Quality-Driven Design of Pandan-Flavored Sponge Cake: Unraveling the Role of Thermal Processing on Typical Pandan Aroma
Abstract
1. Introduction
2. Materials and Methods
2.1. Pandan Leaf Juice Preparation
2.2. Pandan-Flavored Cake Elaboration
2.3. Specific Gravity of the Cake Batter
2.4. Colorimetric Measurement
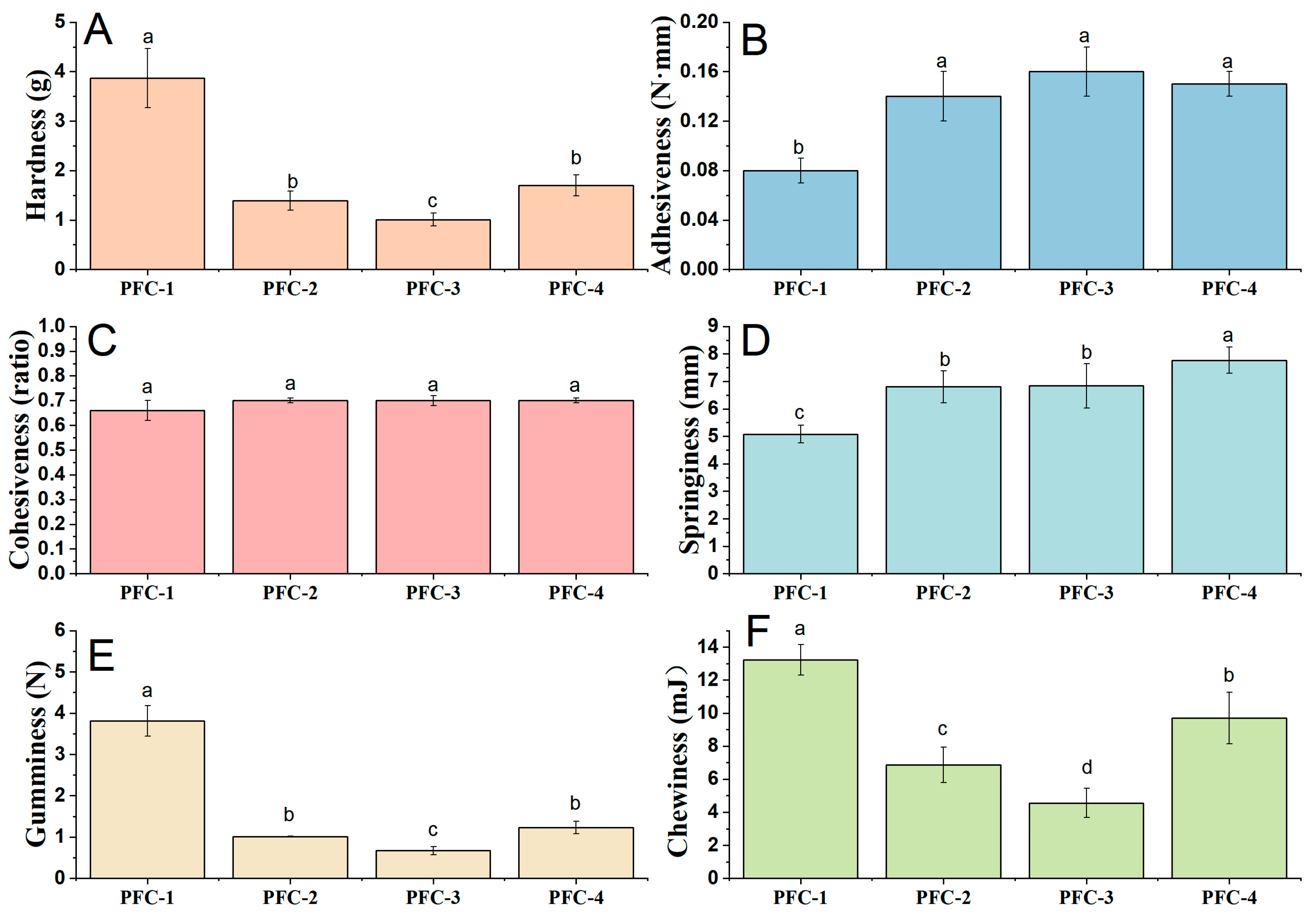
2.5. Texture Evaluation
2.6. Effects of Thermal Treatments on Volatile Compounds of Pandan Leaf Juices
2.6.1. Electronic Nose (E-Nose) Analysis
2.6.2. Gas Chromatography-Ion Mobility Spectrometry (GC-IMS) Analysis
2.6.3. Gas Chromatography-Olfactometry-Mass Spectrometry (GC-O-MS) Analysis
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of Thermal Processing on Typical Pandan Aroma
3.1.1. Comparison of Aroma-Active Compounds in Fresh and Thermal-Treated Pandan Leaf Juice
3.1.2. E-Nose Analysis of Volatile Compounds in Thermal-Treated Pandan Leaf Juices
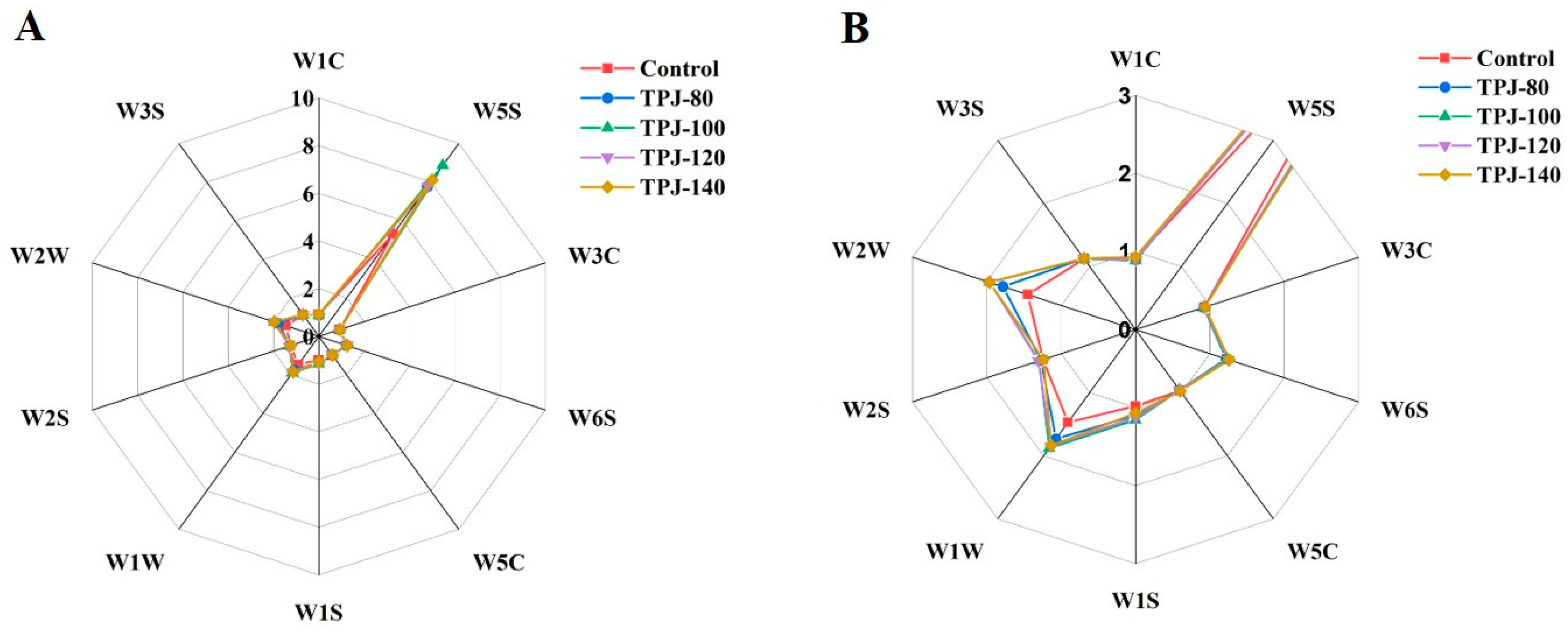
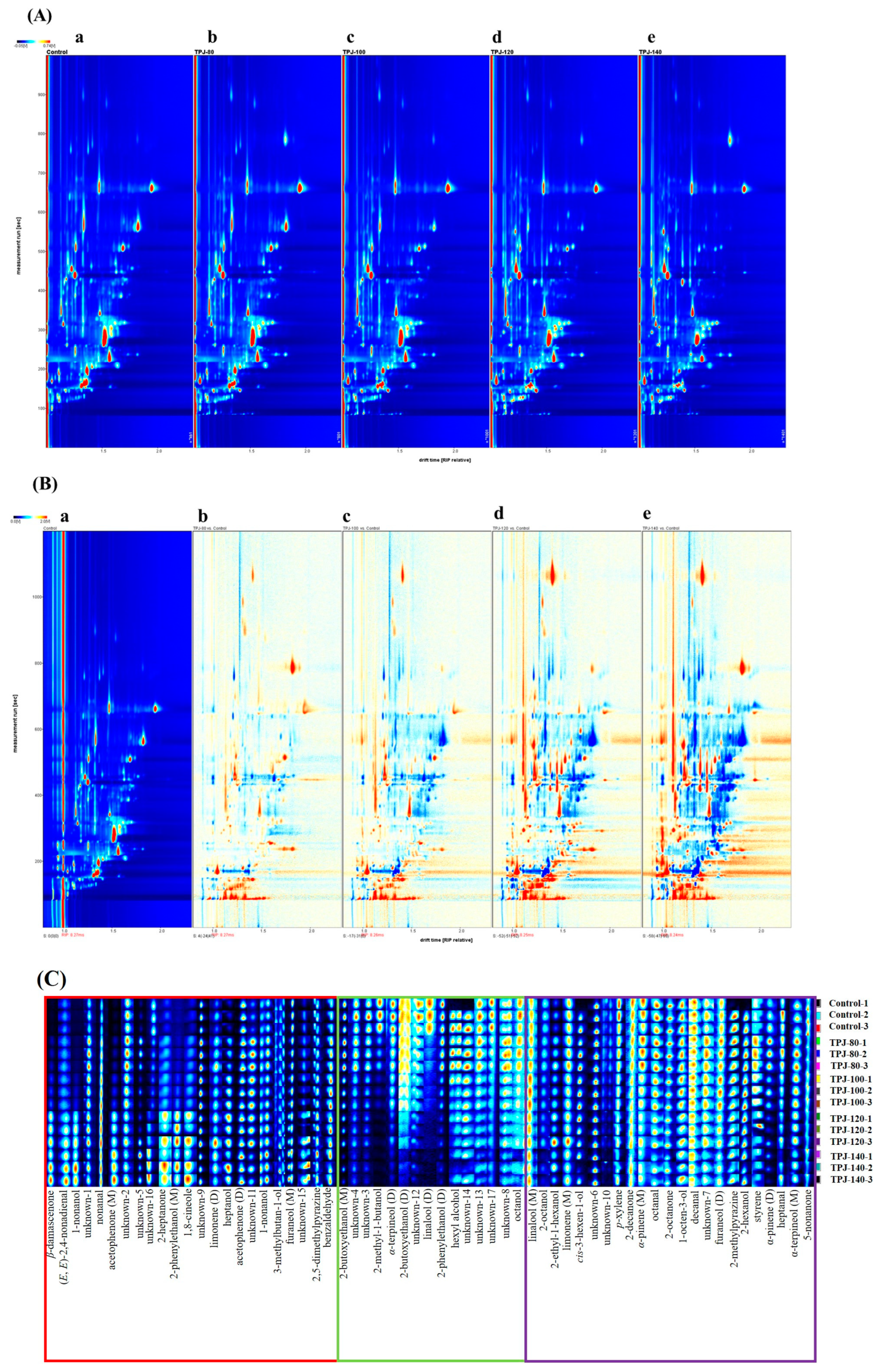
3.1.3. GC-IMS Analysis of Volatiles in Pandan Leaf Juices upon Different Degrees of Thermal Treatments
3.2. Effect of Pandan Leaf Juice on the Physical Properties of Batter and Cake
3.3. The Role of Thermal Processing on Typical Pandan-Featured Aroma in Sponge Cake
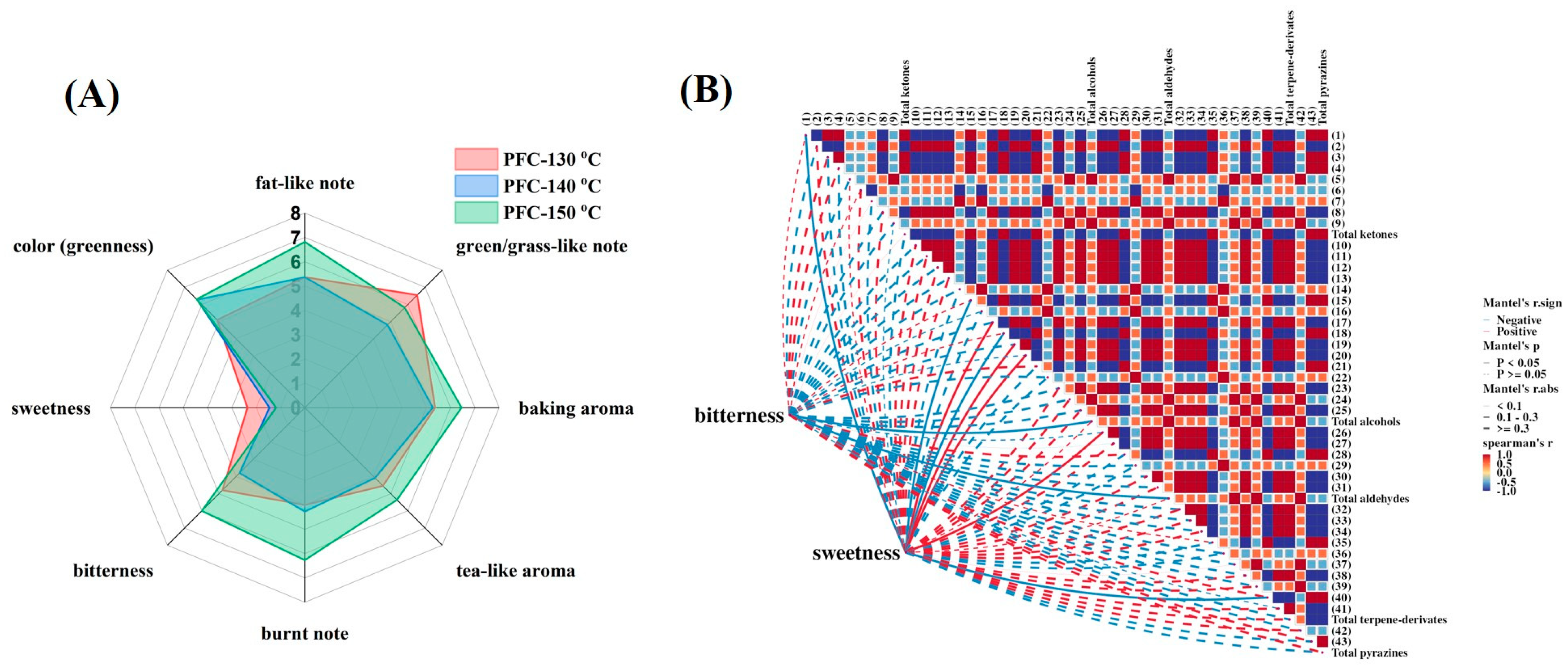
3.3.1. Sensory Evaluation of Pandan-Flavored Cakes Prepared under Various Temperatures
