Extra Virgin Olive Oil from Stoned Olives with Oxygen Supply during Processing: Impact on Volatile and Phenolic Fraction and Sensory Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction Plant Equipped for Oxygen Addition during Crushing or Stoning
2.3. EVOO Chemical Analysis
Legal Quality Parameters
2.4. Phenolic Compounds
2.5. Volatile Compounds
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Legal Quality Parameters
3.2. Phenolic Compounds
3.3. Volatile Compounds
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of storage conditions on the quality of arbequina extra virgin olive oil and the impact on the composition of flavor-related compounds (Phenols and volatiles). Foods 2021, 10, 2161. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Catalane, P. Hammer crushers vs disk crushers: The influence of working temperature on the quality and preservation of virgin olive oil. Eur. Food Res. Technol. 2001, 213, 219–224. [Google Scholar] [CrossRef]
- Di Serio, M.G.; Giansante, L.; Del Re, P.; Pollastri, L.; Panni, F.; Valli, E.; Di Giacinto, L. Characterization of ‘Olivastro di Bucchianico cv’ extra virgin olive oils and its recognition by HS-GC-IMS. J. Sci. Food Agric. 2021, 101, 6074–6082. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Roselli, L.; Cicia, G.; Cavallo, C.; del Giudice, T.; Carlucci, D.; Clodoveo, M.L.; de Gennaro, B.C. Consumers’ willingness to buy innovative traditional food products: The case of extra-virgin olive oil extracted by ultrasound. Food Res. Int. 2018, 108, 482–490. [Google Scholar] [CrossRef]
- Sinesio, F.; Moneta, E.; Raffo, A.; Lucchetti, S.; Peparaio, M.; D’Aloise, A.; Pastore, G. Effect of extraction conditions and storage time on the sensory profile of monovarietal extra virgin olive oil (cv Carboncella) and chemical drivers of sensory changes. LWT-Food Sci. Technol. 2015, 63, 281–288. [Google Scholar] [CrossRef]
- Veneziani, G.; Nucciarelli, D.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Tomasone, R.; Pagano, M.; Servili, M. Application of low temperature during the malaxation phase of virgin olive oil mechanical extraction processes of three different italian cultivars. Foods 2021, 10, 1578. [Google Scholar] [CrossRef]
- Nucciarelli, D.; Esposto, S.; Veneziani, G.; Daidone, L.; Urbani, S.; Taticchi, A.; Selvaggini, R.; Servili, M. The Use of a Cooling Crusher to Reduce the Temperature of Olive Paste and Improve EVOO Quality of Coratina, Peranzana, and Moresca Cultivars: Impact on Phenolic and Volatile Compounds. Food Bioprocess Technol. 2022, 15, 1988–1996. [Google Scholar] [CrossRef]
- Servili, M.; Veneziani, G.; Taticchi, A.; Romaniello, R.; Tamborrino, A.; Leone, A. Low-frequency, high-power ultrasound treatment at different pressures for olive paste: Effects on olive oil yield and quality. Ultrason. Sonochemistry 2019, 59, 104747. [Google Scholar] [CrossRef]
- Tamborrino, A.; Romaniello, R.; Caponio, F.; Squeo, G.; Leone, A. Combined industrial olive oil extraction plant using ultrasounds, microwave, and heat exchange: Impact on olive oil quality and yield. J. Food Eng. 2019, 245, 124–130. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Di Maio, I.; Sordini, B.; Servili, M. Cooling treatment of olive paste during the oil processing: Impact on the yield and extra virgin olive oil quality. Food Chem. 2017, 221, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Romaniello, R.; Zagaria, R.; Tamborrino, A. Development of a prototype malaxer to investigate the influence of oxygen on extra-virgin olive oil quality and yield, to define a new design of machine. Biosyst. Eng. 2014, 118, 95–104. [Google Scholar] [CrossRef]
- Leone, A.; Romaniello, R.; Tamborrino, A.; Xu, X.Q.; Juliano, P. Microwave and megasonics combined technology for a continuous olive oil process with enhanced extractability. Innov. Food Sci. Emerg. Technol. 2017, 42, 56–63. [Google Scholar] [CrossRef]
- Perone, C.; Romaniello, R.; Leone, A.; Catalano, P.; Tamborrino, A. CFD analysis of a tubular heat exchanger for the conditioning of olive paste. Appl. Sci. 2021, 11, 1858. [Google Scholar] [CrossRef]
- Tamborrino, A.; Taticchi, A.; Romaniello, R.; Perone, C.; Esposto, S.; Leone, A.; Servili, M. Assessment of the olive oil extraction plant layout implementing a high-power ultrasound machine. Ultrason. Sonochemistry 2021, 73, 105505. [Google Scholar] [CrossRef]
- Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Official Journal of the European Union L 284/1 4.11. 2022. Available online: http://data.europa.eu/eli/reg_del/2022/2104/oj (accessed on 23 August 2024).
