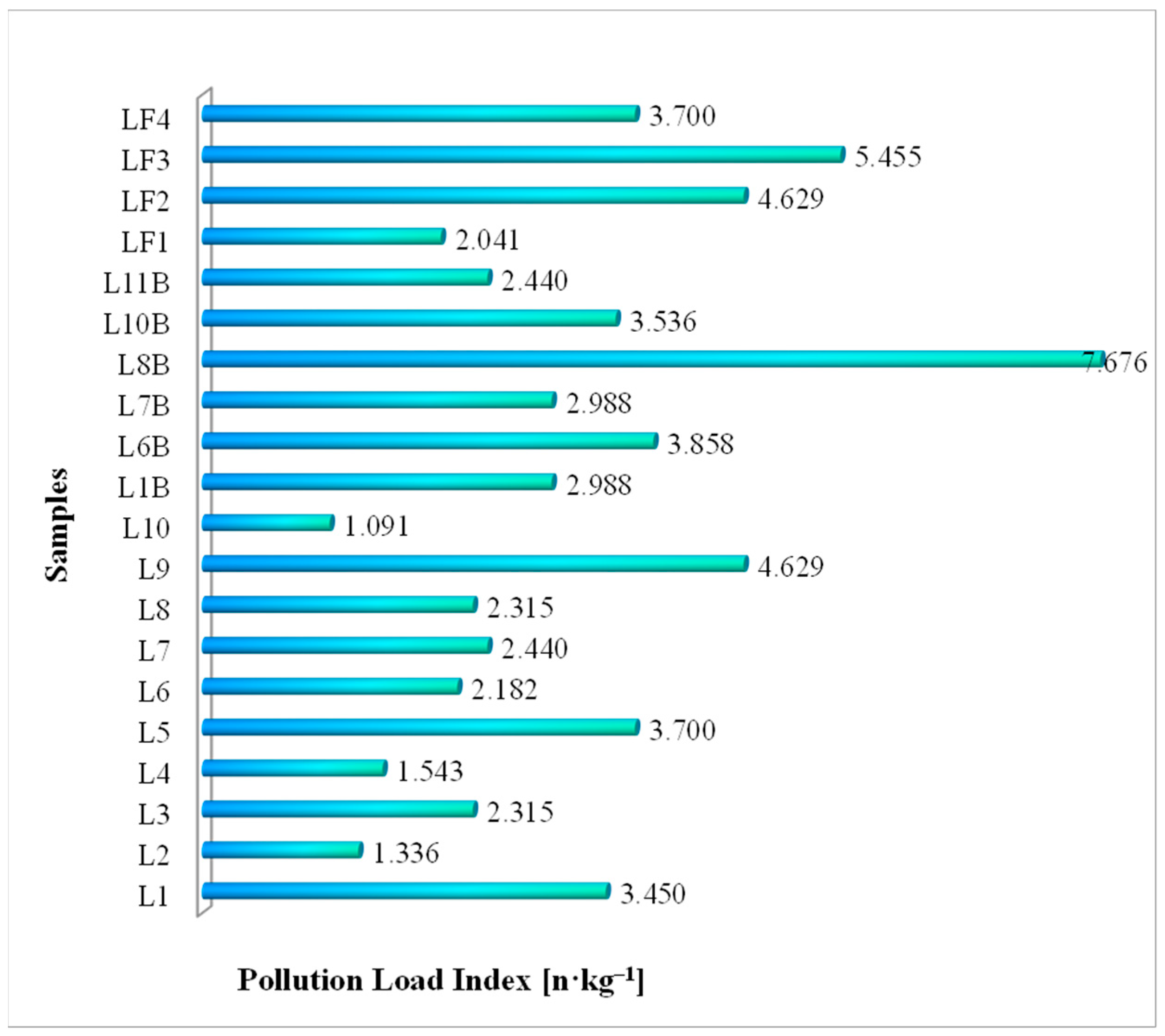
3.7.1. Pollution Load Index (PLI) of Microplastics
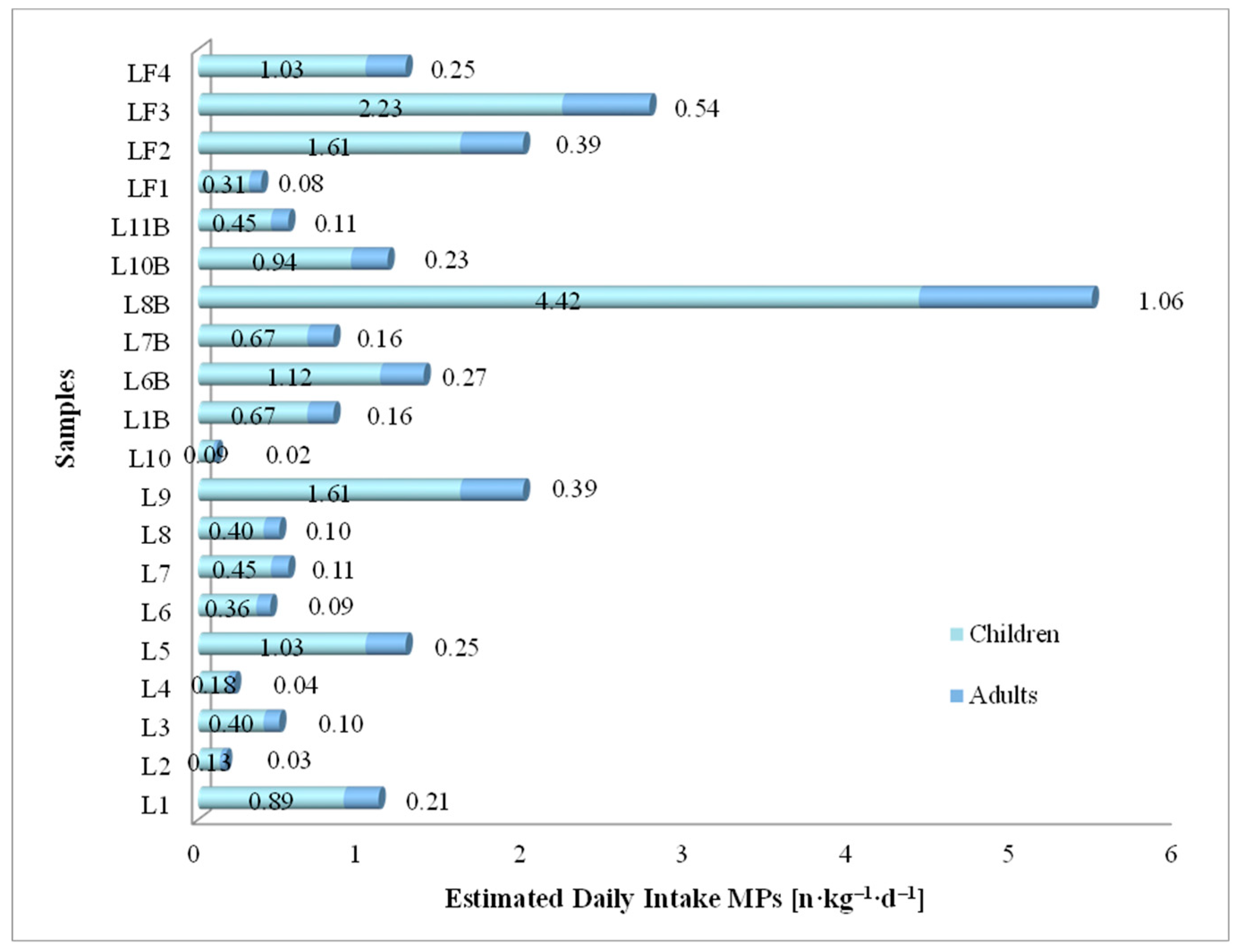
3.7.2. Estimated Daily Intake of MPs
3.7.3. Estimated Daily Intake of Metals
3.7.4. Health Risk Index
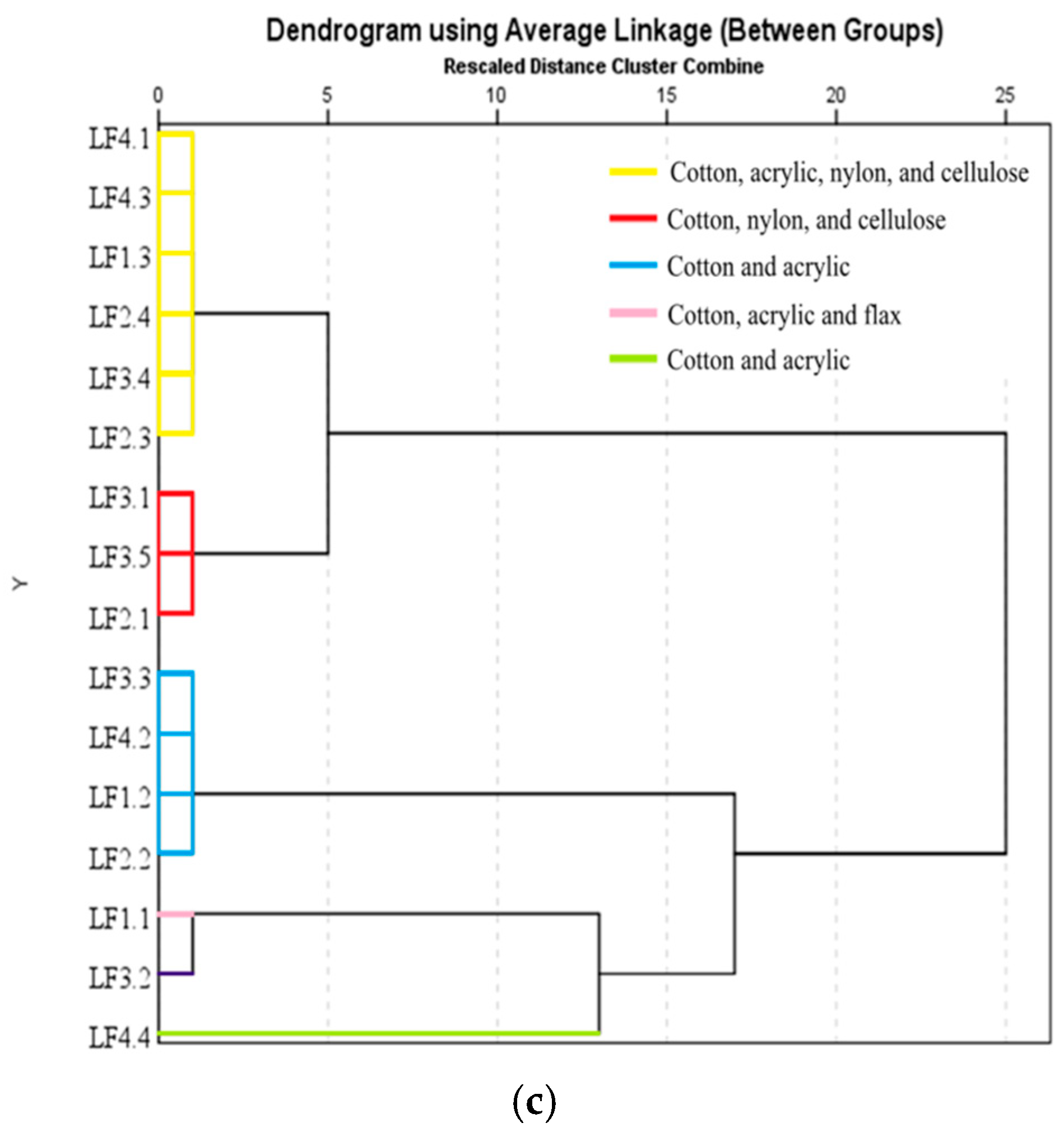
3.7.5. Hierarchical Cluster Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Time [min] | 10 | 30 | 60 | |
|---|---|---|---|---|
| Speed [rpm] | ||||
| 50 | VFG | VFG | VFG | |
| 100 | AH | AH | GH | |
| 150 | GH | GH | GH | |
Appendix B
| Time [min] | 20 | 30 | 60 | 90 | |
|---|---|---|---|---|---|
| Temperature [°C] | |||||
| ~21 | LD | LD | MD | MD | |
| 30 | GD | GD | GD | GD | |
| 45 | GD | GD | GD | GD | |
| 60 | GD | GD | GD | GD | |
| 75 | GD | GD | GD | GD | |
References
- Pathak, H.K.; Seth, C.S.; Chauhan, P.K.; Dubey, G.; Singh, G.; Jain, D.; Upadhyay, S.K.; Dwivedi, P.; Khoo, K.S. Recent advancement of nano-biochar for the remediation of heavy metals and emerging contaminants: Mechanism, adsorption kinetic model, plant growth and development. Environ. Res. 2024, 255, 119136. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.; Fu, R.; Nie, F.; Zuo, W.; Tian, Y.; Zhang, J. Full-chain analysis on emerging contaminants in soil: Source, migration and remediation. Chemosphere 2024, 363, 142854. [Google Scholar] [CrossRef] [PubMed]
- Banica, A.L.; Radulescu, C.; Dulama, I.D.; Bucurica, I.A.; Stirbescu, R.M.; Stanescu, S.G. Microplastic debris in yogurt: Occurrence, characterization, and implications for human health. J. Sci. Arts 2024, 24, 233–248. [Google Scholar] [CrossRef]