3.3.2. The Possible Contributions of Pandan Volatile Compounds to the Sweet and Bitter Tastes of Sponge Cake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nor, F.M.; Mohamed, S.; Idris, N.A.; Ismail, R. Antioxidative properties of Pandanus amaryllifolius leaf extracts in accelerated oxidation and deep frying studies. Food Chem. 2008, 110, 319–327. [Google Scholar] [CrossRef]
- Bhuyan, B.; Sonowal, R. An overview of Pandanus amaryllifolius Roxb. exLindl. and its potential impact on health. Curr. Trends Pharm. Res. 2021, 8, 138–157. [Google Scholar]
- Ooi, L.S.; Sun, S.S.; Ooi, V.E. Purification and characterization of a new antiviral protein from the leaves of Pandanus amaryllifolius (Pandanaceae). Int. J. Biochem. Cell Biol. 2004, 36, 1440–1446. [Google Scholar] [CrossRef]
- Pawin, B.; Norkaew, O.; Sookwong, P.; Puangsombat, P.; Mahatheeranont, S. Determination of 2-acetyl-1-pyrroline in alginate encapsulated pandanus flavorings by static headspace (SHS) and gas chromatography with nitrogen–phosphorus detection (GC-NPD). Anal. Lett. 2021, 54, 2327–2346. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Supercritical carbon dioxide extraction of 2-acetyl-1-pyrroline and volatile components from pandan leaves. Flavour Fragr. J. 2004, 19, 251–259. [Google Scholar] [CrossRef]
- Wakte, K.V.; Thengane, R.J.; Jawali, N.; Nadaf, A.B. Optimization of HS-SPME conditions for quantification of 2-acetyl-1-pyrroline and study of other volatiles in Pandanus amaryllifolius Roxb. Food Chem. 2010, 121, 595–600. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Kshirsagar, A.; Singhal, R.S. Supercritical carbon dioxide extraction of 2-acetyl-1-pyrroline from Pandanus amaryllifolius Roxb. Food Chem. 2005, 91, 255–259. [Google Scholar] [CrossRef]
