Comparison of Protective Effects of Polyphenol-Enriched Extracts from Thinned Immature Kiwifruits and Mature Kiwifruits against Alcoholic Liver Disease in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Polyphenol-Enriched Extracts from Thinned Immature and Mature Kiwifruits
2.3. Determination of Total Polyphenols and Major Phenolic Compounds in Polyphenol-Enriched Extracts
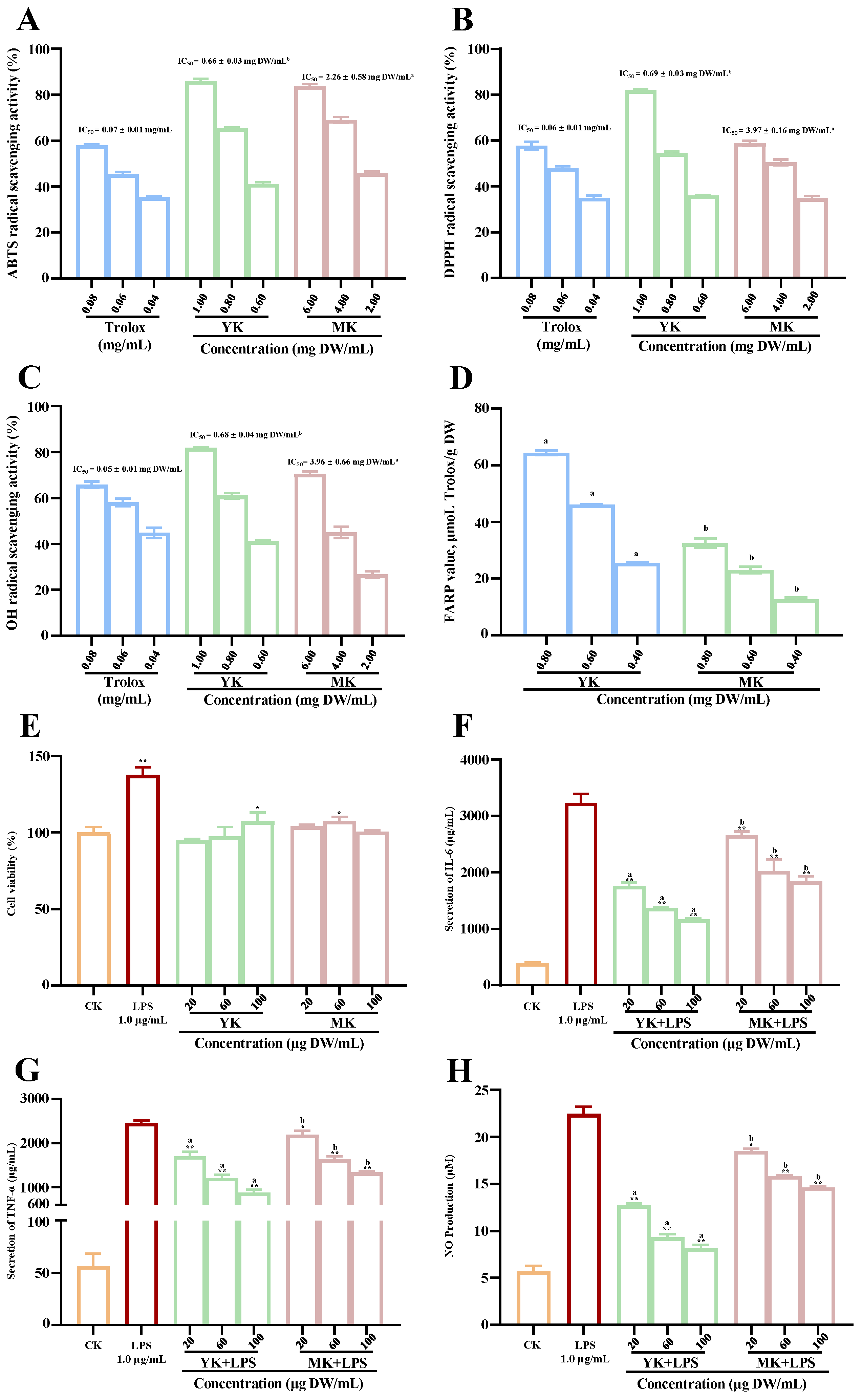
2.4. Evaluation of In Vitro Antioxidant Activities of Polyphenol-Enriched Extracts
2.5. Evaluation of In Vitro Anti-Inflammatory Activities
2.6. Ameliorative Effects of Polyphenol-Enriched Extracts on Alcoholic Liver Disease in Mice
2.6.1. Animals and Experimental Design
2.6.2. Histological Analysis and Biochemical Assays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Comparison of Total Polyphenols and Individual Phenolic Components in Thinned Immature and Mature Kiwifruits
3.2. Comparison of In Vitro Antioxidant and Anti-Inflammatory Activities of Thinned Immature and Mature Kiwifruits
3.3. Comparison of Protective Effects of Polyphenol-Enriched Extracts from Thinned Immature and Mature Kiwifruits against Alcoholic Liver Disease in Mice
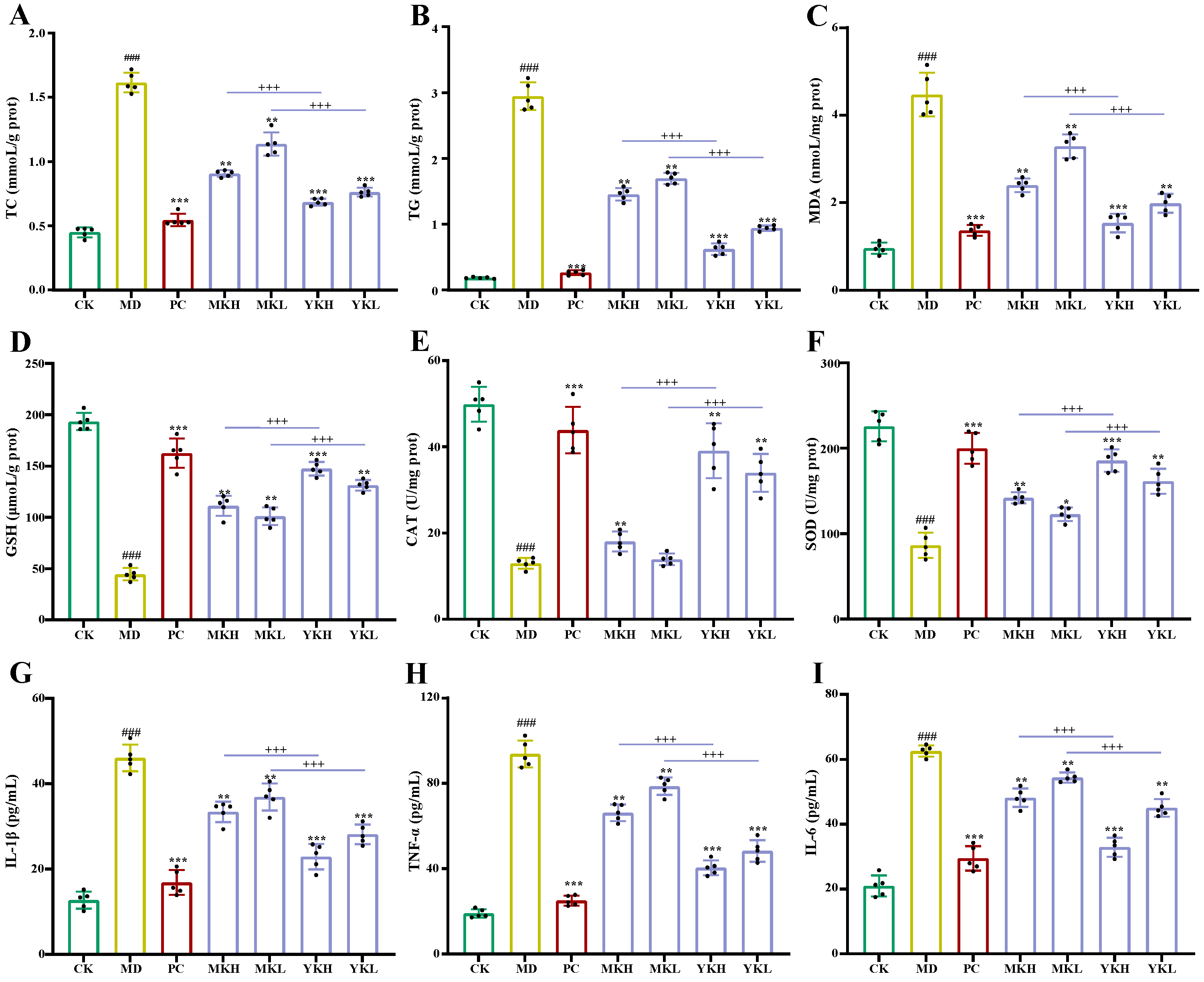
3.3.1. Effects of Polyphenol-Enriched Extracts on Alcohol-Induced Liver Injury in Mice
3.3.2. Effects of Polyphenol-Enriched Extracts on Ethanol-Induced Oxidative Stress in Mice
3.3.3. Effects of Polyphenol-Enriched Extracts on Ethanol-Induced Inflammation in Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between oxidative stress and inflammatory liver injury in the pathogenesis of alcoholic liver disease. Int. J. Mol. Sci. 2022, 23, 774. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G. Gut–liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Yuan, D.D.; Usman, M.; Murtaza, M.A.; Yaqoob, S.; Wang, C.T. Protective mechanism of edible food plants against alcoholic liver disease with special mention to polyphenolic compounds. Nutrients 2021, 13, 1612. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, R.F.; Zhou, Q.Y.; Liu, L.; Huang, F.; Deng, Y.Y.; Ma, Y.X.; Wei, Z.C.; Tang, X.J.; Zhang, M.W. Lychee (Litchi chinensis Sonn.) pulp phenolic extract provides protection against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, intestinal barrier dysfunction, and liver inflammation. J. Agric. Food Chem. 2017, 65, 9675–9684. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, R.; Wu, Y.; Wu, C.; Jia, X.; Dong, L.; Liu, L.; Chen, Y.; Bai, Y.; Zhang, M. Rice bran phenolic extract protects against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, barrier dysfunction, and liver inflammation mediated by the endotoxin–TLR4–NF-κB pathway. J. Agric. Food Chem. 2020, 68, 1237–1247. [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Wang, Q.Y.; Hussain, M.A.; Wang, C.Y.; Hou, J.C.; Jiang, Z.M. Protective effect of polyphenols, protein, peptides, and polysaccharides on alcoholic liver disease: A review of research status and molecular mechanisms. J. Agric. Food Chem. 2023, 71, 5861–5883. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Wu, D.T.; Gen, J.L.; Li, J.; Deng, W.; Zhang, Y.; Hu, Y.C.; Zou, L.; Xia, Y.; Zhuang, Q.G.; Liu, H.Y.; et al. Efficient extraction of pectic polysaccharides from thinned unripe kiwifruits by deep eutectic solvent-based methods: Chemical structures and bioactivities. Food Chem. X 2024, 21, 101083. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, D.; Fan, M.; Quek, S.Y. UPLC-QqQ-MS/MS-based phenolic quantification and antioxidant activity assessment for thinned young kiwifruits. Food Chem. 2019, 281, 97–105. [Google Scholar] [CrossRef]
- Wu, D.T.; Deng, W.; Li, J.; Geng, J.L.; Hu, Y.C.; Zou, L.; Liu, Y.; Liu, H.Y.; Gan, R.-Y. Ultrasound-assisted deep eutectic solvent extraction of phenolic compounds from thinned young kiwifruits and their beneficial effects. Antioxidants 2023, 12, 1475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, K.W.; Wu, D.T.; Liu, H.Y.; Li, H.B.; Zhang, J.R.; Gan, R.Y. Pomegranate peel-derived punicalagin: Ultrasonic-assisted extraction, purification, and its alpha-glucosidase inhibitory mechanism. Food Chem. 2022, 374, 131635. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yuan, Q.; Yang, Y.L.; Han, Q.H.; He, J.L.; Zhao, L.; Zhang, Q.; Liu, S.X.; Lin, D.R.; Wu, D.T.; et al. Phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of different kiwifruits. Molecules 2018, 23, 2957. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Chen, L.; Liu, R.; Chang, M.; Wang, X. Identification and in vitro anti-inflammatory activity of different forms of phenolic compounds in Camellia oleifera oil. Food Chem. 2021, 344, 128660. [Google Scholar] [CrossRef]
- Zheng, L.; Aimaiti, Z.; Long, L.; Xia, C.; Wang, W.; Zhou, Z.-Z. Discovery of 4-Ethoxy-6-chloro-5-azaindazoles as novel PDE4 inhibitors for the treatment of alcohol use disorder and alcoholic liver diseases. J. Med. Chem. 2024, 67, 728–753. [Google Scholar] [CrossRef]
- Fang, X.; Cao, J.; Tao, Z.; Yang, Z.; Dai, Y.; Zhao, L. Hydroxytyrosol attenuates ethanol-induced liver injury by ameliorating steatosis, oxidative stress and hepatic inflammation by interfering STAT3/iNOS pathway. Redox Rep. 2023, 28, 2187564. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Vaidya, S.N.; Telrandhe, U.B.; Agrawal, S. Nutritional and health benefits of kiwifruit: An overview. Ann. Phytomed. 2022, 11, 176–185. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Chen, X.; Zhao, Z.; Li, Y.; Meng, Y.; Huang, L. Actinidia chinensis planch: A review of chemistry and pharmacology. Front. Pharmacol. 2019, 10, 1236. [Google Scholar] [CrossRef]
- Mai, Y.H.; Zhuang, Q.G.; Li, Q.H.; Du, K.; Wu, D.T.; Li, H.B.; Xia, Y.; Zhu, F.; Gan, R.Y. Ultrasound-assisted extraction, identification, and quantification of antioxidants from ‘Jinfeng’ kiwifruit. Foods 2020, 11, 827. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative stress in liver pathophysiology and disease. Antioxidant 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Petrasek, J. Gut–liver axis and sterile signals in the development of alcoholic liver disease. Alcohol. Alcoholism. 2017, 52, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Miranda, R.G.; Dorta, D.J.; Rolo, A.P.; Palmeira, C.M. Targeting oxidative stress with polyphenols to fight liver diseases. Antioxidant 2023, 12, 1212. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhao, Q.Y.; Lan, T.; Geng, T.H.; Gao, C.X.; Yuan, Q.Y.; Zhang, Q.W.; Xu, P.K.; Sun, X.Y.; Liu, X.B.; et al. Comparative analysis of physicochemical characteristics, nutritional and functional components and antioxidant capacity of fifteen kiwifruit (Actinidia) cultivars-comparative analysis of fifteen kiwifruit (Actinidia) cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef]
- Liu, Y.F.; Qi, Y.W.; Chen, X.; He, H.H.; Liu, Z.D.; Zhang, Z.; Ren, Y.M.; Ren, X.L. Phenolic compounds and antioxidant activity in red- and in green-fleshed kiwifruits. Food Res. Int. 2019, 2019, 291–301. [Google Scholar] [CrossRef]
- Alim, A.; Li, T.; Nisar, T.; Ren, D.; Zhai, X.; Pang, Y.; Yang, X. Antioxidant, antimicrobial, and antiproliferative activity-based comparative study of peel and flesh polyphenols from Actinidia chinensis. Food Nutr. Res. 2019, 63, 1577. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Jing, Y.; Liu, S.; Qin, S.; Peng, F.; Li, D.; Peng, C. Procyanidin B2: A promising multi-functional food-derived pigment for human diseases. Food Chem. 2023, 420, 136101. [Google Scholar] [CrossRef]
- Terra, X.; Palozza, P.; Fernandez-Larrea, J.; Ardevol, A.; Blade, C.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M.T. Procyanidin dimer B1 and trimer C1 impair inflammatory response signaling in human monocytes. Free Radic. Res. 2011, 45, 611–619. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Yi, E.H.; Kim, Y.; Park, G. Neochlorogenic acid inhibits against LPS-activated inflammatory responses through up-regulation of Nrf2/HO-1 and involving AMPK pathway. Environ. Toxicol. Pharmacol. 2018, 62, 1–10. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K.; Zeng, M.; Qiao, B.; Zhou, B. Serum metabolomics analysis of the anti-inflammatory effects of gallic acid on rats with acute inflammation. Front. Pharmacol. 2022, 13, 830439. [Google Scholar] [CrossRef]
- Iweala, E.E.J.; Evbakhavbokun, W.O.; Maduagwu, E.N. Antioxidant and hepatoprotective effect of Cajanus cajan in N-Nitrosodiethylamine-induced liver damage. Sci. Pharm. 2019, 83, 24. [Google Scholar] [CrossRef]