- Bai, R.; Fan, R.; Xie, C.; Liu, Q.; Liu, Q.; Yan, C.; Cui, J.; He, W. Microplastics are overestimated due to poor quality control of reagents. J. Hazard. Mater. 2023, 459, 132068. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, S.; Shrivas, K.; Kant, T. Progress in Analytical Methods for Monitoring of Heavy Metals and Metalloid in Milk and Global Health Risk Assessment. J. Food Compos. Anal. 2024, 135, 106568. [Google Scholar] [CrossRef]
- Permigiani, I.S.; Vallejo, N.K.; Hasuoka, P.E.; Gil, R.A.; Romero, M.C. Arsenic speciation analysis in cow’s milk and plant-based imitation milks by HPLC-ICP-MS. J. Food Compos. Anal. 2024, 126, 105898. [Google Scholar] [CrossRef]
- Guo, X.; Ji, X.; Liu, Z.; Feng, Z.; Zhang, Z.; Du, S.; Li, X.; Ma, J.; Sun, Z. Complex impact of metals on the fate of disinfection by-products in drinking water pipelines: A systematic review. Water Res. 2024, 261, 121991. [Google Scholar] [CrossRef]
- Banica, A.L.; Bucur, R.M.; Dulama, I.D.; Bucurica, I.A.; Stirbescu, R.M.; Radulescu, C. Assessment of microplastics in personal care products by microscopic methods and vibrational spectroscopy. Sci. Study Res. Chem. Chemic. Eng. Biotechnol. Food Ind. 2023, 24, 155–171. [Google Scholar]