- Amirante, P.; Clodoveo, M.L.; Tamborrino, A.; Leone, A.; Dugo, G. Oxygen concentration control during olive oil extraction process: A New System to emphasize the organoleptic and healthy properties of virgin olive oil. Acta Hortic. 2012, 949, 473–480. [Google Scholar] [CrossRef]
- Masella, P.; Angeloni, G.; Guerrini, L.; Spadi, A.; Corti, F.; Parenti, A. Pumping contribution to dissolved oxygen in virgin olive oil during processing. Chem. Eng. Trans. 2021, 87, 307–312. [Google Scholar]
- Sánchez-Ortiz, A.; Romero, C.; Pérez, A.G.; Sanz, C. Oxygen concentration affects volatile compound biosynthesis during virgin olive oil production. J. Agric. Food Chem. 2008, 56, 4681–4685. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Bejaoui, M.A.; Herrera, M.P.A.; Márquez, A.J.; Maza, G.B. Application of oxygen during olive fruit crushing impacts on the characteristics and sensory profile of the virgin olive oil. Eur. J. Lipid Sci. Technol. 2016, 118, 1018–1029. [Google Scholar] [CrossRef]
- Raffo, A.; Bucci, R.; D’Aloise, A.; Pastore, G. Combined effects of reduced malaxation oxygen levels and storage time on extra-virgin olive oil volatiles investigated by a novel chemometric approach. Food Chem. 2015, 182, 257–267. [Google Scholar] [CrossRef]
- Veneziani, G.; García-González, D.L.; Esposto, S.; Nucciarelli, D.; Taticchi, A.; Boudebouz, A.; Servili, M. Effect of Controlled Oxygen Supply during Crushing on Volatile and Phenol Compounds and Sensory Characteristics in Coratina and Ogliarola Virgin Olive Oils. Foods 2023, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.P.; Aust, S.D. On the Mechanism of Peroxidase-Catalized Oxygen Production. Arch. Biochem. Biophys. 1993, 303, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Taticchi, A.; Esposto, S.; Urbani, S.; Selvaggini, R.; Montedoro, G. Influence of the decrease in oxygen during malaxation of olive paste on the composition of volatiles and phenolic compounds in virgin olive oil. J. Agric. Food Chem. 2008, 56, 10048–10055. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Romero-Segura, C.; Sanz, C.; Sánchez-Ortiz, A.; Pérez, A.G. Role of polyphenol oxidase and peroxidase in shaping the phenolic profile of virgin olive oil. Food Res. Int. 2011, 44, 629–635. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Cecchini, M.; Massantini, R.; Monarca, D. Extra Virgin Olive Oil from Destoned Fruits to Improve the Quality of the Oil and Environmental Sustainability. Foods 2022, 11, 1479. [Google Scholar] [CrossRef]
- Restuccia, D.; Clodoveo, M.L.; Corbo, F.; Loizzo, M.R. De-stoning technology for improving olive oil nutritional and sensory features: The right idea at the wrong time. Food Res. Int. 2018, 106, 636–646. [Google Scholar] [CrossRef]
- Servili, M.; Taticchi, A.; Esposto, S.; Urbani, S.; Selvaggini, R.; Montedoro, G.F. Effect of olive stoning on the volatile and phenolic composition of virgin olive oil. J. Agric. Food Chem. 2007, 55, 7028–7035. [Google Scholar] [CrossRef]