- Albasher, G.; Almeer, R.; Al-Otibi, F.O.; Al-Kubaisi, N.; Mahmoud, A.M. Ameliorative effect of beta vulgaris root extract on chlorpyrifos-induced oxidative stress, inflammation and liver injury in rats. Biomolecules 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Alcoholic liver disease: Alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, N.H.; Yang, D.; Yang, M.Y.; Guo, X.X.; He, J.G.; Wu, W.; Ji, B.P.; Cheng, Q.; Zhou, F. Protective effects of five structurally diverse flavonoid subgroups against chronic alcohol-induced hepatic damage in a mouse model. Nutrients 2018, 10, 1754. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The role of gut-derived lipopolysaccharides and the intestinal barrier in fatty liver diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Hou, X.; Yang, L.; Chu, H. The role of gut bacteria and fungi in alcohol-associated liver disease. Front. Med. 2022, 9, 840752. [Google Scholar] [CrossRef]
- Torres, S.; Segalés, P.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria and the NLRP3 inflammasome in alcoholic and nonalcoholic steatohepatitis. Cells 2022, 11, 1475. [Google Scholar] [CrossRef]
- Xia, T.; Duan, W.; Zhang, Z.; Li, S.; Zhao, Y.; Geng, B.; Zheng, Y.; Yu, J.; Wang, M. Polyphenol-rich vinegar extract regulates intestinal microbiota and immunity and prevents alcohol-induced inflammation in mice. Food Res. Int. 2021, 140, 110064. [Google Scholar] [CrossRef]


| Compounds | Regression Equation | R2 | Linear Range (μg/mL) | YK | MK |
|---|---|---|---|---|---|
| GA | y = 14.9049x − 45.5985 | 0.9942 | 0.56–71.43 | 652.92 ± 23.31 a | 19.25 ± 0.99 b |
| PA | y = 12.812 − 30.4955 | 0.9957 | 0.56–71.43 | 713.05 ± 22.70 a | 51.22 ± 1.54 b |
| NCL | y = 30.4434x − 172.967 | 0.9983 | 5.73–71.43 | 6209.89 ± 197.53 a | 314.46 ± 7.71 b |
| B1 | y = 4.6754x − 9.235 | 0.9991 | 5.73–71.43 | 3115.16 ± 141.69 a | 731.49 ± 25.88 b |
| CN | y = 10.972x − 61.241 | 0.9987 | 5.73–71.43 | 921.14 ± 42.91 a | 509.74 ± 22.67 b |
| CL | y = 19.5361x − 109.581 | 0.9964 | 5.73–71.43 | 227.76 ± 10.41 a | 7.92 ± 0.22 b |
| CA | y = 137.59x − 63.175 | 0.9947 | 5.73–71.43 | 78.69 ± 3.31 a | 23.79 ± 1.04 b |