- Seetha, B.S.; Bhandi, M.M.; Shaikh, A.; Matta, S.; Mudiam, M.K.R.M. Natural hydrophobic deep eutectic solvent based dispersive liquid-liquid microextraction followed by liquid chromatography with tandem mass spectrometry analysis of multi-class metabolites of pesticides, phthalates, and polycyclic aromatic hydrocarbons in animal-originated foods. Microchem. J. 2024, 199, 110196. [Google Scholar]
- Yurtsever, M.; Cuvelek, M.A. Abundance of microplastics in the agro-industrial product beet sugar; food or plastifood. Process Saf. Environ. Prot. 2024, 188, 467–479. [Google Scholar] [CrossRef]
- Agus, B.A.P.; Rajentran, K.; Selamat, J.; Lestari, S.D.; Umar, N.B.; Hussain, N. Determination of 16 EPA PAHs in food using gas and liquid chromatography. J. Food Compos. Anal. 2023, 116, 105038. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 92: Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Radulescu, C.; Dulama, I.D.; Banica, A.L.; Bucurica, I.A.; Stirbescu, R.M.; Gorghiu, L.M. Fast Method of Isolating Microplastics from Milk, Yoghurt, Sour Cream and. Butter. Patent Application RO137927A0, 26 September 2023. [Google Scholar]
- Radulescu, C.; Dulama, I.D.; Banica, A.L.; Bucurica, I.A.; Stirbescu, R.M.; Gorghiu, L.M. Rapid Method for Isolation of Microplastics from Milk, Yogurt, Sour Cream, and. Butter. Patent Application No. PCT/RO2024/000010, 15 April 2024. [Google Scholar]
- ISO 14644-1:2015; Cleanrooms and Associated Controlled Environments—Part 1. Classification of air Cleanliness by Particle Concentration. Second Edition; International Organization for Standardization: Geneva, Switzerland, 2015.