- Manganiello, R.; Pagano, M.; Nucciarelli, D.; Ciccoritti, R.; Tomasone, R.; di Serio, M.G.; Giansante, L.; Del Re, P.; Servili, M.; Veneziani, G. Effects of ultrasound technology on the qualitative properties of Italian extra virgin olive oil. Foods 2021, 10, 2884. [Google Scholar] [CrossRef]
- Vetter, G.; Wirth, W. Understand progressive cavity pumps characteristics and avoid abrasive wear. In Proceedings of the Twelfth International Pump User Symposium, Houston, TX, USA, 14–16 March 1995. [Google Scholar]
- Zheng, L.; Wu, X.; Zhao, R.; Li, H.; Liu, M. Study on performance of progressing cavity pumps (PCPs) in different fit modes. Int. J. Comput. Exp. Sci. Eng. 2018, 4, 25–29. [Google Scholar] [CrossRef]
- Bartocci, P.; D’Amico, M.; Moriconi, N.; Bidini, G.; Fantozzi, F. Pyrolysis of olive stone for energy purposes. Energy Procedia 2015, 82, 374–380. [Google Scholar] [CrossRef]
- Calvano, C.D.; Tamborrino, A. Valorization of Olive By-Products: Innovative Strategies for Their Production, Treatment and Characterization. Foods 2022, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Gul, E.; Al Bkoor Alrawashdeh, K.; Masek, O.; Skreiberg, Ø.; Corona, A.; Zampilli, M.; Wang, L.; Samaras, P.; Yang, Q.; Zhou, H.; et al. Production and use of biochar from lignin and lignin-rich residues (such as digestate and olive stones) for wastewater treatment. J. Anal. Appl. Pyrolysis 2021, 158, 105263. [Google Scholar] [CrossRef]
- Tamborrino, A.; Servili, M.; Leone, A.; Romaniello, R.; Perone, C.; Veneziani, G. Partial De-Stoning of Olive Paste to Increase Olive Oil Quality, Yield, and Sustainability of the Olive Oil Extraction Process. Eur. J. Lipid Sci. Technol. 2020, 122, 2000129. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Olive (Olea europaea L.) Seeds, From Chemistry to Health Benefits. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 847–853. [Google Scholar]
- Sadeghi, H.; Teimouri Yansari, A.; Ansari-Pirsarai, Z. Effects of Different Olive Cake by Products on Dry Matter Intake, Nutrient Digestibility and Performance of Zel Sheep. Int. J. Agric. Biol. 2013, 11, 39–43. [Google Scholar]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Australas. J. Anim. Sci. 2013, 26, 971–980. [Google Scholar] [CrossRef]
- Ortega-García, F.; Balnco, S.; Ángeles Peinado, M.; Peragón, J. Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. ‘Picual’ trees during fruit ripening. Tree Physiol. 2008, 28, 45–54. [Google Scholar] [CrossRef]