| B2 | y = 9.89x − 17.1126 | 0.9963 | 5.73–71.43 | 9857.14 ± 384.42 a | 1971.01 ± 87.66 b |
| EPC | y = 9.184x − 30.653 | 0.9974 | 5.73–71.43 | 5890.79 ± 153.76 a | 1638.87 ± 29.57 b |
| p-CA | y = 217.0018x − 119.512 | 0.9970 | 0.56–71.43 | 181.78 ± 2.99 a | 8.89 ± 0.34 b |
| FA | y = 203.622x − 22.17 | 0.9984 | 0.56–71.43 | 15.59 ± 0.71 a | 5.12 ± 0.22 b |
| QG | y = 51.0929x − 33.0198 | 0.9959 | 0.56–71.43 | 820.29 ± 13.71 a | 67.21 ± 1.15 b |
| QR | y = 33.099x − 93.358 | 0.9963 | 2.85–15.18 | 521.63 ± 1.66 a | 85.90 ± 2.31 b |
| C1 | y = 206.91x − 517.43 | 0.9960 | 1.20–14.28 | 352.36 ± 5.15 a | 61.72 ± 1.25 b |
| Total content (μg/g DW) | 29,558.19 ± 1170.58 a | 5496.59 ± 174.86 b | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, W.; Yang, Q.-N.; Wu, D.-T.; Li, J.; Liu, H.-Y.; Hu, Y.-C.; Zou, L.; Gan, R.-Y.; Yan, H.-L.; Huang, J.-W. Comparison of Protective Effects of Polyphenol-Enriched Extracts from Thinned Immature Kiwifruits and Mature Kiwifruits against Alcoholic Liver Disease in Mice. Foods 2024, 13, 3072. https://doi.org/10.3390/foods13193072
Deng W, Yang Q-N, Wu D-T, Li J, Liu H-Y, Hu Y-C, Zou L, Gan R-Y, Yan H-L, Huang J-W. Comparison of Protective Effects of Polyphenol-Enriched Extracts from Thinned Immature Kiwifruits and Mature Kiwifruits against Alcoholic Liver Disease in Mice. Foods. 2024; 13(19):3072. https://doi.org/10.3390/foods13193072
Chicago/Turabian StyleDeng, Wen, Qian-Ni Yang, Ding-Tao Wu, Jie Li, Hong-Yan Liu, Yi-Chen Hu, Liang Zou, Ren-You Gan, Hui-Ling Yan, and Jing-Wei Huang. 2024. "Comparison of Protective Effects of Polyphenol-Enriched Extracts from Thinned Immature Kiwifruits and Mature Kiwifruits against Alcoholic Liver Disease in Mice" Foods 13, no. 19: 3072. https://doi.org/10.3390/foods13193072
APA StyleDeng, W., Yang, Q.-N., Wu, D.-T., Li, J., Liu, H.-Y., Hu, Y.-C., Zou, L., Gan, R.-Y., Yan, H.-L., & Huang, J.-W. (2024). Comparison of Protective Effects of Polyphenol-Enriched Extracts from Thinned Immature Kiwifruits and Mature Kiwifruits against Alcoholic Liver Disease in Mice. Foods, 13(19), 3072. https://doi.org/10.3390/foods13193072