- Ningrum, A.; Schreiner, M. Pandan leaves: “Vanilla of the East”. Agro. Food Ind. Hi Tech. 2014, 25, 56–60. [Google Scholar]
- Gurmeet, S.; Amrita, P. Unique pandanus-flavour, food and medicine. J. Pharmacogn. Phytochem. 2016, 5, 08–14. [Google Scholar]
- Minh, N.P.; Vo, T.T.; Phong, T.D.; Van Toan, N.; Nam, V.H. Green pigment extraction from pandan (Pandanus amaryllifolius) and its application in food industry. J. Pharm. Sci. Res. 2019, 11, 925–929. [Google Scholar]
- Papageorgiou, M.; Paraskevopoulou, A.; Pantazi, F.; Skendi, A. Cake perception, texture and aroma profile as affected by wheat flour and cocoa replacement with carob flour. Foods 2020, 9, 1586. [Google Scholar] [CrossRef]
- Huang, M.; Yang, H. Eucheuma powder as a partial flour replacement and its effect on the properties of sponge cake. LWT—Food Sci. Technol. 2019, 110, 262–268. [Google Scholar] [CrossRef]
- Kazanci, M.; Guner, K.G.; Durakli Velioglu, S. The effect of the use of pekmez and honey as sugar substitutes on the quality characteristics and the acrylamide content of sponge cakes and cookies. J. Food Meas. Charact. 2024, 18, 1392–1411. [Google Scholar] [CrossRef]
- Rodríguez-García, J.; Salvador, A.; Hernando, I. Replacing fat and sugar with inulin in cakes: Bubble size distribution, physical and sensory properties. Food Bioprocess Technol. 2014, 7, 964–974. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Lan, W.; Sun, H.; Chen, K.; Zhao, L. Maillard reaction products of hydrolyzed Copyinds comatus protein with various sugar-amino acid combinations: Focusing on their meaty aroma notes and salt reduction abilities. Food Biosci. 2024, 61, 104503. [Google Scholar] [CrossRef]
- Chen, X.; Kilmartin, P.A.; Fedrizzi, B.; Quek, S.Y. Elucidation of endogenous aroma compounds in tamarillo (Solanum betaceum) using a molecular sensory approach. J. Agric. Food Chem. 2021, 69, 9362–9375. [Google Scholar] [CrossRef]
- Charles, A.P.R.; Gu, Z.; Archer, R.; Auwarter, C.; Hatterman-Valenti, H.; Rao, J.; Chen, B. Effect of high-tunnel and open-field production on the yield, cannabinoids, and volatile profiles in industrial hemp (Cannabis sativa L.) inflorescence. J. Agric. Food Chem. 2024, 72, 12975–12987. [Google Scholar] [CrossRef]
- Gu, Z.; Jin, Z.; Schwarz, P.; Rao, J.; Chen, B. Uncovering aroma boundary compositions of barley malts by untargeted and targeted flavoromics with HS-SPME-GC-MS/olfactometry. Food Chem. 2022, 394, 133541. [Google Scholar] [CrossRef]
- Chen, X.; Gu, Z.; Peng, Y.; Quek, S.Y. What happens to commercial camembert cheese under packaging? Unveiling biochemical changes by untargeted and targeted metabolomic approaches. Food Chem. 2022, 383, 132437. [Google Scholar] [CrossRef]
- ISO 8586:2023; Sensory Analysis—Selection and Training of Sensory Assessors. ISO: Geneva, Switzerland, 2023.
- ISO 5496:2006; Methodology—Initiation and Training of Assessors in the Detection and Recognition of Odours. ISO: Geneva, Switzerland, 2023.