- Servili, M.; Taticchi, A.; Esposto, S.; Sordini, B.; Urbani, S. Technological Aspects of Olive Oil Production. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.F.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Gambacorta, G.; Faccia, M.; Previtali, M.A.; Pati, S.; Notte, E.L.; Baiano, A. Effects of olive maturation and stoning on quality indices and antioxidant content of extra virgin oils (cv. Coratina) during storage. J. Food Sci. 2010, 75, C229–C235. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The influence of the malaxation temperature on the activity of polyphenoloxidase and peroxidase and on the phenolic composition of virgin olive oil. Food Chem. 2013, 136, 975–983. [Google Scholar] [CrossRef]
- Amirante, P.; Clodoveo, M.L.; Dugo, G.; Leone, A.; Tamborrino, A. Advance technology in virgin olive oil production from traditional and de-stoned pastes: Influence of the introduction of a heat exchanger on oil quality. Food Chem. 2006, 98, 797–805. [Google Scholar] [CrossRef]
- Pérez, M.; López-yerena, A.; Lozano-castellón, J.; Olmo-cunillera, A.; Lamuela-raventós, R.M.; Martin-belloso, O.; Vallverdú-queralt, A. Impact of novel technologies on virgin olive oil processing, consumer acceptance, and the valorization of olive mill wastes. Antioxidants 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Selvaggini, R.; Esposto, S.; Taticchi, A.; Urbani, S.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Optimization of the temperature and oxygen concentration conditions in the malaxation during the oil mechanical extraction process of four Italian olive cultivars. J. Agric. Food Chem. 2014, 62, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, G.; Uceda, M.; Jiménez, A.; Aguilera, M.P. Olive oil extractability index as a parameter for olive cultivar characterisation. J. Sci. Food Agric. 2003, 83, 503–506. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Minnocci, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Sebastiani, L.; Servili, M. High vacuum-assisted extraction affects virgin olive oil quality: Impact on phenolic and volatile compounds. Food Chem. 2021, 342, 128369. [Google Scholar] [CrossRef]
- Selvaggini, R.; Servili, M.; Urbani, S.; Esposto, S.; Taticchi, A.; Montedoro, G.F. Evaluation of phenolic compounds in virgin olive oil by direct injection in high-performance liquid chromatography with fluorometric detection. J. Agric. Food Chem. 2006, 54, 2832–2838. [Google Scholar] [CrossRef]
- Ranalli, A.; Contento, S. Analytical assessment of destoned and organic-destoned extra-virgin olive oil. Eur. Food Res. Technol. 2010, 230, 965–971. [Google Scholar] [CrossRef]
- Ranalli, F.; Ranalli, A.; Contento, S.; Casanovas, M.; Antonucci, M.; Di Simone, G. Concentrations of Bioactives and Functional Factors in Destoned Virgin Olive Oil: The Case Study of the Oil from Olivastra di Seggiano Cultivar. J. Pharm. Nutr. Sci. 2012, 2, 83–93. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Dminić, I.; Kosic, U.; Majetić, V.; Godena, S.; Valenčič, V. Dynamics of oil quality parameters changes related to olive fruit fly attack. Eur. J. Lipid Sci. Technol. 2010, 112, 1033–1040. [Google Scholar] [CrossRef]
