In Vitro Digestion and Fermentation of Cowpea Pod Extracts and Proteins Loaded in Ca(II)-Alginate Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extract Preparation and Encapsulation
2.3. Morphology Analysis
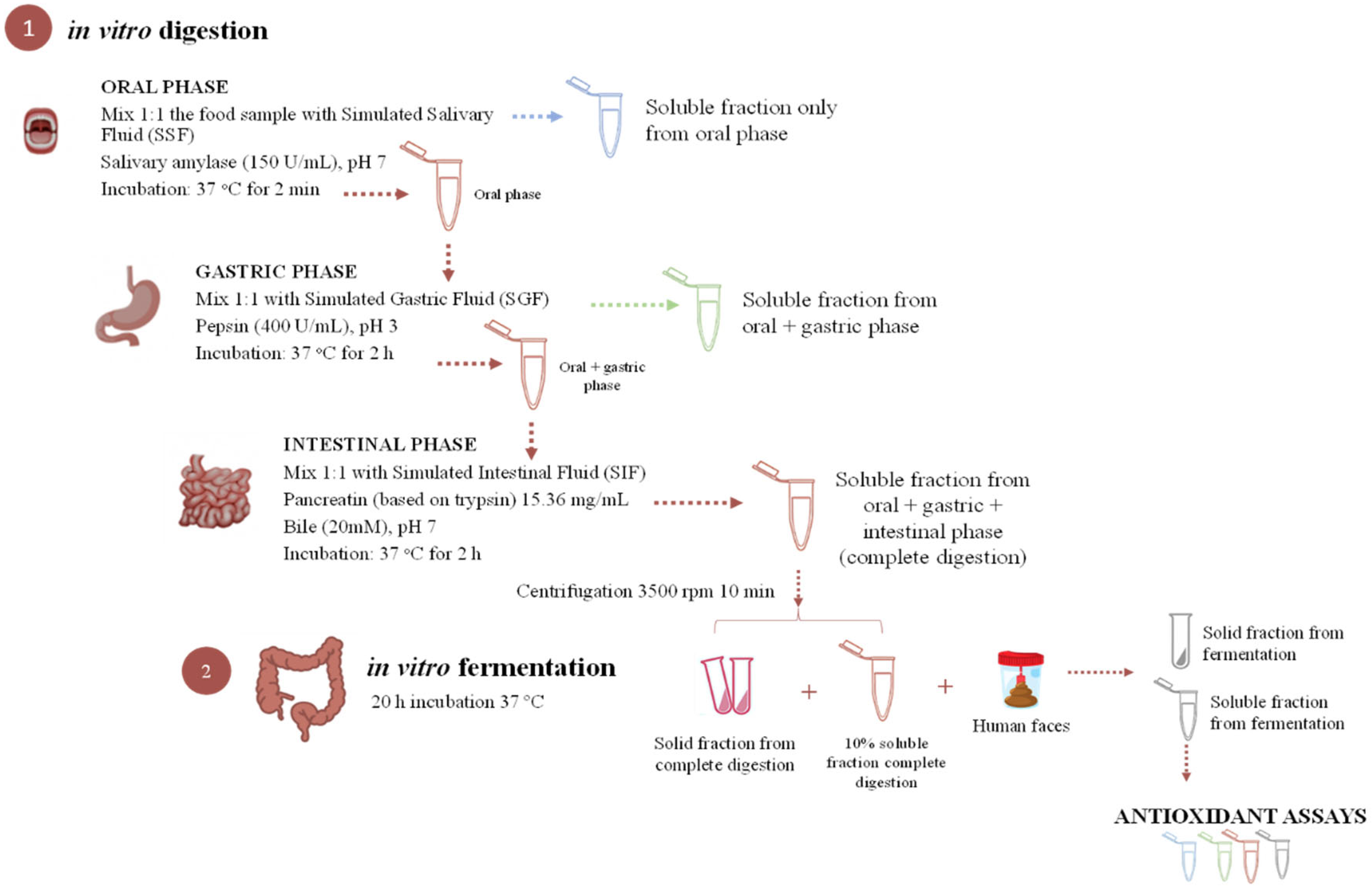
2.4. In Vitro Gastrointestinal Simulation
2.4.1. Antioxidant Capacity Methods, Loading Efficiency, and Remaining Antioxidant Activity
2.4.2. Short-Chain Fatty Acids (SCFAs) Analysis
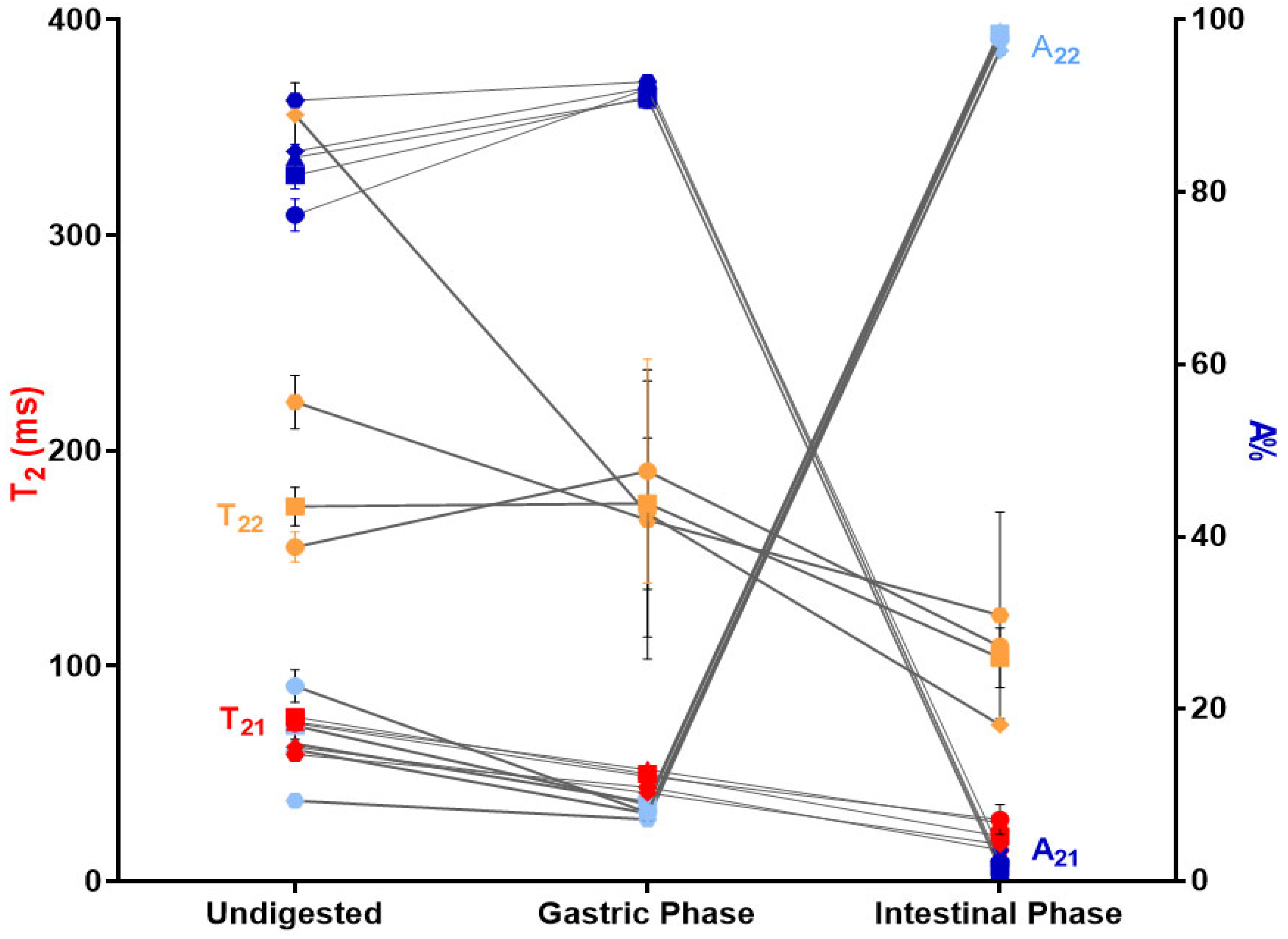
2.4.3. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Phcog. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya, Á.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Effect of cooking methods on the antioxidant capacity of foods of animal origin submitted to in vitro digestion-fermentation. Antioxidants 2021, 10, 445. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Banerjee, N.; Noratto, G.D.; Angel-Morales, G.; Hachibamba, T.; Awika, J.M.; Mertens-Talcott, S.U. Polyphenolic extracts from cowpea (Vigna unguiculata) protect colonic myofibroblasts (CCD18Co cells) from lipopolysaccharide (LPS)-induced inflammation–modulation of microRNA 126. Food Funct. 2015, 6, 145–153. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Maia, F.M.M.; Farias, D.F.; Campello, C.C.; Carvalho, A.F.U.; de Azevedo Moreira, R.; de Oliveira, J.T.A. Protein fractions, amino acid composition and antinutritional constituents of high-yielding cowpea cultivars. J. Food Compos. Anal. 2010, 23, 54–60. [Google Scholar] [CrossRef]
- Rengadu, D.; Gerrano, A.S.; Mellem, J.J. Physicochemical and structural characterization of resistant starch isolated from Vigna unguiculata. Int. J. Biol. Macromol. 2020, 147, 268–275. [Google Scholar] [CrossRef]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Seke, F.; Moloto, M.R.; Shoko, T.; Sultanbawa, Y.; Sivakumar, D. Comparative study of the functional compounds and antioxidant properties of different cowpea (Vigna unguiculata) leaf cultivars after in vitro digestion. Int. J. Food Sci. Technol. 2023, 58, 1089–1097. [Google Scholar] [CrossRef]