- Azhar, A.N.H.; Amran, N.A.; Yusup, S.; Mohd Yusoff, M.H. Ultrasonic extraction of 2-acetyl-1-pyrroline (2AP) from Pandanus amaryllifolius Roxb. using ethanol as solvent. Molecules 2022, 27, 4906. [Google Scholar] [CrossRef]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Rayaguru, K. Chemical constituents and post-harvest prospects of Pandanus amaryllifolius leaves: A review. Food Rev. Int. 2010, 26, 230–245. [Google Scholar] [CrossRef]
- Peng, Q.; Li, S.; Zheng, H.; Meng, K.; Jiang, X.; Shen, R.; Xue, J.; Xie, G. Characterization of different grades of Jiuqu hongmei tea based on flavor profiles using HS-SPME-GC-MS combined with E-nose and E-tongue. Food Res. Int. 2023, 172, 113198. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, L.; Tu, K. Aroma in freshly squeezed strawberry juice during cold storage detected by E-nose, HS–SPME–GC–MS and GC-IMS. J. Food Meas. Charact. 2023, 17, 3309–3322. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhu, Y.; Ben, A.; Qi, J. A novel strategy for discriminating different cultivation and screening odor and taste flavor compounds in Xinhui tangerine peel using E-nose, E-tongue, and chemometrics. Food Chem. 2022, 384, 132519. [Google Scholar] [CrossRef]
- Wakte, K.V.; Zanan, R.L.; Thengane, R.J.; Jawali, N.; Nadaf, A.B. Identification of elite population of Pandanus amaryllifolius Roxb. for higher 2-acetyl-1-pyrroline and other volatile contents by HS-SPME/GC-FID from Peninsular India. Food Anal. Methods 2012, 5, 1276–1288. [Google Scholar] [CrossRef]
- Wang, D.; Deng, Y.; Chen, X.; Wang, K.; Zhao, L.; Wang, Z.; Liu, X.; Hu, Z. Elucidating the effects of Lactobacillus plantarum fermentation on the aroma profiles of pasteurized litchi juice using multi-scale molecular sensory science. Curr. Res. Food Sci. 2023, 6, 100481. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; del Mar Dobao-Prieto, M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC–IMS to assign the quality of virgin olive oil. Food Chem. 2015, 187, 572–579. [Google Scholar]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar]
- Yao, W.; Ma, S.; Wu, H.; Liu, D.; Liu, J.; Zhang, M. Flavor profile analysis of grilled lamb seasoned with classic salt, chili pepper, and cumin (Cuminum cyminum) through HS-SPME-GC-MS, HS-GC-IMS, E-nose techniques, and sensory evaluation on Sonit sheep. Food Chem. 2024, 454, 139514. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Formation of damascenone and its related compounds from carotenoids in tea. Tea Health Dis. Prev. 2013, 31, 375–386. [Google Scholar]
- Díaz-Ramírez, M.; Calderón-Domínguez, G.; García-Garibay, M.; Jiménez-Guzmán, J.; Villanueva-Carvajal, A.; de la Paz Salgado-Cruz, M.; Arizmendi-Cotero, D.; Del Moral-Ramírez, E. Effect of whey protein isolate addition on physical, structural and sensory properties of sponge cake. Food Hydrocoll. 2016, 61, 633–639. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Anagnostara, I.; Bezati, G.; Rizou, T.; Pavlidou, E.; Vouvoudi, E.; Kiosseoglou, V. Water extraction residue from maize milling by-product as a potential functional ingredient for the enrichment with fibre of cakes. LWT-Food Sci. Technol. 2020, 129, 109604. [Google Scholar] [CrossRef]
- Lu, T.M.; Lee, C.C.; Mau, J.L.; Lin, S.D. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010, 119, 1090–1095. [Google Scholar] [CrossRef]
- Lee, J.; Roux, S.; Le Roux, E.; Keller, S.; Rega, B.; Bonazzi, C. Unravelling caramelization and Maillard reactions in glucose and glucose+ leucine model cakes: Formation and degradation kinetics of precursors, α-dicarbonyl intermediates and furanic compounds during baking. Food Chem. 2022, 376, 131917. [Google Scholar] [CrossRef]
- Wang, W.; Ren, Z.; Zheng, S.; Wu, H.; Li, P.; Peng, W.; Su, W.; Wang, Y. Botany, Phytochemistry, pharmacology, and applications of Pandanus amaryllifolius Roxb.: A review. Fitoterapia 2024, 177, 106144. [Google Scholar] [CrossRef]
- Spence, C. Multisensory flavor perception. Cell 2015, 161, 24–35. [Google Scholar] [CrossRef]