- Nokthai, P.; Lee, V.S.; Shank, L. Molecular modeling of peroxidase and polyphenol Oxidase: Substrate specificity and active site comparison. Int. J. Mol. Sci. 2010, 11, 3266–3276. [Google Scholar] [CrossRef]
- Notario, A.; Sánchez, R.; Luaces, P.; Sanz, C.; Pérez, A.G. The Infestation of Olive Fruits by Bactrocera oleae (Rossi) Modifies the Expression of Key Genes in the Biosynthesis of Volatile and Phenolic Compounds and Alters the Composition of Virgin Olive Oil. Molecules 2022, 27, 1650. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Improvements in the malaxation process to enhance the aroma quality of extra virgin olive oils. Food Chem. 2014, 158, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Cultivar influence on the volatile components of olive oil formed in the lipoxygenase pathway. LWT-Food Sci. Technol. 2021, 147, 111485. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Calderón-Santiago, M.; Priego-Capote, F. Metabolic patterns in the lipoxygenase pathway associated to fruitiness attributes of extra virgin olive oil. J. Food Compos. Anal. 2022, 109, 104478. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin olive oil volatile compounds: Composition, sensory characteristics, analytical approaches, quality control, and authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef]
- Kotti, F.; Jaziri, K.; Arab, F.; Mater, Y.; Sifi, S.; Fares, N.; Hammami, M.; Gargouri, M. Lipoxygenase: Optimization of extraction and evaluation of its contribution to virgin olive oil aroma. Food Biotechnol. 2010, 24, 95–105. [Google Scholar] [CrossRef]
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Bubola, K.B. Inter-varietal diversity of typical volatile and phenolic profiles of Croatian extra virgin olive oils as revealed by GC-IT-MS and UPLC-DAD analysis. Foods 2019, 8, 565. [Google Scholar] [CrossRef]
- Cecchi, L.; Parenti, A.; Bellumori, M.; Migliorini, M.; Mulinacci, N.; Guerrini, L. Clustering Monovarietal Extra Virgin Olive Oil According to Sensory Profile, Volatile Compounds, and k-Mean Algorithm. Eur. J. Lipid Sci. Technol. 2022, 124, 2200038. [Google Scholar] [CrossRef]
- Olías, J.M.; Pérez, A.G.; Ríos, J.J.; Sanz, L.C. Aroma of Virgin Olive Oil: Biogenesis of the “Green” Odor Notes. J. Agric. Food Chem. 1993, 41, 2368–2373. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Aroma biogenesis and distribution between olive pulps and seeds with identification of aroma trends among cultivars. Food Chem. 2013, 141, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Mikrou, T.; Litsa, M.; Papantoni, A.; Kapsokefalou, M.; Gardeli, C.; Mallouchos, A. Effect of Cultivar and Geographical Origin on the Volatile Composition of Greek Monovarietal Extra Virgin Olive Oils. Chemosensors 2023, 11, 80. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Bejaoui, M.A.; Quintero-Flores, A.; Jiménez, A.; Beltrán, G. “Biosynthesis of volatile compounds by hydroperoxide lyase enzymatic activity during virgin olive oil extraction process”. Food Res. Int. 2018, 111, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Pouliarekou, E.; Badeka, A.; Tasioula-Margari, M.; Kontakos, S.; Longobardi, F.; Kontominas, M.G. Characterization and classification of Western Greek olive oils according to cultivar and geographical origin based on volatile compounds. J. Chromatogr. A 2011, 1218, 7534–7542. [Google Scholar] [CrossRef]
- Lechhab, T.; Lechhab, W.; Trovato, E.; Salmoun, F.; Mondello, L.; Cacciola, F. Screening of the Volatile Composition of Moroccan Olive Oils by Using SPME/GC-MS-FID over a Two-Year Period: A Pedoclimatic Discrimination. Horticulturae 2022, 8, 925. [Google Scholar] [CrossRef]
- Noordermeer, M.A.; Veldink, G.A.; Vliegenthart, J.F.G. Alfalfa contains substantial 9-hydroperoxide lyase activity and a 3Z:2E-enal isomerase. FEBS Lett. 1999, 443, 201–204. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Gómez-Rico, A.; Salvador, M.D.; Fregapane, G. Influence of malaxation conditions on virgin olive oil yield, overall quality and composition. Eur. Food Res. Technol. 2009, 228, 671–677. [Google Scholar] [CrossRef]
- Di Serio, M.G.; Giansante, L.; di Loreto, G.; di Giacinto, L. Shelf life of extra-virgin olive oils: First efforts toward a prediction model. J. Food Process. Preserv. 2018, 42, e13663. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; O’Keefe, A.J.; Slade, L.; Beauchamp, G.K. Protein suppresses both bitterness and oleocanthal-elicited pungency of extra virgin olive oil. Sci. Rep. 2021, 11, 11851. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of agroclimatic parameters on phenolic and volatile compounds of Chilean virgin olive oils and characterization based on geographical origin, cultivar and ripening stage. J. Sci. Food Agric. 2016, 96, 583–592. [Google Scholar] [CrossRef]
- Serrano, A.; De la Rosa, R.; Sánchez-Ortiz, A.; Cano, J.; Pérez, A.G.; Sanz, C.; Arias-Calderón, R.; Velasco, L.; León, L. Chemical components influencing oxidative stability and sensorial properties of extra virgin olive oil and effect of genotype and location on their expression. LWT-Food Sci. Technol. 2021, 136, 110257. [Google Scholar] [CrossRef]
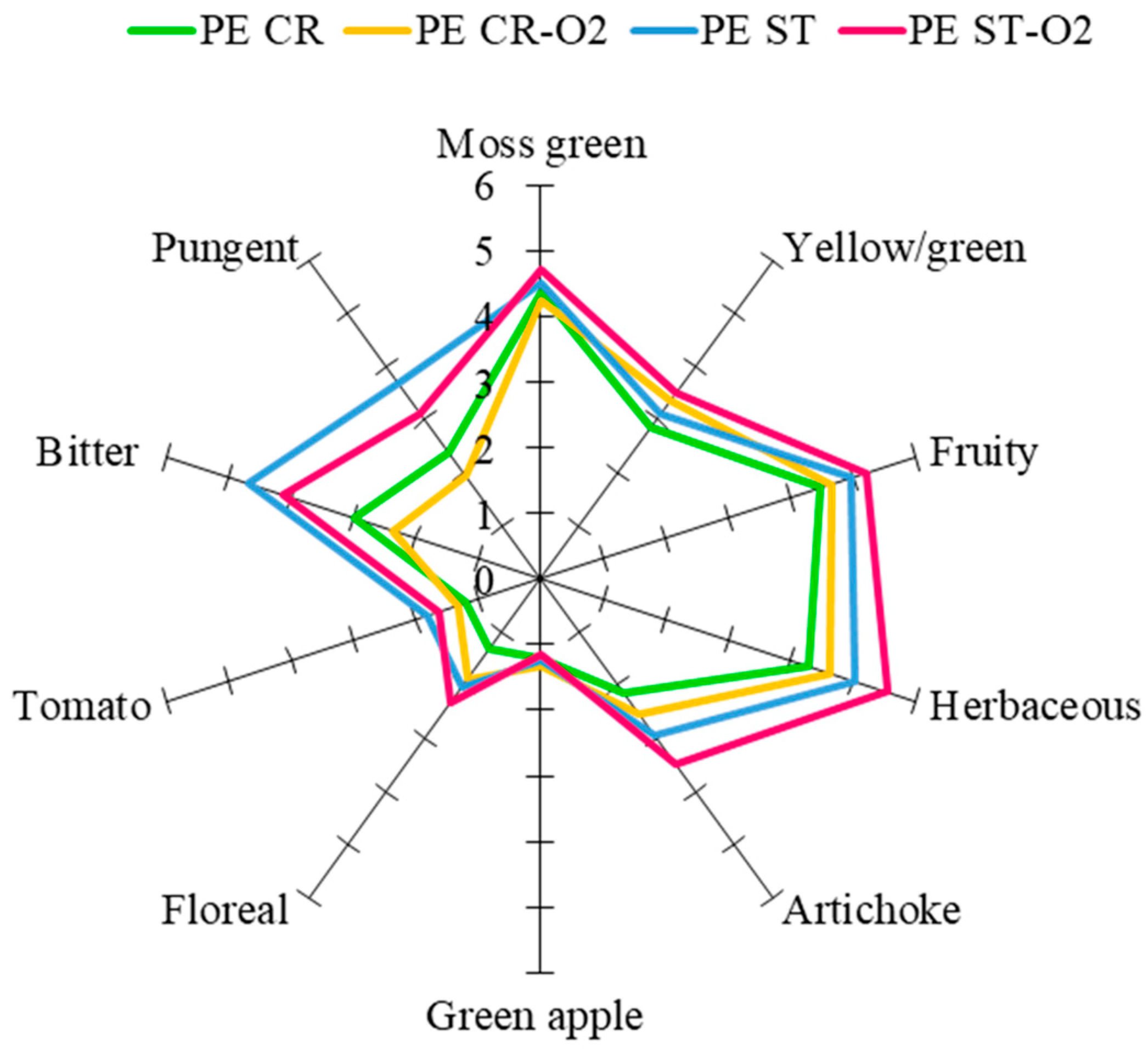
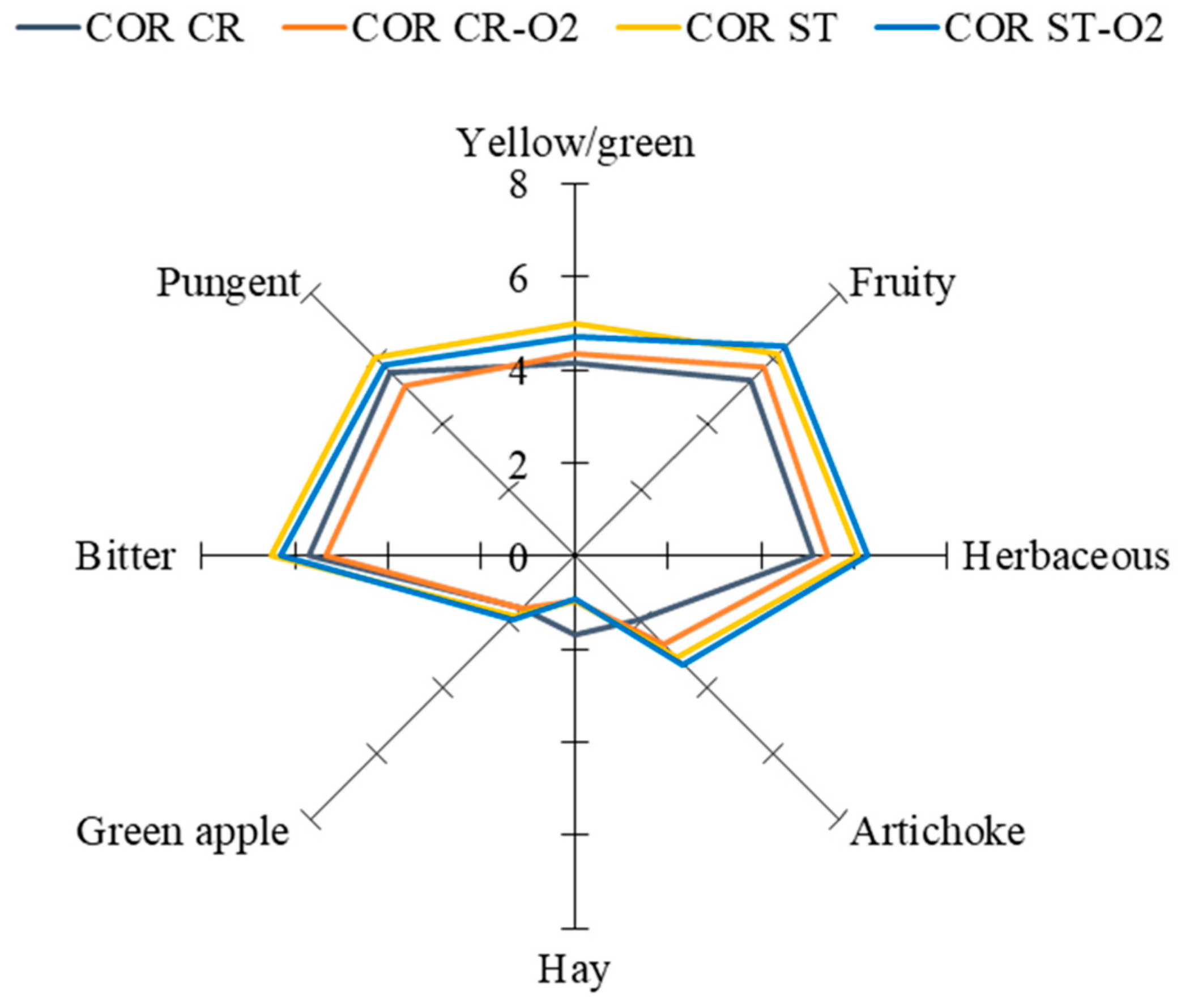
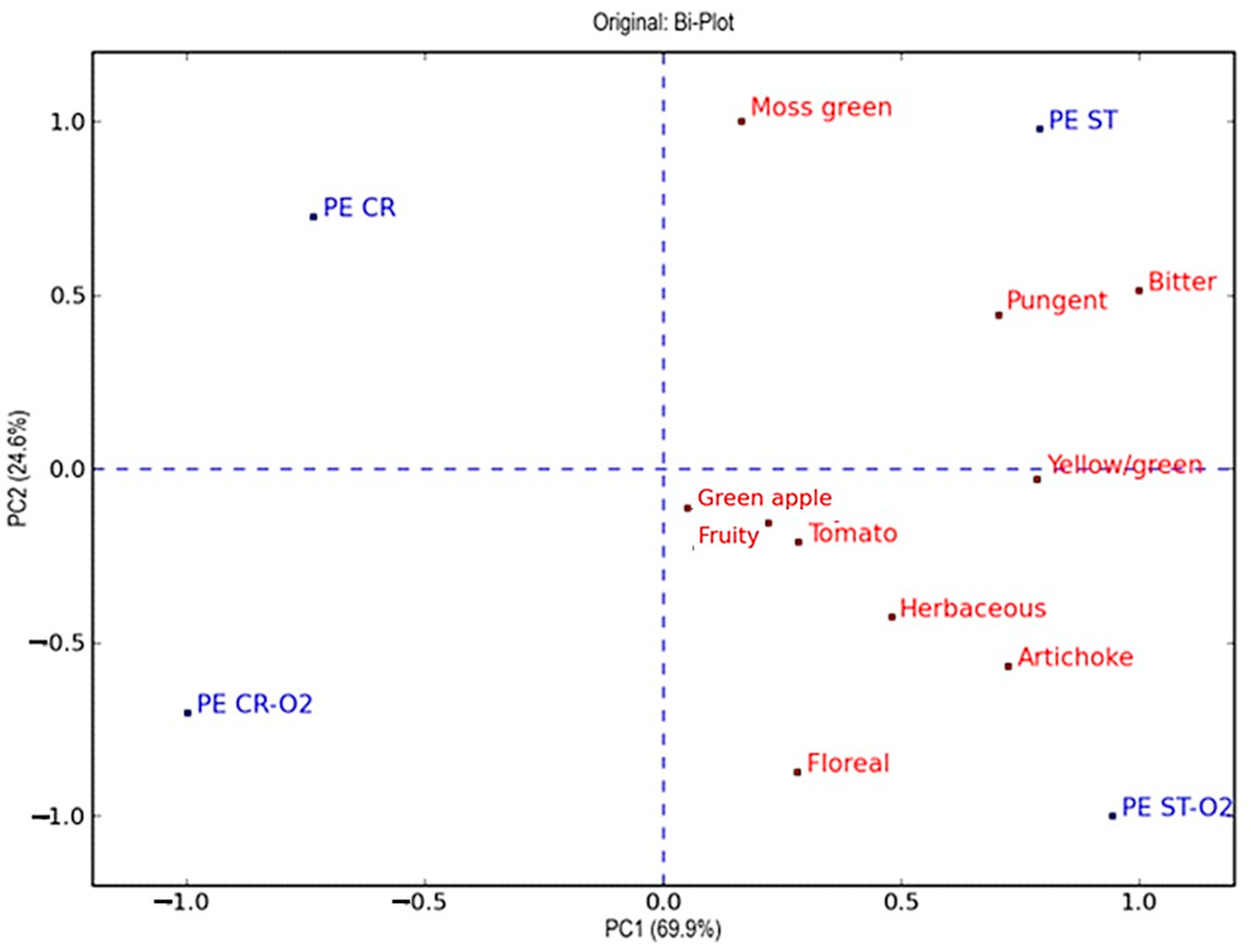

| Parameters | Peranzana | Coratina | ||||||
|---|---|---|---|---|---|---|---|---|
| PE CR | PE CR-O2 | PE ST | PE ST-O2 | COR CR | COR CR-O2 | COR ST | COR ST-O2 | |
| Acidity (% oleic acid) | 0.28 ± 0.01a | 0.28 ± 0.01a | 0.28 ± 0.01a | 0.27 ± 0.01a | 0.23 ± 0.01a | 0.23 ± 0.01a | 0.25 ± 0.01a | 0.24 ± 0.01a |
| Peroxide value (meq O2/kg of oil) | 5.93 ± 0.15a | 6.2 ± 0.3a | 5.93 ± 0.45a | 6.07 ± 0.21a | 3.27 ± 0.46a | 2.7 ± 0.52a | 3.2 ± 0.52a | 2.73 ± 0.12a |
| K232 | 1.74 ± 0.06a | 1.74 ± 0.04a | 1.71 ± 0.01a | 1.73 ± 0.02a | 1.64 ± 0.07a | 1.58 ± 0.07a | 1.54 ± 0.05a | 1.54 ± 0.15a |