- Moloto, M.R.; Phan, A.D.T.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. Comparison of phenolic compounds, carotenoids, amino acid composition, in vitro antioxidant and anti-diabetic activities in the leaves of seven cowpea (Vigna unguiculata) cultivars. Foods 2020, 9, 1285. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Aguirre-Calvo, T.R.; Avanza, M.V.; Santagapita, P.R. High-intensity ultrasound-assisted extraction of phenolic compounds from cowpea pods and its encapsulation in hydrogels. Heliyon 2020, 6, e04410. [Google Scholar] [CrossRef]
- Karapanos, I.; Papandreou, A.; Skouloudi, M.; Makrogianni, D.; Fernández, J.A.; Rosa, E.; Ntatsi, G.; Bebeli, P.J.; Savvas, D. Cowpea fresh pods–a new legume for the market: Assessment of their quality and dietary characteristics of 37 cowpea accessions grown in southern Europe. J. Sci. Food Agric. 2017, 97, 4343–4352. [Google Scholar] [CrossRef]
- Avanza, M.V.; Álvarez-Rivera, G.; Cifuentes, A.; Mendiola, J.A.; Ibáñez, E. Phytochemical and functional characterization of phenolic compounds from cowpea (Vigna unguiculata (L.) Walp.) obtained by green extraction technologies. Agronomy 2021, 11, 162. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Sosa, N.; López, T.A.; Quintanilla-Carvajal, M.X.; Perullini, M.; Santagapita, P.R. Bioaccessibility assay, antioxidant activity and consumer-oriented sensory analysis of Beta vulgaris by-product encapsulated in Ca (II)-alginate beads for different foods. Food Chem. Mol. Sci. 2022, 5, 100140. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, I.A.; Seal, C.J.; Wilcox, M.; Dettmar, P.W.; Pearson, J.P. Applications of Alginates in Food. In Alginates: Biology and Applications; Rehm, B.H.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 211–228. [Google Scholar]