- Stuckey, B. Taste What You’re Missing: The Passionate Eater’s Guide to Why Good Food Tastes Good: Simon and Schuster; Simon and Schuster: New York, NY, USA, 2012. [Google Scholar]
- Chen, Y.P.; Ding, Z.; Yu, Y.; He, P.; Zhou, Y.; Liu, Y.; Feng, X. Recent advances in investigating odor-taste interactions: Psychophysics, neuroscience, and microfluidic techniques. Trends Food Sci. Technol. 2023, 138, 500–510. [Google Scholar] [CrossRef]
- Liu, J.; Wan, P.; Xie, C.; Chen, D.W. Key aroma-active compounds in brown sugar and their influence on sweetness. Food Chem. 2021, 345, 128826. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Chen, H.; Niu, Y.; Zhu, J. Characterization of the aroma-active compounds in banana (Musa AAA Red green) and their contributions to the enhancement of sweetness perception. J. Agric. Food Chem. 2021, 69, 15301–15313. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Campo, E.; Fernández-Zurbano, P.; Valentin, D.; Ferreira, V. An assessment of the effects of wine volatiles on the perception of taste and astringency in wine. Food Chem. 2010, 121, 1139–1149. [Google Scholar] [CrossRef]
- Oladokun, O.; Tarrega, A.; James, S.; Cowley, T.; Dehrmann, F.; Smart, K.; Cook, D.; Hort, J. Modification of perceived beer bitterness intensity, character and temporal profile by hop aroma extract. Food Res. Int. 2016, 86, 104–111. [Google Scholar] [CrossRef]
- Caporale, G.; Policastro, S.; Monteleone, E. Bitterness enhancement induced by cut grass odorant (cis-3-hexen-1-ol) in a model olive oil. Food Qual. Prefer. 2004, 15, 219–227. [Google Scholar] [CrossRef]
| Ingredient | Manufacture | Control a | PFC-1 | PFC-2 | PFC-3 | PFC-4 |
|---|---|---|---|---|---|---|
| pandan leaf juice | — | 0 | 8.4 | 11.2 | 14 | 16.8 |
| whole egg (g) (egg yolk and egg white were then separated for usage) | Hangzhou Qiandao Lake Zhikang Agricultural Development Co., Ltd., Hangzhou, China | 43 | 43 | 43 | 43 | 43 |
| corn starch (g) | Yantai Shuangta Foods Co., Ltd., Yantai, China | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| low gluten flour (g) | Shandong Fushikang Noodle Group Co., Ltd., Feicheng, China | 12 | 12 | 12 | 12 | 12 |
| corn oil (g) | Golden Sun Cereals and Oils Co., Ltd., Nantong, China | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 |
| butter (g) | Fonterra Trading Co., Ltd., Shanghai, China | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 |
| sodium chloride (g) | Jiangsu Salt Industry Group Co., Ltd., Nanjing, China | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| sugar | Shanghai Delfu Sugar Co., Ltd., Shanghai, China | 9 | 9 | 9 | 9 | 9 |
| No. | CAS | Compound | Odor Description | Odor Intensity | |
|---|---|---|---|---|---|
| Control | TPJ-130 a | ||||
| 1 | 928-96-1 | leaf alcohol | green, grassy | 3 | 4 |
| 2 | 85213-22-5 | 2AP | popcorn-, steamed rice-like | 4 | 4 |
| 3 | 122-78-1 | benzeneacetaldehyde | green, grassy | 4 | 4 |
| 4 | 2167-14-8 | 1-ethyl-2-pyrrolecarboxaldehyde | roasted, steamed rice-like | 2 | 4 |
| 5 | 472-66-2 | β-homocyclocitral | tea-like, camphoreous, mint-like | 4 | 3 |
| 6 | 1517-69-7 | (R)-styralyl alcohol | matcha-like, floral | 3 | 3 |
| 7 | 1568-20-3 | 5-methyl-2-pyrazoline | milky | 2 | — |
| 8 | — | unknown-1 | sweet | 3 | — |
| 9 | — | unknown-2 | bitter melon | 3 | — |
| 10 | 763-32-6 | 3-methyl-3-buten-1-ol | burnt | — | 2 |
| 11 | 1551-06-0 | 2-ethyl-1H-pyrrole | woody | — | 3 |
| 12 | 3777-69-3 | 2-pentylfuran | green bean-, vegetable-like, grassy | — | 4 |
| 13 | 100-86-7 | dimethyl benzyl carbinol | bitter, herbaceous, mild floral | — | 3 |
| 14 | 3913-81-3 | (E)-2-decenal | green, cucumber-like | — | 4 |
| 15 | — | unknown-3 | raw chili pepper-like | — | 4 |
| 16 | 7289-52-3 | 1-methoxy-docane | fresh, matcha-like | — | 3 |
| 17 | 13679-41-9 | 3-phenylfuran | milky, caramel-like | — | 4 |
| 18 | — | unknown-4 | raw rice-like | — | 2 |
| 19 | 23726-93-4 | (E)-beta-damascenone | fruity, sweet, honey-like | — | 3 |
| 20 | — | unknown-5 | floral, sweet | — | 4 |
| Compounds | GC-IMS Peak Volume | ||||
|---|---|---|---|---|---|
| Control a | TPJ-80 | TPJ-100 | TPJ-120 | TPJ-140 | |