- Girelli, A.M.; Sperati, D.; Tarola, A.M. Determination of polycyclic aromatic hydrocarbons in Italian milk by HPLC with fluorescence detection. Food Addit. Contam. Part A 2014, 31, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Altun, S.; Aydemir, M.E. Determination of some minerals and heavy metal levels in Urfa cheese and cow’s milk. Food Health 2021, 7, 185–193. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Mar. Res. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okffo, E.D.; Olasehinde, E.F. Microparticles and microplastics contamination in African table salts. Mar. Pollut. Bull. 2021, 164, 112006. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, S.; Pang, L.; Sun, C.; Chen, L.; Li, F. Potential risk of microplastics in processed foods: Preliminary risk assessment concerning polymer types, abundance, and human exposure of microplastics. Ecotoxicol. Environ. Saf. 2022, 247, 114260. [Google Scholar] [CrossRef]
- Khan, S.; Farooq, R.; Shahbaz, S.; Khan, M.A.; Sadique, M. Health risk assessment of heavy metals for population via consumption of vegetables. World Appl. Sci. J. 2009, 6, 1602–1606. [Google Scholar]
- Linkuku, A.S.; Obuseng, G. Health risk assessment of heavy metals via dietary intake of vegetables irrigated with treated wastewater around Gaborone, Botswana. In Proceedings of the International Conference on Plant, Marine and Environmental Sciences (PMES–2015), Kuala Lumpur, Malaysia, 1–2 January 2015. [Google Scholar]
- Pehoiu, G.; Murarescu, O.; Radulescu, C.; Dulama, I.D.; Teodorescu, S.; Stirbescu, R.M.; Bucurica, I.A.; Stanescu, S.G. Heavy metals accumulation and translocation in native plants grown on tailing dumps and human health risk. Plant Sci. 2020, 456, 405–424. [Google Scholar] [CrossRef]
- Atique Ullah, A.K.M.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Quraishi, S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef]
- Kohn, H.F.; Hubert, L.J. Hierarchical cluster analysis. In Wiley StatsRef: Statistics Reference Online (WSR); John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; pp. 1–13. [Google Scholar]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between Bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Perez-Guevara, F.; Elizalde-Martinez, I.; Shruti, V.C. Branded milks—Are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef] [PubMed]
- Basaran, B.; Ozcifci, Z.; Akcay, H.T.; Aytan, U. Microplastics in branded milk: Dietary exposure and risk assessment. J. Food Compos. Anal. 2023, 123, 105611. [Google Scholar] [CrossRef]
- Buyukunal, S.K.; Zipak, S.R.; Muratoglu, K. Microplastics in a Traditional Turkish Dairy Product: Ayran. Pol. J. Food Nutr. Sci. 2023, 73, 139–150. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in honey, beer, milk and refreshments in Ecuador as emerging contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Da Costa Filho, P.A.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; Panchaud, A.; et al. Detection and characterization of small-sized microplastics (≥ 5 μm) in milk products. Sci. Rep. 2021, 11, 24046. [Google Scholar] [CrossRef]