- Rayment, P.; Wright, P.; Hoad, C.; Ciampi, E.; Haydock, D.; Gowland, P.; Butler, M.F. Investigation of alginate beads for gastro-intestinal functionality, Part 1: In vitro characterisation. Food Hydrocoll. 2009, 23, 816–822. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Fito, P.J.; Perullini, M.; Santagapita, P.R. Gums induced microstructure stability in Ca (II)-alginate beads containing lactase analyzed by SAXS. Carbohydr. Polym. 2018, 179, 402–407. [Google Scholar] [CrossRef]
- Aguirre Calvo, T.R.; Perullini, M.; Santagapita, P.R. Encapsulation of betacyanins and polyphenols extracted from leaves and stems of beetroot in Ca (II)-alginate beads: A structural study. J. Food Eng. 2018, 235, 32–40. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Molino, S.; Perullini, M.; Rufián-Henares, J.Á.; Santagapita, P.R. Effect of in vitro digestion-fermentation of Ca (II)-alginate beads containing sugar and biopolymers over global antioxidant response and short chain fatty acids production. Food Chem. 2020, 333, 127483. [Google Scholar] [CrossRef]
- Peyrano, F.; De Lamballerie, M.; Speroni, F.; Avanza, M.V. Rheological characterization of thermal gelation of cowpea protein isolates: Effect of processing conditions. LWT 2019, 109, 406–414. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.; Santagapita, P. Physicochemical characterization of alginate beads containing sugars and biopolymers. J. Qual. Reliab. Eng. 2016, 2016, 9184039. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Brodkorb, A. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Effect of cooking methods on the antioxidant capacity of plant foods submitted to in vitro digestion–fermentation. Antioxidants 2020, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Rufián-Henares, J.A.; Pastoriza, S. Towards an improved global antioxidant response method (GAR+): Physiological-resembling in vitro digestion-fermentation method. Food Chem. 2018, 239, 1253–1262. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Pérez-Burillo, S.; Pastoriza, S.; Martín, M.Á.; Cerruti, P.; Goya, L.; Ramos, S.; Rufián-Henares, J.Á.; Napolitano, A.; d’Ischia, M. High Antioxidant Action and Prebiotic Activity of Hydrolyzed Spent Coffee Grounds (HSCG) in a Simulated Digestion–Fermentation Model: Toward the Development of a Novel Food Supplement. J. Agric. Food Chem. 2017, 65, 6452–6459. [Google Scholar] [CrossRef] [PubMed]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Encapsulation of lactase in Ca (II)-alginate beads: Effect of stabilizers and drying methods. Food Res. Int. 2017, 100, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Zazzali, I.; Aguirre Calvo, T.R.; Ruíz-Henestrosa, V.M.P.; Santagapita, P.R.; Perullini, M. Effects of pH, extrusion tip size and storage protocol on the structural properties of Ca (II)-alginate beads. Carbohydr. Polym. 2019, 206, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Qin, W.; Ketnawa, S.; Ogawa, Y. Impact of particle size of pulverized citrus peel tissue on changes in antioxidant properties of digested fluids during simulated in vitro digestion. Food Sci. Hum. Well. 2020, 9, 58–63. [Google Scholar] [CrossRef]
- Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends. Biotechnol. 2012, 30, 591–600. [Google Scholar] [CrossRef]