| K270 | 0.18 ± 0.02a | 0.18 ± 0.01a | 0.18 ± 0.001a | 0.19 ± 0.01a | 0.16 ± 0.02a | 0.17 ± 0.01a | 0.18 ± 0.01a | 0.18 ± 0.03a |
| Phenolic Compounds | Peranzana | Coratina | ||||||
|---|---|---|---|---|---|---|---|---|
| PE CR | PE CR-O2 | PE ST | PE ST-O2 | COR CR | COR CR-O2 | COR ST | COR ST-O2 | |
| Hydroxytyrosol | 1.0 ± 0.1a | 1.2 ± 0.5a | 1.4 ± 0.8a | 3.3 ± 1.8a | 2.0 ± 0.5ab | 1.6 ± 0a | 2.1 ± 0.4ab | 2.5 ± 0.4b |
| Tyrosol | 1.8 ± 0.3a | 1.8 ± 0.1a | 1.8 ± 0.2a | 2.2 ± 0.3a | 2.4 ± 0.4a | 2.4 ± 0.1a | 2.5 ± 0.5a | 3.2 ± 0.2a |
| Vanillic acid | 0.5 ± 0a | 0.5 ± 0a | 0.6 ± 0.1a | 0.7 ± 0.1a | 0.3 ± 0a | 0.3 ± 0a | 0.3 ± 0a | 0.3 ± 0a |
| Oleacein | 264.2 ± 18.8b | 258.7 ± 20.4b | 395.3 ± 24.4a | 382.7 ± 19.1a | 576.8 ± 26.5bc | 562.2 ± 33.9c | 671.8 ± 37.9a | 645.7 ± 22.7ab |
| Oleocanthal | 49.8 ± 8.4b | 46.3 ± 0.9b | 67.8 ± 14.6ab | 73.1 ± 0.4a | 137.9 ± 11.7a | 130.9 ± 1.6a | 162.2 ± 3.3b | 161.6 ± 6.3b |
| (+)-1-acetoxypinoresinol | 4.7 ± 0.2a | 4.8 ± 0.1a | 5.1 ± 0.5a | 4.6 ± 0.3a | 24.8 ± 1.6a | 24.4 ± 0.9a | 25.1 ± 0.8a | 25.1 ± 1.1a |
| (+)-pinoresinol | 7 ± 0.1a | 6.6 ± 0.1a | 7.3 ± 0.6a | 7.1 ± 0.3a | 10.3 ± 1.8a | 10.4 ± 1.9a | 10.6 ± 0.3a | 10.2 ± 1.3a |
| Oleuropein aglycone | 59 ± 4.2b | 44.4 ± 2.7c | 84.2 ± 3.8a | 83.7 ± 4.9a | 108.6 ± 3.9ab | 106.8 ± 7.7b | 129.8 ± 5.3a | 121.6 ± 13.1ab |
| Ligstroside aglicone | 8.2 ± 1.5b | 6.4 ± 0.3b | 11.7 ± 0.9a | 12.2 ± 0.6a | 20 ± 3.3a | 22.6 ± 4a | 19.1 ± 2a | 20 ± 2.6a |
| Total phenols | 396.1 ± 21.5a | 370.7 ± 20.8a | 575.1 ± 29b | 569.5 ± 20.4b | 883.1 ± 29.8b | 861.6 ± 35.9b | 1023.5 ± 38.8a | 990.2 ± 30.1a |
| Oleuropein derivatives | 324.2 ± 19.3b | 304.3 ± 20.6b | 480.9 ± 24.7a | 469.7 ± 19.8a | 687.4 ± 26.8bc | 670.6 ± 34.7c | 803.8 ± 38.3a | 769.8 ± 26.2ab |
| Ligustroside derivatives | 59.8 ± 8.6b | 54.5 ± 1b | 81.3 ± 14.7a | 87.5 ± 0.8a | 160.3 ± 12.1b | 155.9 ± 4.4b | 183.8 ± 4a | 184.8 ± 6.8a |
| Lignans | 11.7 ± 0.3a | 11.4 ± 0.1a | 12.4 ± 0.7a | 11.7 ± 0.4a | 35.1 ± 2.4a | 34.8 ± 2.1a | 35.7 ± 0.8a | 35.3 ± 1.7a |
| Volatile Compounds | Peranzana | Coratina | ||||||
|---|---|---|---|---|---|---|---|---|
| PE CR | PE CR-O2 | PE ST | PE ST-O2 | COR CR | COR CR-O2 | COR ST | COR ST-O2 | |
| Aldehydes | ||||||||
| Pentanal | n.d. | n.d. | n.d. | n.d. | 15 ± 2a | 11 ± 3a | 14 ± 7a | 10 ± 1a |
| (E)-2-Pentenal | 44 ± 4b | 37 ± 4b | 68 ± 16a | 77 ± 5a | 31 ± 2ab | 23 ± 2b | 47 ± 5a | 40 ± 15ab |
| Hexanal | 1052 ± 58a | 969 ± 42ab | 990 ± 13ab | 912 ± 20b | 1012 ± 85a | 1006 ± 41a | 818 ± 147a | 902 ± 71a |
| (E)-2-Hexenal | 15,358 ± 1111d | 23,319 ± 915c | 28,465 ± 896b | 31,759 ± 1214a | 38,908 ± 2650b | 41,783 ± 1558ab | 44,370 ± 1335a | 45,895 ± 1006a |
| (E,E)-2,4-Hexadienal | 247 ± 8b | 268 ± 6b | 308 ± 9a | 300 ± 16a | 174 ± 7a | 160 ± 9a | 176 ± 13a | 178 ± 12a |