- Zarafu, I.; Matei, L.; Bleotu, C.; Ionita, P.; Tatibouët, A.; Paun, A.; Nicolau, I.; Hanganu, A.; Limban, C.; Nuta, D.C.; et al. Synthesis, Characterization, and Biologic Activity of New Acyl Hydrazides and 1,3,4-Oxadiazole Derivatives. Molecules 2020, 25, 3308. [Google Scholar] [CrossRef]
- Mecozzi, M.; Pietroletti, M.; Monakhova, Y.B. FTIR spectroscopy supported by statistical techniques for the structural characterisation of plastic debris in the marine environment: Application to monitoring studies. Mar. Poll. Bull. 2016, 106, 155–161. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR-FTIR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Poll. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Noda, I.; Dowrey, A.E.; Haynes, J.L.; Marcott, C. Group frequency assignments for major infrared bands observed in common synthetic polymers. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 395–406. [Google Scholar]
- Bonello, G.; Varrella, P.; Pane, L. First Evaluation of Microplastic Content in Benthic Filter-feeders of the Gulf of La Spezia (Ligurian Sea). J. Aquat. Food Prod. Technol. 2018, 27, 284–291. [Google Scholar] [CrossRef]
- Torres-Moreno, C.; Puente-DelaCruz, L.; Codling, G.; Villa, A.L.; Cabo, M.; Klanova, J.; Johnson-Restrepo, J. Polycyclic aromatic hydrocarbons (PAHs) in human breast milk from Colombia: Spatial occurrence, sources and probabilistic risk assessment. Environ. Res. 2022, 204, 111981. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar] [PubMed]
- Huber, L. Validation and Qualification in Analytical Laboratories, 2nd ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2007. [Google Scholar]
- Ali, M.; Xu, D.; Yang, X.; Hu, J. Microplastics and PAHs mixed contamination: An in-depth review on the sources, co-occurrence, and fate in marine ecosystems. Water Res. 2024, 257, 121622. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, S. Approach to an answer to “How dangerous microplastics are to the human body”: A systematic review of the quantification of MPs and simultaneously exposed chemicals. J. Hazard. Mater. 2023, 460, 132404. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, J.; Zuo, R.; Xu, Q.; Qian, Y.; An, L. Identification of microplastics in human placenta using laser direct infrared spectroscopy. Sci. Total Environ. 2023, 856, 159060. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’Avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
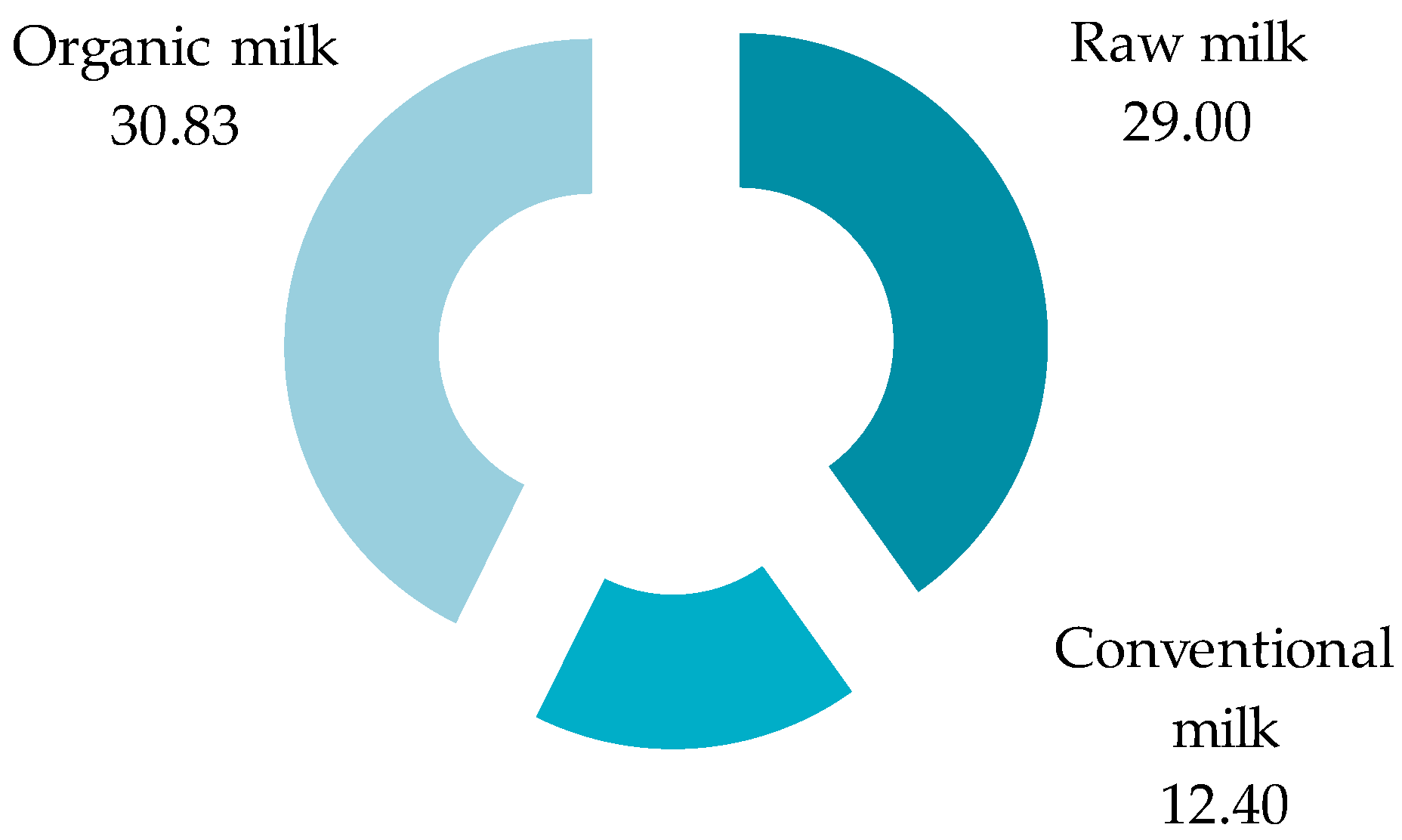




| Parameters/Step | 1 | 2 | 3 |
|---|---|---|---|
| Temperature [°C] | 145 | 170 | 190 |
| Pressure [bar] | 40 | 40 | 40 |
| Power [%] | 80 | 80 | 80 |
| Ramp [min] | 2 | 5 | 2 |
| Time [min] | 5 | 10 | 15 |
| PLI | Hazard Level |
|---|---|
| <10 | Very low hazard |
| 10–20 | Low hazard |
| 20–30 | Medium hazard |
| >30 | High hazard |
| - | Very high hazard |
| Ir [kg·d−1] | Ef [d·y−1] | Ed [y] | Bw [kg] | At [d] | |
|---|---|---|---|---|---|