- Wongverawattanakul, C.; Suklaew, P.O.; Chusak, C.; Adisakwattana, S.; Thilavech, T. Encapsulation of Mesona chinensis benth extract in alginate beads enhances the stability and antioxidant activity of polyphenols under simulated gastrointestinal digestion. Foods 2022, 11, 2378. [Google Scholar] [CrossRef]
- Aguirre-Calvo, T.R.; Molino, S.; Perullini, M.; Rufián-Henares, J.Á.; Santagapita, P.R. Effects of in vitro digestion–fermentation over global antioxidant response and short chain fatty acid production of beet waste extracts in Ca (II)–alginate beads. Food Funct. 2020, 11, 10645–10654. [Google Scholar] [CrossRef]
- Gómez, A.; Gay, C.; Tironi, V.; Avanza, M.V. Structural and antioxidant properties of cowpea protein hydrolysates. Food Biosci. 2021, 41, 101074. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Liu, H.; Ma, R.; Ma, J.; Fang, H. Fermentation by multiple bacterial strains improves the production of bioactive compounds and antioxidant activity of goji juice. Molecules 2019, 24, 3519. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D.; Verzelloni, E.; Conte, A. Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion. J. Funct. Foods. 2014, 7, 506–516. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Espinosa-García, L.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Effect of enzymatic hydrolysis on solubility, hydrophobicity, and in vivo digestibility in cowpea (Vigna unguiculata). Int. J. Food Prop. 2012, 15, 770–780. [Google Scholar] [CrossRef]
- Rodríguez, M.; Tironi, V.A. Chemical and cell antioxidant activity of amaranth flour and beverage after simulated gastrointestinal digestion. Role of peptides. Food Res. Int. 2023, 173, 113410. [Google Scholar] [CrossRef] [PubMed]
- Alain, M.; Mune, M. Prediction and Evaluation of Bioactive Properties of Cowpea Protein Hydrolysates. J. Food Biochem. 2023, 2023, 9095113. [Google Scholar] [CrossRef]