| Ketones | |||||
| (1) β-damascenone | 256.53 ± 31.36 c | 532.54 ± 25.13 bc | 827.06 ± 33.02 b | 2299.5 ± 148.12 a | 2615.21 ± 509.57 a |
| (2) 2-decanone | 683.21 ± 20.4 a | 633.14 ± 16.54 b | 612.8 ± 16.07 b | 604.77 ± 12.72 bc | 577.77 ± 22.44 c |
| (3) acetophenone (M) | 45.58 ± 2.5 d | 89.97 ± 7.2 d | 147.44 ± 8.68 c | 452.46 ± 15.45 b | 656.26 ± 59.75 a |
| (4) acetophenone (D) | 1668.46 ± 23.6 c | 1865.68 ± 45.56 a | 1496.95 ± 18.05 d | 1773.98 ± 50.37 b | 1930.2 ± 81.02 a |
| (5) 2-octanone | 486.61 ± 18.17 a | 483.59 ± 12.34 a | 413.88 ± 9.16 a | 421.12 ± 29.76 a | 407.69 ± 95.38 a |
| (6) 5-nonanone | 318.91 ± 25.73 a | 228.7 ± 3.02 b | 306.11 ± 5.45 a | 230.68 ± 11.8 b | 256.91 ± 42.02 b |
| (7) 2-heptanone | 194.03 ± 26.49 c | 333.42 ± 20.01 b | 332.98 ± 10.12 b | 642.89 ± 31.47 a | 637.73 ± 118.41 a |
| (8) furaneol (M) | 276.26 ± 2.65 b | 303.1 ± 10.37 a | 299.15 ± 5.26 a | 264.44 ± 6.75 b | 248.01 ± 9.11 c |
| (9) furaneol (D) | 3570.36 ± 187.71 a | 3787.35 ± 72.6 a | 3620.59 ± 26.26 a | 4072.94 ± 110.59 a | 3571.72 ± 769.08 a |
| Total ketones | 7499.95 ± 10.02 e | 8257.49 ± 10.02 c | 8056.96 ± 10.02 d | 10,762.79 ± 10.02 b | 10,901.49 ± 10.02 a |
| Alcohols | |||||
| (10) octanol | 95.99 ± 4.95 a | 103.96 ± 6.68 a | 80.2 ± 3.92 b | 71.41 ± 3.14 c | 56.05 ± 3.08 d |
| (11) 2-octanol | 400.4 ± 25.9 a | 389.49 ± 14.97 ab | 348.75 ± 15.81 bc | 342.47 ± 33.86 c | 252.41 ± 26.93 d |
| (12) 2-hexanol | 447.6 ± 328.28 a | 665.35 ± 53.09 a | 670.04 ± 8.8 a | 615.11 ± 34.32 a | 506.36 ± 128.35 a |
| (13) cis-3-hexen-1-ol | 150.83 ± 100.74 a | 231.51 ± 34.36 a | 248.33 ± 18.09 a | 220.88 ± 30.07 a | 161.56 ± 58.76 a |
| (14) 2-ethyl-1-hexanol | 423.18 ± 76.09 b | 795.94 ± 135.39 ab | 709.47 ± 55.39 ab | 1005.74 ± 412 a | 780.86 ± 236.49 ab |
| (15) 2-phenylethanol (M) | 91.98 ± 3.19 b | 100.88 ± 13.39 b | 209.74 ± 42.4 b | 807.47 ± 92.27 a | 945.32 ± 217.94 a |
| (16) 1-pentanol | 77.64 ± 3.84 c | 168.52 ± 8.88 bc | 271.66 ± 30.52 b | 729.32 ± 82.71 a | 620.65 ± 155.35 a |
| (17) 2-phenylethanol (D) | 1061.48 ± 83.62 a | 963.97 ± 38.83 b | 558.75 ± 2.98 c | 389.46 ± 17.73 d | 318.39 ± 62.3 d |
| (18) 1-nonanol | 496.25 ± 34.85 bc | 422.97 ± 23.81 c | 455.58 ± 40.78 c | 605.29 ± 30.93 ab | 657.11 ± 117.76 a |
| (19) 2-methyl-1-butanol | 537.67 ± 21.17 a | 227.76 ± 16.3 b | 106.18 ± 6.24 c | 67.94 ± 9.55 d | 54.44 ± 4.54 d |
| (20) hexyl alcohol | 88.02 ± 64.79 ab | 132.2 ± 16.23 a | 77.4 ± 17.49 ab | 61.2 ± 9.78 b | 55.96 ± 24.6 b |
| (21) 3-methylbutan-1-ol | 694.19 ± 24.44 c | 690.84 ± 8.5 c | 746.54 ± 8.99 c | 909.47 ± 25.66 b | 1189.17 ± 127.88 a |
| (22) heptanol | 668.4 ± 41.84 c | 1235.78 ± 115.11 bc | 1893.25 ± 115.64 ab | 2469.77 ± 303.29 a | 2257.36 ± 878.16 a |
| (23) 2-butoxyethanol (D) | 4509.06 ± 605.68 b | 5547.11 ± 107.05 a | 3423.92 ± 101.07 c | 2560.82 ± 195.84 d | 1352.17 ± 462.99 e |
| (24) 2-butoxyethanol (M) | 1794.25 ± 107.52 bc | 2118.78 ± 41.2 a | 2045.24 ± 39.64 ab | 2053.73 ± 89.41 ab | 1674.17 ± 337.83 c |
| (25) 1-octen-3-ol | 445.87 ± 21.9 a | 429.77 ± 19.8 a | 412.8 ± 12.87 a | 403.38 ± 28.03 a | 394.09 ± 58.78 a |
| Total alcohols | 11,982.82 ± 833.01 ab | 14,224.85 ± 50.74 a | 12,257.85 ± 223.69 ab | 13,313.47 ± 989.98 a | 11,276.07 ± 2038.28 b |
| Aldehydes | |||||
| (26) decanal | 848.05 ± 28.49 a | 759.64 ± 4.18 bc | 806.3 ± 38.78 ab | 738.44 ± 19.65 c | 717.73 ± 45.3 c |
| (27) benzaldehyde | 984.61 ± 7.33 b | 1245.57 ± 42.05 a | 1435.46 ± 18.84 a | 1291 ± 102.74 a | 1286.89 ± 270.84 a |
| (28) (E,E)-2,4-nonadienal | 414.42 ± 18.48 d | 482.43 ± 5.31 cd | 521.52 ± 11.65 c | 742.84 ± 74.74 b | 872.93 ± 80.16 a |
| (29) nonanal | 1781.13 ± 94.72 d | 2730.86 ± 97.77 c | 3375.85 ± 211.45 b | 3861.62 ± 73.73 a | 3485.29 ± 226.02 b |
| (30) octanal | 1774.66 ± 57.58 a | 1809.86 ± 56.02 a | 1531.91 ± 6.47 b | 1335.12 ± 82.3 c | 1042.07 ± 167.92 d |
| (31) heptanal | 554.83 ± 139.89 a | 567.86 ± 38.52 a | 425.46 ± 25.99 ab | 392.42 ± 79.66 ab | 350.68 ± 163.42 b |
| Total aldehydes | 6357.7 ± 217.36 d | 7596.23 ± 126.51 c | 8096.5 ± 164.07 ab | 8361.43 ± 231.89 a | 7755.58 ± 291.64 bc |