| Ʃ of C5 and C6 aldehydes | 16,701 ± 1113d | 24,592 ± 0c | 29,832 ± 896b | 33,048 ± 1214a | 40,139 ± 2652b | 42,984 ± 1559ab | 45,425 ± 1343a | 47,024 ± 1008a |
| Alcohols | ||||||||
| 1-Pentanol | 24 ± 2a | 24 ± 1a | 23 ± 1a | 24 ± 1a | 12 ± 2a | 6 ± 3b | 8 ± 1ab | 7 ± 1b |
| 1-Penten-3-ol | 282 ± 22a | 232 ± 15b | 130 ± 4c | 104 ± 2c | 240 ± 19a | 217 ± 17a | 116 ± 11b | 155 ± 13b |
| (E)-2-Penten-1-ol | 20 ± 2a | 18 ± 3ab | 13 ± 4b | 15 ± 1ab | 26 ± 3a | 22 ± 2ab | 14 ± 1bc | 12 ± 6c |
| (Z)-2-Penten-1-ol | 240 ± 13a | 201 ± 7b | 199 ± 8b | 169 ± 9c | 242 ± 16a | 239 ± 6a | 173 ± 18ab | 204 ± 32b |
| 1-Hexanol | 188 ± 14a | 188 ± 12a | 155 ± 2b | 153 ± 5b | 189 ± 13b | 164 ± 11b | 221 ± 8a | 186 ± 14b |
| (E)-2-Hexen-1-ol | 126 ± 8a | 118 ± 8a | 79 ± 21b | 103 ± 8ab | 477 ± 52a | 475 ± 35a | 493 ± 29a | 460 ± 20a |
| (Z)-3-Hexen-1-ol | 210 ± 16a | 165 ± 10b | 119 ± 14c | 115 ± 12c | 159 ± 17a | 119 ± 8ab | 82 ± 5bc | 72 ± 29c |
| Ʃ of C5 and C6 alcohols | 1091 ± 35a | 947 ± 0b | 717 ± 27c | 683 ± 18c | 1345 ± 62a | 1242 ± 42a | 1106 ± 37b | 1095 ± 52b |
| Esters | ||||||||
| Hexyl acetate | 180 ± 8c | 229 ± 16b | 307 ± 18a | 305 ± 19a | 57 ± 3a | 57 ± 4a | 59 ± 4a | 57 ± 4a |
| (Z)-3-Hexenyl acetate | 191 ± 14b | 213 ± 6b | 248 ± 9a | 244 ± 11a | 14 ± 0a | 13 ± 2a | 14 ± 1a | 15 ± 3a |
| Ʃ of esters at C6 | 371 ± 16c | 4421 ± 0b | 556 ± 20a | 549 ± 22a | 71 ± 3a | 70 ± 4a | 74 ± 5a | 72 ± 6a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nucciarelli, D.; García-González, D.L.; Veneziani, G.; Urbani, S.; Daidone, L.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Servili, M. Extra Virgin Olive Oil from Stoned Olives with Oxygen Supply during Processing: Impact on Volatile and Phenolic Fraction and Sensory Characteristics. Foods 2024, 13, 3073. https://doi.org/10.3390/foods13193073
Nucciarelli D, García-González DL, Veneziani G, Urbani S, Daidone L, Esposto S, Taticchi A, Selvaggini R, Servili M. Extra Virgin Olive Oil from Stoned Olives with Oxygen Supply during Processing: Impact on Volatile and Phenolic Fraction and Sensory Characteristics. Foods. 2024; 13(19):3073. https://doi.org/10.3390/foods13193073
Chicago/Turabian StyleNucciarelli, Davide, Diego L. García-González, Gianluca Veneziani, Stefania Urbani, Luigi Daidone, Sonia Esposto, Agnese Taticchi, Roberto Selvaggini, and Maurizio Servili. 2024. "Extra Virgin Olive Oil from Stoned Olives with Oxygen Supply during Processing: Impact on Volatile and Phenolic Fraction and Sensory Characteristics" Foods 13, no. 19: 3073. https://doi.org/10.3390/foods13193073
APA StyleNucciarelli, D., García-González, D. L., Veneziani, G., Urbani, S., Daidone, L., Esposto, S., Taticchi, A., Selvaggini, R., & Servili, M. (2024). Extra Virgin Olive Oil from Stoned Olives with Oxygen Supply during Processing: Impact on Volatile and Phenolic Fraction and Sensory Characteristics. Foods, 13(19), 3073. https://doi.org/10.3390/foods13193073