- Hu, K.; Huang, H.; Li, H.; Wei, Y.; Yao, C. Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges. Nutrients 2023, 15, 1096. [Google Scholar] [CrossRef]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Willemsen, L.E.M.; Koetsier, M.A.; Van Deventer, S.J.H.; Van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Barber, X.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res. Int. 2015, 78, 169–176. [Google Scholar] [CrossRef]
- Zambell, K.L.; Fitch, M.D.; Fleming, S.E. Acetate and butyrate are the major substrates for de novo lipogenesis in rat colonic epithelial cells. J. Nutr. 2003, 133, 3509–3515. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Gibson, G.R. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends. Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Alonso, V.R.; Guarner, F. Linking the gut microbiota to human health. Br. J. Nutr. 2013, 109 (Suppl. S2), S21–S26. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Liu, W.; Yin, Z.; Wu, J.; Yu, X.; Zhan, X. The specificity of ten non-digestible carbohydrates to enhance butyrate-producing bacteria and butyrate production in vitro fermentation. Food Sci. Hum. Well. 2023, 12, 2344–2354. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC. Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Xing, P.Y.; Pettersson, S.; Kundu, P. Microbial metabolites and intestinal stem cells tune intestinal homeostasis. Proteomics 2020, 20, 1800419. [Google Scholar] [CrossRef]
- Pi, X.E.; Fu, H.; Yang, X.X.; Yu, Z.C.; Teng, W.L.; Zhang, Y.; Liu, W. Bacterial, short-chain fatty acid and gas profiles of partially hydrolyzed guar gum in vitro fermentation by human fecal microbiota. Food Chem. 2024, 430, 137006. [Google Scholar] [CrossRef]