| Terpene-derivates | |||||
| (32) α-pinene (M) | 281.44 ± 27.31 a | 289.95 ± 9.39 a | 224.52 ± 6.72 b | 187.87 ± 15.02 bc | 169.46 ± 36.71 c |
| (33) α-pinene (D) | 730.91 ± 27.61 c | 1084.56 ± 20.74 a | 945.84 ± 19.75 b | 865.14 ± 38.31 b | 712.13 ± 111.62 c |
| (34) styrene | 553.31 ± 53.71 b | 692.97 ± 40.58 a | 563.07 ± 19.57 b | 413.21 ± 28.49 c | 328.91 ± 114.61 c |
| (35) 1,8-cineole | 266.15 ± 47.73 b | 479.34 ± 14.16 b | 506.04 ± 17.35 b | 959.95 ± 223.53 a | 1035.79 ± 307.85 a |
| (36) limonene (M) | 315.41 ± 62.81 b | 519.99 ± 86.43 ab | 464.77 ± 41.03 ab | 613.76 ± 230.81 a | 528.8 ± 107.24 ab |
| (37) limonene (D) | 1445.07 ± 53.67 a | 1480.19 ± 74.13 a | 1154.34 ± 32.53 b | 1311.73 ± 265.12 ab | 1077.21 ± 76.98 b |
| (38) α-terpineol(D) | 8512.63 ± 661.4 a | 7957.81 ± 142.84 a | 3322.49 ± 121.13 b | 1868.37 ± 180.87 c | 1231.98 ± 226.75 d |
| (39) α-terpineol (M) | 5380.16 ± 114.13 b | 6009.12 ± 45.76 a | 5576.21 ± 82.96 b | 5607.12 ± 25.56 ab | 5297.91 ± 471.92 b |
| (40) linalool (M) | 1633.42 ± 32.32 d | 2057.24 ± 36.88 ab | 1734.18 ± 37.72 c | 2024.16 ± 24.57 b | 2134.82 ± 70.44 a |
| (41) linalool (D) | 129.17 ± 6.08 a | 49.26 ± 1.23 b | 36.87 ± 2.38 c | 20.58 ± 5.23 d | 18.5 ± 3.77 d |
| Total terpene-derivates | 19,247.67 ± 1003.87 a | 20,620.41 ± 224.58 a | 14,528.33 ± 229.45 b | 13,871.89 ± 920.58 bc | 12,535.5 ± 974.71 c |
| Pyrazines | |||||
| (42) 2-methylpyrazine | 153.65 ± 109.18 a | 202.45 ± 5.6 a | 193.84 ± 5.74 a | 195.21 ± 6.24 a | 189.74 ± 18.33 a |
| (43) 2,5-dimethylpyrazine | 2877.64 ± 189.37 a | 2939.17 ± 89.4 a | 2957.26 ± 67.68 a | 3068.18 ± 167.17 a | 3137.65 ± 170.8 a |
| Total pyrazines | 3031.29 ± 206.13 a | 3141.62 ± 90.89 a | 3151.1 ± 71.26 a | 3263.38 ± 163.47 a | 3327.39 ± 188.99 a |
| Others | |||||
| (44) p-xylene | 1140.36 ± 97.68 a | 1033.57 ± 20.7 ab | 908.7 ± 4.35 bc | 889.61 ± 33.5 bc | 769.88 ± 140.8 c |
| Total identified volatiles | 49,259.78 ± 2368.08 ab | 54,874.17 ± 523.44 a | 46,999.43 ± 702.84 b | 50,462.57 ± 2349.44 ab | 46,565.92 ± 3644.44 b |
| Measurement Site | Sample | L* | a* | b* | △E | Appearance |
|---|---|---|---|---|---|---|
| crust | a Control | 66.91 ± 2.73 a | 12.02 ± 1.75 a | 38.33 ± 0.93 a | — |  |
| PFC-1 | 64.08 ± 1.60 a | 9.49 ± 0.54 b | 36.36 ± 1.07 b | 4.53 ± 0.75 d |  | |
| PFC-2 | 61.08 ± 0.78 b | 8.42 ± 0.91 bc | 39.09 ± 0.97 a | 7.00 ± 0.29 c |  | |
| PFC-3 | 59.96 ± 0.55 b | 7.90 ± 0.55 bc | 38.54 ± 0.30 a | 8.10 ± 0.22 b |  | |
| PFC-4 | 59.26 ± 1.26 b | 7.35 ± 1.04 c | 39.08 ± 0.95 a | 9.12 ± 0.35 a |  | |
| crumb | Control | 64.44 ± 0.98 a | 6.27 ± 0.63 a | 42.49 ± 1.58 c | — |  |
| PFC-1 | 62.73 ± 1.30 ab | −8.41 ± 0.42 b | 45.28 ± 0.63 b | 25.54 ± 0.39 c |  | |
| PFC-2 | 61.46 ± 1.75 b | −8.65 ± 0.38 b | 48.13 ± 0.58 a | 28.15 ± 0.57 ab |  | |
| PFC-3 | 60.94 ± 0.95 b | −9.06 ± 0.12 bc | 48.42 ± 0.73 a | 28.65 ± 0.62 a |  | |
| PFC-4 | 58.41 ± 0.29 c | −9.61 ± 0.34 c | 45.93 ± 0.69 b | 27.36 ± 0.64 b |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Cao, Y.; Lan, W.; Gu, Z.; He, W.; He, J.; Zhao, L. Quality-Driven Design of Pandan-Flavored Sponge Cake: Unraveling the Role of Thermal Processing on Typical Pandan Aroma. Foods 2024, 13, 3074. https://doi.org/10.3390/foods13193074
Chen X, Cao Y, Lan W, Gu Z, He W, He J, Zhao L. Quality-Driven Design of Pandan-Flavored Sponge Cake: Unraveling the Role of Thermal Processing on Typical Pandan Aroma. Foods. 2024; 13(19):3074. https://doi.org/10.3390/foods13193074
Chicago/Turabian StyleChen, Xiao, Ying Cao, Weijie Lan, Zixuan Gu, Wenjia He, Jianfei He, and Liyan Zhao. 2024. "Quality-Driven Design of Pandan-Flavored Sponge Cake: Unraveling the Role of Thermal Processing on Typical Pandan Aroma" Foods 13, no. 19: 3074. https://doi.org/10.3390/foods13193074
APA StyleChen, X., Cao, Y., Lan, W., Gu, Z., He, W., He, J., & Zhao, L. (2024). Quality-Driven Design of Pandan-Flavored Sponge Cake: Unraveling the Role of Thermal Processing on Typical Pandan Aroma. Foods, 13(19), 3074. https://doi.org/10.3390/foods13193074