| Adults | 0.750 | 365 | 70 | 70 | 25,550 |
| Children | 0.625 | 365 | 10 | 14 | 3650 |
| Cr | Mn | Ni | Cu | Zn | Sr | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|
| RfD | 1.500 | 0.140 | 0.020 | 0.040 | 0.300 | 0.600 | 0.001 | 0.035 |
| Sample Code | Color and Number of Microparticles | Total [Microparticles·L−1] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black | Blue | Red | Brown | Gray | Yellow | Golden | Green | Turquoise | ||
| Conventional milk | ||||||||||
| L1 | 12 | 8 | nd * | nd * | nd * | nd * | nd * | nd * | nd * | 20 |
| L2 | 2 | nd * | 1 | nd * | nd * | nd * | nd * | nd * | nd * | 3 |
| L3 | 8 | 1 | nd * | nd * | nd * | nd * | nd * | nd * | nd * | 9 |
| L4 | 4 | nd * | nd * | nd * | nd * | nd * | nd * | nd * | nd * | 4 |
| L5 | 15 | 3 | 1 | 4 | nd * | nd * | nd * | nd * | nd * | 23 |
| L6 | 8 | nd * | nd * | nd * | nd * | nd * | nd * | nd * | nd * | 8 |
| L7 | 10 | nd * | nd * | nd * | nd * | nd * | nd * | nd * | nd * | 10 |
| L8 | 7 | 2 | nd * | nd * | nd * | nd * | nd * | nd * | nd * | 9 |
| L9 | 24 | 6 | 2 | nd * | 1 | nd * | 2 | nd * | 1 | 36 |
| L10 | 1 | nd * | 1 | nd * | nd * | nd * | nd * | nd * | nd * | 2 |
| Organic milk | ||||||||||
| L1B | 7 | 6 | 2 | nd * | nd * | nd* | nd * | nd * | nd * | 15 |
| L6B | 18 | 4 | 2 | 1 | nd * | nd* | nd * | nd * | nd * | 25 |
| L7B | 9 | 3 | 2 | 1 | nd * | nd* | nd * | nd * | nd * | 15 |
| L8B | 71 | 16 | 10 | 1 | nd * | 1 | nd * | nd * | nd * | 99 |
| L10B | 18 | 2 | nd * | 1 | nd * | nd * | nd * | nd * | nd * | 21 |
| L11B | 7 | 3 | nd * | nd * | nd * | nd * | nd * | nd * | nd * | 10 |
| Raw milk | ||||||||||
| LF1 | 2 | 3 | nd * | nd * | 2 | nd * | nd * | nd * | nd * | 7 |
| LF2 | 21 | 5 | 4 | nd * | 5 | nd * | nd * | 1 | nd * | 36 |
| LF3 | 33 | 8 | 2 | 1 | 4 | 2 | nd * | nd * | nd * | 50 |
| LF4 | 18 | 3 | 2 | nd * | nd * | nd * | nd * | nd * | nd * | 23 |
| Cr | Mn | Ni | Cu | Zn | Sr | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|
| LOD | 2.766 | 5.337 | 4.041 | 11.073 | 9.741 | 2.488 | 2.242 | 10.954 |
| LOQ | 6.556 | 14.871 | 10.284 | 24.926 | 27.449 | 5.824 | 4.441 | 28.295 |
| Metals | Certified Value [mg/kg] | Determined Value [mg/kg] | Recovery [%] |
|---|---|---|---|
| Cr | 0.859 ± 0.066 | 0.861 ± 0.001 | 100.23 |
| Mn | 46.000 ± 1.600 | 44.577 ± 2.061 | 96.91 |
| Cu | 19.000 ± 0.380 | 18.937 ± 0.261 | 99.67 |
| Zn | 144.000 ± 3.200 | 144.226 ± 3.745 | 100.16 |
| Code | Cr | Mn | Ni | Cu | Zn | Sr | Cd | Pb |
|---|---|---|---|---|---|---|---|---|
| MAL 1 | nr * | nr * | nr * | nr * | nr * | nr * | nr * | 0.020 |
| MAL 2 | nr * | nr * | nr * | 0.500 | 5.000 | nr * | 0.010 | 0.100 |
| EDI | Sample Cod | Cr | Mn | Ni | Cu | Zn | Sr | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Children | L1 | 0.00324 | 0.00130 | 0.00060 | 0.00447 | 0.15703 | 0.01539 | nd | 0.00263 |
| L2 | 0.00274 | 0.00091 | 0.00052 | 0.00386 | 0.13703 | 0.01410 | nd | 0.00218 | |
| L3 | 0.00211 | 0.00125 | 0.00052 | 0.00443 | 0.15129 | 0.01662 | nd | 0.00070 | |
| L4 | 0.00258 | 0.00138 | 0.00095 | 0.00576 | 0.15964 | 0.01802 | 0.00025 | 0.00376 | |