- Li, H.; Lane, J.A.; Chen, J.; Lu, Z.; Wang, H.; Dhital, S.; Zhang, B. In vitro fermentation of human milk oligosaccharides by individual Bifidobacterium longum-dominant infant fecal inocula. Carbohydr. Polym. 2022, 287, 119322. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut fermentation of dietary fibres: Physico-chemistry of plant cell walls and implications for health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Zwolińska-Wcisło, M. Fecal levels of lactic, succinic and short-chain fatty acids in patients with ulcerative colitis and Crohn disease: A pilot study. J. Clin. Med. 2021, 10, 4701. [Google Scholar] [CrossRef] [PubMed]
- Högberg, A.; Lindberg, J.E. Influence of cereal non-starch polysaccharides and enzyme supplementation on digestion site and gut environment in weaned piglets. Anim. Feed. Sci. Technol. 2004, 116, 113–128. [Google Scholar] [CrossRef]
- Aguirre Calvo, T.R.; Busch, V.M.; Santagapita, P.R. Stability and release of an encapsulated solvent-free lycopene extract in alginate-based beads. LWT-Food Sci. Technol. 2017, 77, 406–412. [Google Scholar] [CrossRef]
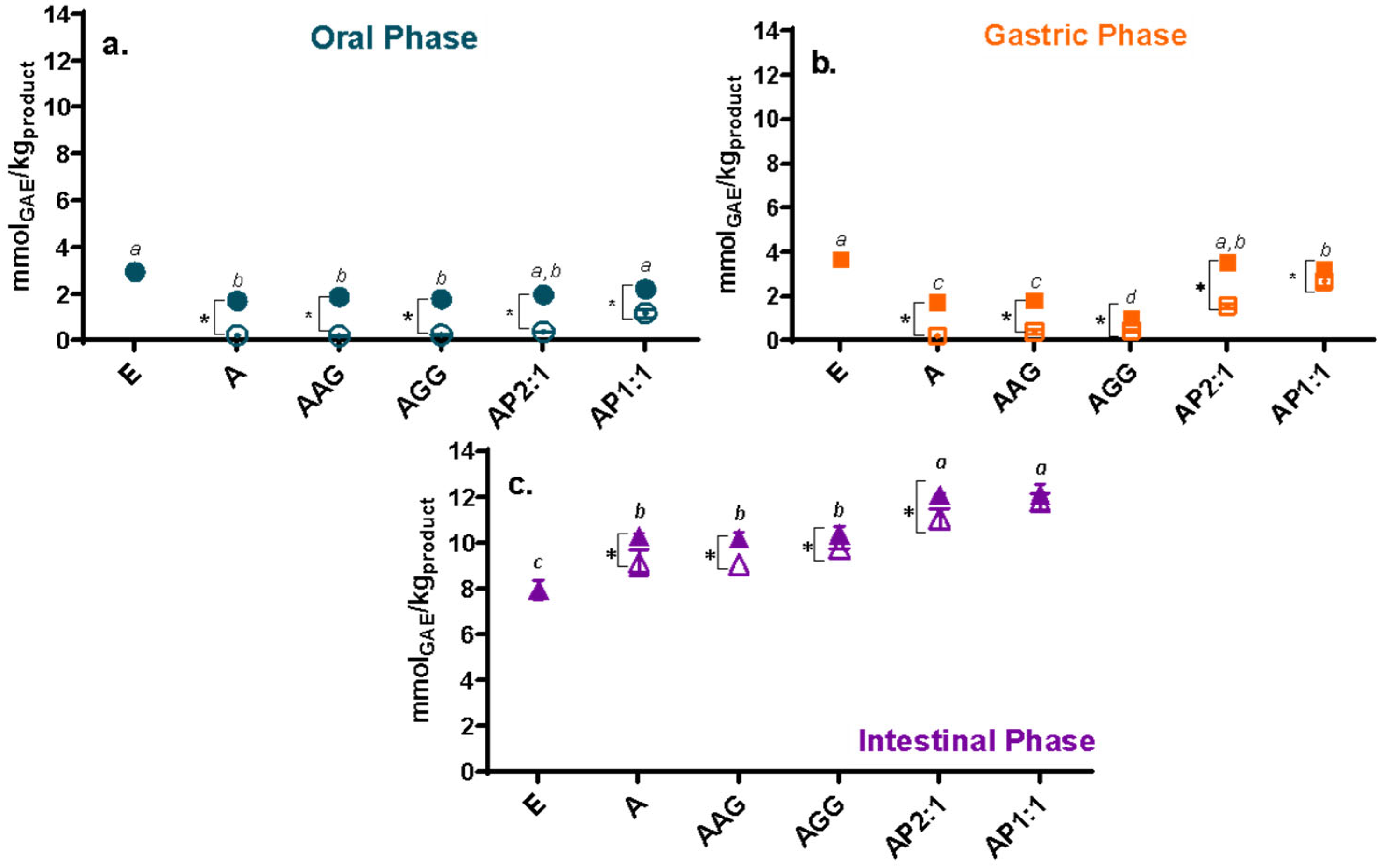

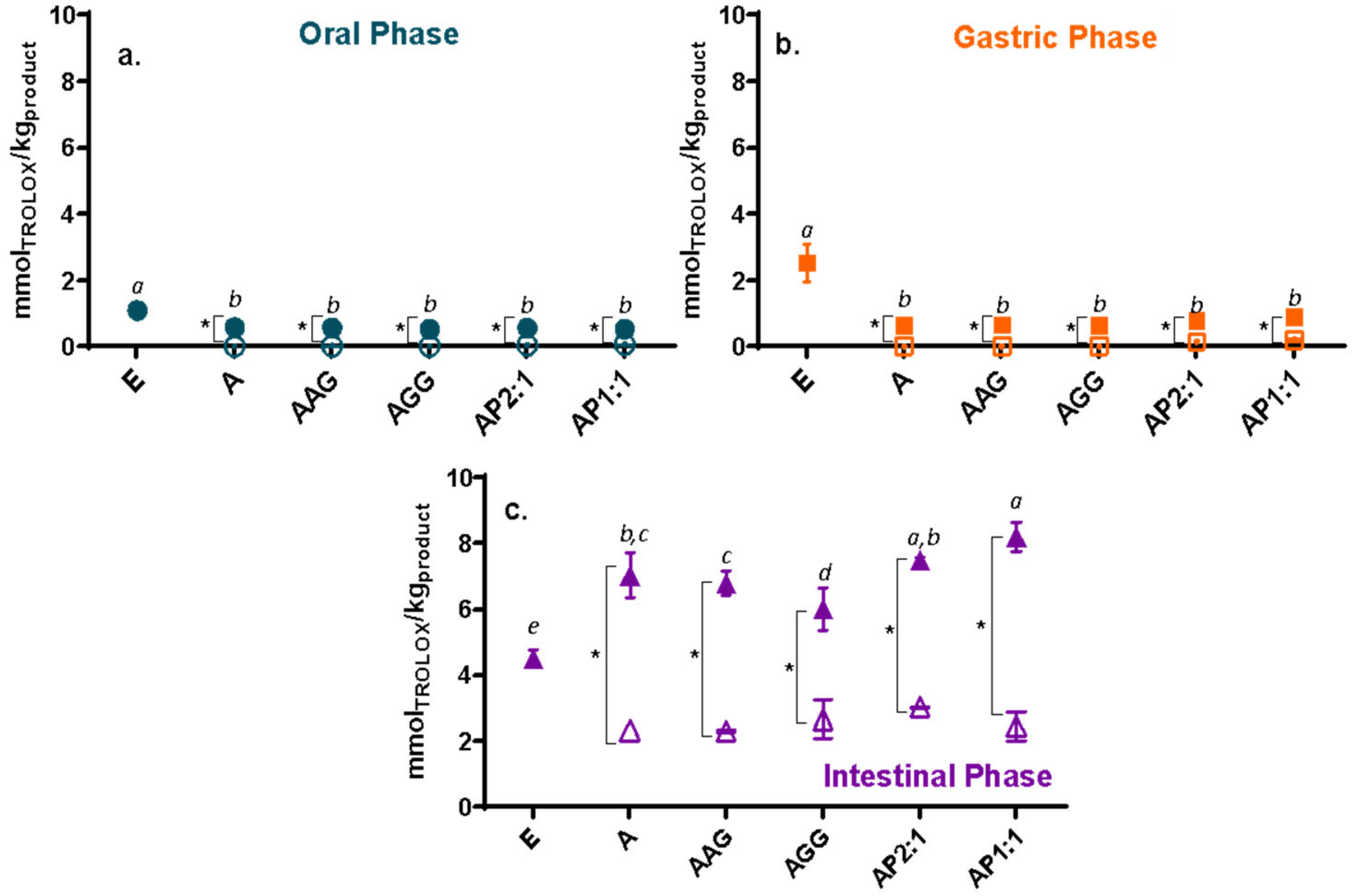

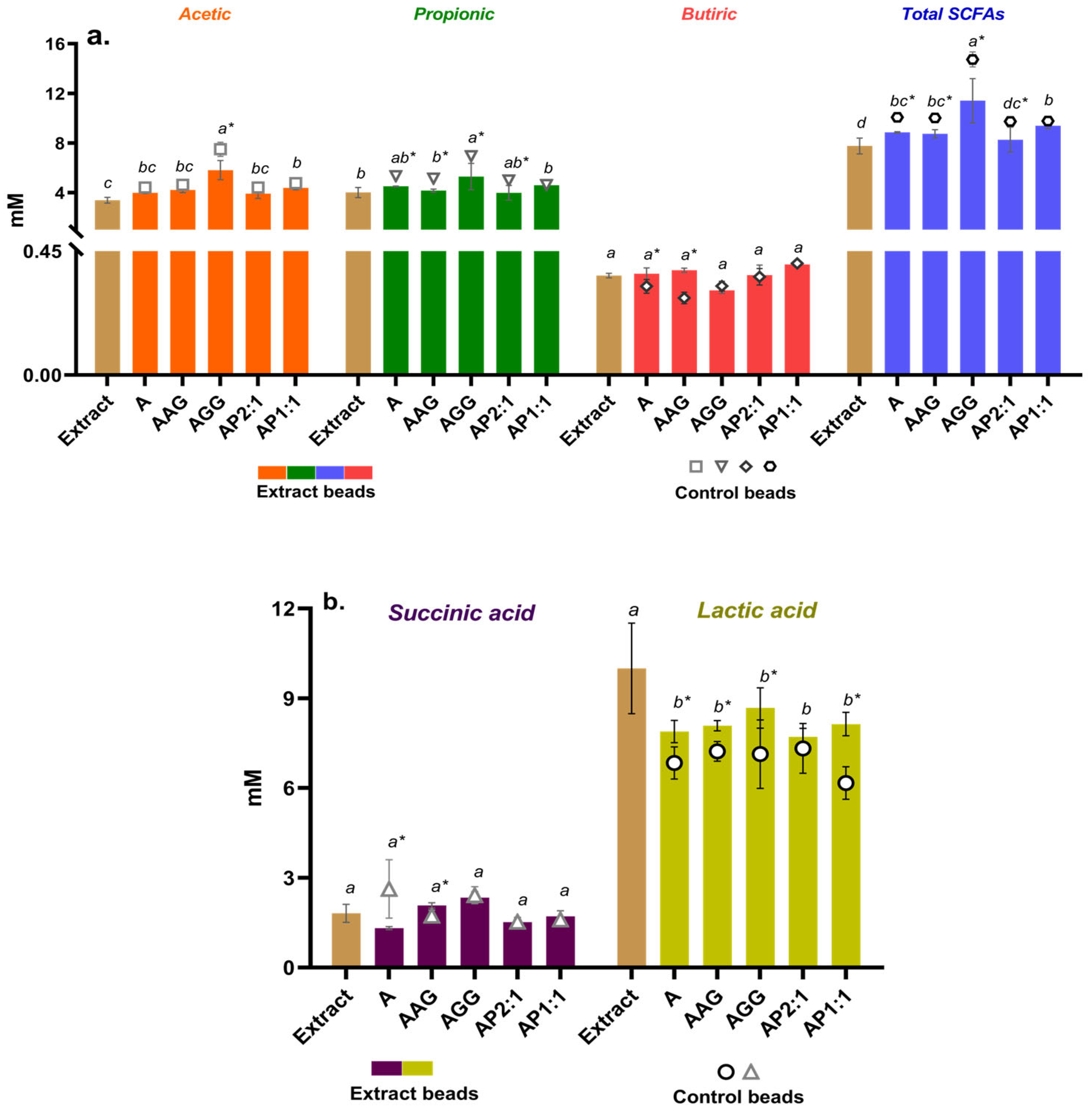
| System | L.E.TP (%) | R.A.A.ABTS (%) | R.A.A.FRAP (%) |
|---|---|---|---|
| A | 19 ± 1 c | 36.4 ± 0.6 c | 1.9 ± 0.5 d |
| AAG | 49 ± 2 a | 32 ± 1 d | 7 ± 1 c |
| AGG | 45 ± 3 a | 25 ± 3 e | 4.5 ± 0.6 c,d |
| AP2:1 | 36 ± 1 b | 55 ± 2 a | 18 ± 1 b |
| AP1:1 | 47 ± 3 a | 47 ± 3 b | 26 ± 3 a |
| Global Antioxidant Response (GAR) | ||||
|---|---|---|---|---|
| System | Folin–Ciocalteu (mmolGAE/kgproduct) | TEACABTS (mmolTROLOX/kgproduct) | TEACFRAP (mmolTROLOX/kgproduct) | |
| E | 20 ± 2 c | 17.1 ± 0.4 b | 9.4 ± 0.1 b | |
| Beads with extract | A | 47 ± 1 b,A | 51 ± 4 a,A | 25 ± 1 a,A |
| AAG | 50 ± 2 a,b,A | 52 ± 3 a,A | 24.8 ± 0.5 a,A | |
| AGG | 49 ± 2 a,b,A | 48 ± 2 a,A | 22.0 ± 0.7 a,A | |
| AP2:1 | 52.3 ± 0.5 a,b,A | 50.9 ± 0.8 a,A | 26.7 ± 0.3 a,A | |
| AP1:1 | 54 ± 2 a,A | 54 ± 6 a,A | 26 ± 1 a,A | |
| Beads without extract | A | 45 ± 2 b,A | 35 ± 1 b,B | 20.1 ± 0.3 a,B |
| AAG | 43.6 ± 0.7 b,B | 28 ± 1 c,B | 20.3 ± 0.6 a,B | |
| AGG | 43 ± 2 b,B | 32 ± 3 b,c,B | 20.5 ± 0.8 a,A | |
| AP2:1 | 45 ± 1 b,B | 35 ± 2 b,B | 20 ± 1 a,B | |
| AP1:1 | 49.7 ± 0.5 a,A | 51 ± 1 a,A | 22.3 ± 0.3 a,B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traffano-Schiffo, M.V.; Aguirre-Calvo, T.R.; Navajas-Porras, B.; Avanza, M.V.; Rufián-Henares, J.Á.; Santagapita, P.R. In Vitro Digestion and Fermentation of Cowpea Pod Extracts and Proteins Loaded in Ca(II)-Alginate Hydrogels. Foods 2024, 13, 3071. https://doi.org/10.3390/foods13193071
Traffano-Schiffo MV, Aguirre-Calvo TR, Navajas-Porras B, Avanza MV, Rufián-Henares JÁ, Santagapita PR. In Vitro Digestion and Fermentation of Cowpea Pod Extracts and Proteins Loaded in Ca(II)-Alginate Hydrogels. Foods. 2024; 13(19):3071. https://doi.org/10.3390/foods13193071
Chicago/Turabian StyleTraffano-Schiffo, Maria Victoria, Tatiana Rocio Aguirre-Calvo, Beatriz Navajas-Porras, María Victoria Avanza, José Ángel Rufián-Henares, and Patricio Román Santagapita. 2024. "In Vitro Digestion and Fermentation of Cowpea Pod Extracts and Proteins Loaded in Ca(II)-Alginate Hydrogels" Foods 13, no. 19: 3071. https://doi.org/10.3390/foods13193071
APA StyleTraffano-Schiffo, M. V., Aguirre-Calvo, T. R., Navajas-Porras, B., Avanza, M. V., Rufián-Henares, J. Á., & Santagapita, P. R. (2024). In Vitro Digestion and Fermentation of Cowpea Pod Extracts and Proteins Loaded in Ca(II)-Alginate Hydrogels. Foods, 13(19), 3071. https://doi.org/10.3390/foods13193071