| L5 | 0.00417 | 0.00114 | 0.00175 | 0.00603 | 0.18223 | 0.02067 | 0.00047 | 0.00386 | |
| L6 | 0.00216 | 0.00131 | 0.00334 | 0.00393 | 0.16221 | 0.01884 | nd | 0.02082 | |
| L7 | 0.00229 | 0.00104 | 0.00103 | 0.00361 | 0.16636 | 0.01896 | nd | 0.00838 | |
| L8 | 0.00441 | 0.00140 | 0.00233 | 0.00441 | 0.16749 | 0.01955 | nd | 0.00891 | |
| L9 | 0.00232 | 0.00105 | 0.00098 | 0.00390 | 0.17325 | 0.01368 | nd | 0.00733 | |
| L10 | 0.00222 | 0.00130 | 0.00437 | 0.00315 | 0.16507 | 0.02747 | nd | 0.01391 | |
| L1B | 0.00212 | 0.00129 | 0.00148 | 0.00319 | 0.14590 | 0.00923 | nd | 0.00233 | |
| L6B | 0.00444 | 0.00101 | 0.00121 | 0.00632 | 0.15935 | 0.02077 | nd | 0.00228 | |
| L7B | 0.00360 | 0.00250 | 0.00083 | 0.00511 | 0.16469 | 0.02205 | nd | 0.00218 | |
| L8B | 0.00242 | 0.00183 | 0.00151 | 0.00561 | 0.16978 | 0.01693 | nd | 0.00319 | |
| L10B | 0.00432 | 0.00119 | 0.00130 | 0.00574 | 0.15613 | 0.02372 | nd | 0.00287 | |
| L11B | 0.00439 | 0.00096 | 0.00127 | 0.00390 | 0.16617 | 0.01472 | nd | 0.00185 | |
| Adults | L1 | 0.00078 | 0.00031 | 0.00014 | 0.00107 | 0.03769 | 0.00369 | nd | 0.00063 |
| L2 | 0.00066 | 0.00022 | 0.00012 | 0.00093 | 0.03289 | 0.00339 | nd | 0.00052 | |
| L3 | 0.00051 | 0.00030 | 0.00012 | 0.00106 | 0.03631 | 0.00399 | nd | 0.00017 | |
| L4 | 0.00062 | 0.00033 | 0.00023 | 0.00138 | 0.03831 | 0.00432 | 0.00006 | 0.00090 | |
| L5 | 0.00100 | 0.00027 | 0.00042 | 0.00145 | 0.04374 | 0.00496 | 0.00011 | 0.00093 | |
| L6 | 0.00052 | 0.00031 | 0.00080 | 0.00094 | 0.03893 | 0.00452 | nd | 0.00500 | |
| L7 | 0.00055 | 0.00025 | 0.00025 | 0.00087 | 0.03993 | 0.00455 | nd | 0.00201 | |
| L8 | 0.00106 | 0.00034 | 0.00056 | 0.00106 | 0.04020 | 0.00469 | nd | 0.00214 | |
| L9 | 0.00056 | 0.00025 | 0.00023 | 0.00093 | 0.04158 | 0.00328 | nd | 0.00176 | |
| L10 | 0.00053 | 0.00031 | 0.00105 | 0.00076 | 0.03962 | 0.00659 | nd | 0.00334 | |
| L1B | 0.00051 | 0.00031 | 0.00035 | 0.00077 | 0.03502 | 0.00222 | nd | 0.00056 | |
| L6B | 0.00107 | 0.00024 | 0.00029 | 0.00152 | 0.03824 | 0.00498 | nd | 0.00055 | |
| L7B | 0.00086 | 0.00060 | 0.00020 | 0.00123 | 0.03952 | 0.00529 | nd | 0.00052 | |
| L8B | 0.00058 | 0.00044 | 0.00036 | 0.00135 | 0.04075 | 0.00406 | nd | 0.00077 | |
| L10B | 0.00104 | 0.00029 | 0.00031 | 0.00138 | 0.03747 | 0.00569 | nd | 0.00069 | |
| L11B | 0.00105 | 0.00023 | 0.00030 | 0.00094 | 0.03988 | 0.00353 | nd | 0.00044 |
| HRI | Sample Cod | Cr | Mn | Ni | Cu | Zn | Sr | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Children | L1 | 0.002 | 0.009 | 0.030 | 0.112 | 0.523 | 0.026 | nd | 0.075 |
| L2 | 0.002 | 0.007 | 0.026 | 0.097 | 0.457 | 0.024 | nd | 0.062 | |
| L3 | 0.001 | 0.009 | 0.026 | 0.111 | 0.504 | 0.028 | nd | 0.020 | |
| L4 | 0.002 | 0.010 | 0.047 | 0.144 | 0.532 | 0.030 | 0.253 | 0.107 | |
| L5 | 0.003 | 0.008 | 0.087 | 0.151 | 0.607 | 0.034 | 0.471 | 0.110 | |
| L6 | 0.001 | 0.009 | 0.167 | 0.098 | 0.541 | 0.031 | nd | 0.595 | |
| L7 | 0.002 | 0.007 | 0.051 | 0.090 | 0.555 | 0.032 | nd | 0.239 | |
| L8 | 0.003 | 0.010 | 0.116 | 0.110 | 0.558 | 0.033 | nd | 0.254 | |
| L9 | 0.002 | 0.007 | 0.049 | 0.097 | 0.577 | 0.023 | nd | 0.209 | |
| L10 | 0.001 | 0.009 | 0.218 | 0.079 | 0.550 | 0.046 | nd | 0.397 | |
| L1B | 0.001 | 0.009 | 0.074 | 0.080 | 0.486 | 0.015 | nd | 0.067 | |
| L6B | 0.003 | 0.007 | 0.060 | 0.158 | 0.531 | 0.035 | nd | 0.065 | |
| L7B | 0.002 | 0.018 | 0.041 | 0.128 | 0.549 | 0.037 | nd | 0.062 | |
| L8B | 0.002 | 0.013 | 0.076 | 0.140 | 0.566 | 0.028 | nd | 0.091 | |
| L10B | 0.003 | 0.009 | 0.065 | 0.143 | 0.520 | 0.040 | nd | 0.082 | |
| L11B | 0.003 | 0.007 | 0.064 | 0.097 | 0.554 | 0.025 | nd | 0.053 | |
| Adults | L1 | 0.001 | 0.002 | 0.007 | 0.027 | 0.126 | 0.006 | nd | 0.018 |
| L2 | 0.000 | 0.002 | 0.006 | 0.023 | 0.110 | 0.006 | nd | 0.015 | |
| L3 | 0.000 | 0.002 | 0.006 | 0.027 | 0.121 | 0.007 | nd | 0.005 | |
| L4 | 0.000 | 0.002 | 0.011 | 0.035 | 0.128 | 0.007 | 0.061 | 0.026 | |
| L5 | 0.001 | 0.002 | 0.021 | 0.036 | 0.146 | 0.008 | 0.113 | 0.026 | |
| L6 | 0.000 | 0.002 | 0.040 | 0.024 | 0.130 | 0.008 | nd | 0.143 | |
| L7 | 0.000 | 0.002 | 0.012 | 0.022 | 0.133 | 0.008 | nd | 0.057 | |
| L8 | 0.001 | 0.002 | 0.028 | 0.026 | 0.134 | 0.008 | nd | 0.061 | |
| L9 | 0.000 | 0.002 | 0.012 | 0.023 | 0.139 | 0.005 | nd | 0.050 | |
| L10 | 0.000 | 0.002 | 0.052 | 0.019 | 0.132 | 0.011 | nd | 0.095 | |
| L1B | 0.000 | 0.002 | 0.018 | 0.019 | 0.117 | 0.004 | nd | 0.016 | |
| L6B | 0.001 | 0.002 | 0.015 | 0.038 | 0.127 | 0.008 | nd | 0.016 | |
| L7B | 0.001 | 0.004 | 0.010 | 0.031 | 0.132 | 0.009 | nd | 0.015 | |
| L8B | 0.000 | 0.003 | 0.018 | 0.034 | 0.136 | 0.007 | nd | 0.022 | |
| L10B | 0.001 | 0.002 | 0.016 | 0.034 | 0.125 | 0.009 | nd | 0.020 | |
| L11B | 0.001 | 0.002 | 0.015 | 0.023 | 0.133 | 0.006 | nd | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banica, A.L.; Radulescu, C.; Dulama, I.D.; Bucurica, I.A.; Stirbescu, R.M.; Stanescu, S.G. Microplastics, Polycyclic Aromatic Hydrocarbons, and Heavy Metals in Milk: Analyses and Induced Health Risk Assessment. Foods 2024, 13, 3069. https://doi.org/10.3390/foods13193069
Banica AL, Radulescu C, Dulama ID, Bucurica IA, Stirbescu RM, Stanescu SG. Microplastics, Polycyclic Aromatic Hydrocarbons, and Heavy Metals in Milk: Analyses and Induced Health Risk Assessment. Foods. 2024; 13(19):3069. https://doi.org/10.3390/foods13193069
Chicago/Turabian StyleBanica, Andreea Laura, Cristiana Radulescu, Ioana Daniela Dulama, Ioan Alin Bucurica, Raluca Maria Stirbescu, and Sorina Geanina Stanescu. 2024. "Microplastics, Polycyclic Aromatic Hydrocarbons, and Heavy Metals in Milk: Analyses and Induced Health Risk Assessment" Foods 13, no. 19: 3069. https://doi.org/10.3390/foods13193069
APA StyleBanica, A. L., Radulescu, C., Dulama, I. D., Bucurica, I. A., Stirbescu, R. M., & Stanescu, S. G. (2024). Microplastics, Polycyclic Aromatic Hydrocarbons, and Heavy Metals in Milk: Analyses and Induced Health Risk Assessment. Foods, 13(19), 3069. https://doi.org/10.3390/foods13193069















