The Potential of Thymus serpyllum Essential Oil as an Antibacterial Agent against Pseudomonas aeruginosa in the Preservation of Sous Vide Red Deer Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Essential Oil
2.2. Chemical Composition of TSEO
2.3. Antimicrobial Activity
2.3.1. Pseudomonas Strain Preparation
2.3.2. Disc Diffusion Method
2.3.3. Minimal Inhibition Concentration
2.4. Antibiofilm Activity
2.4.1. Crystal Violet Assay
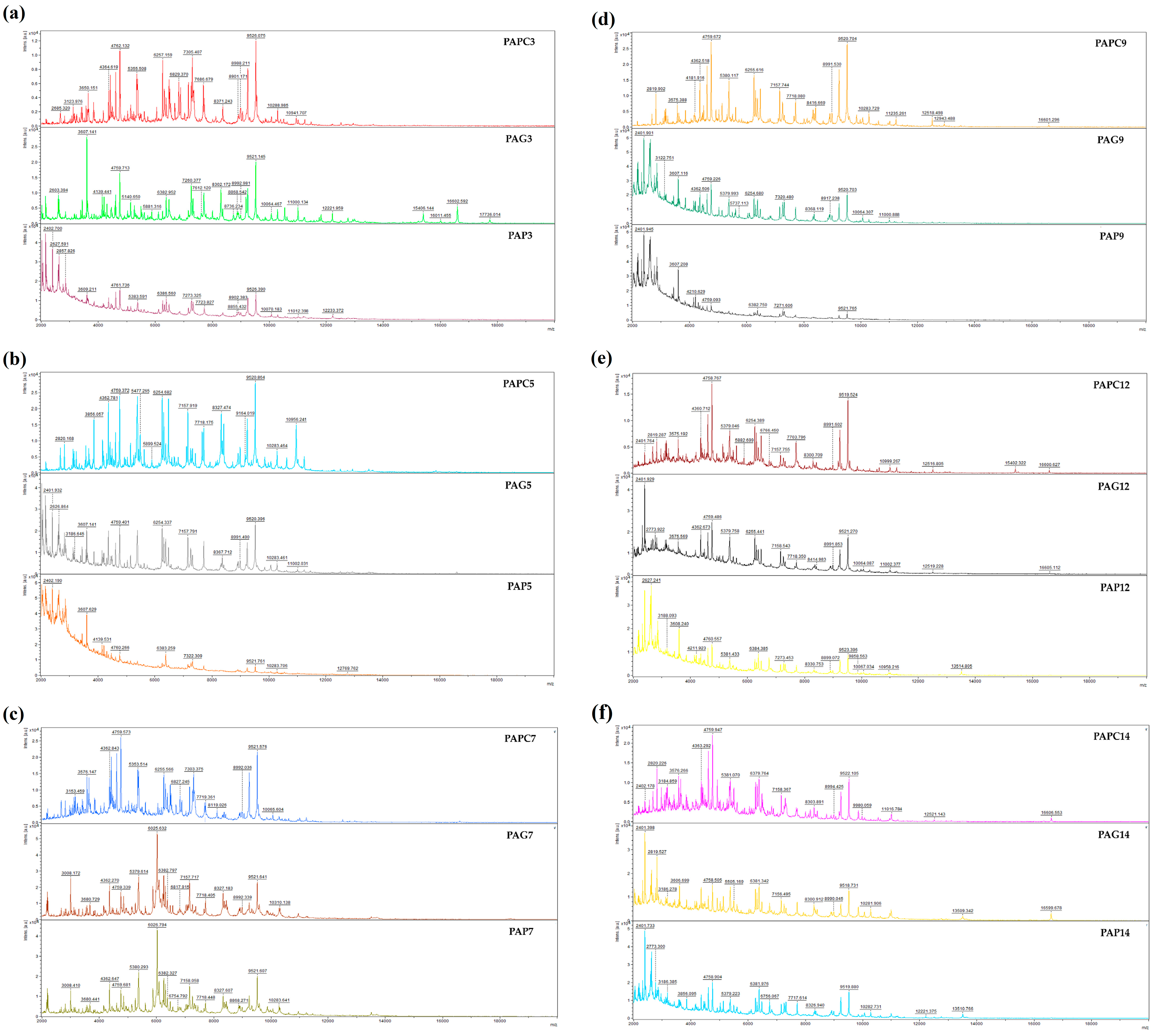
2.4.2. Antibiofilm Detection by MALDI-TOF MS Biotyper
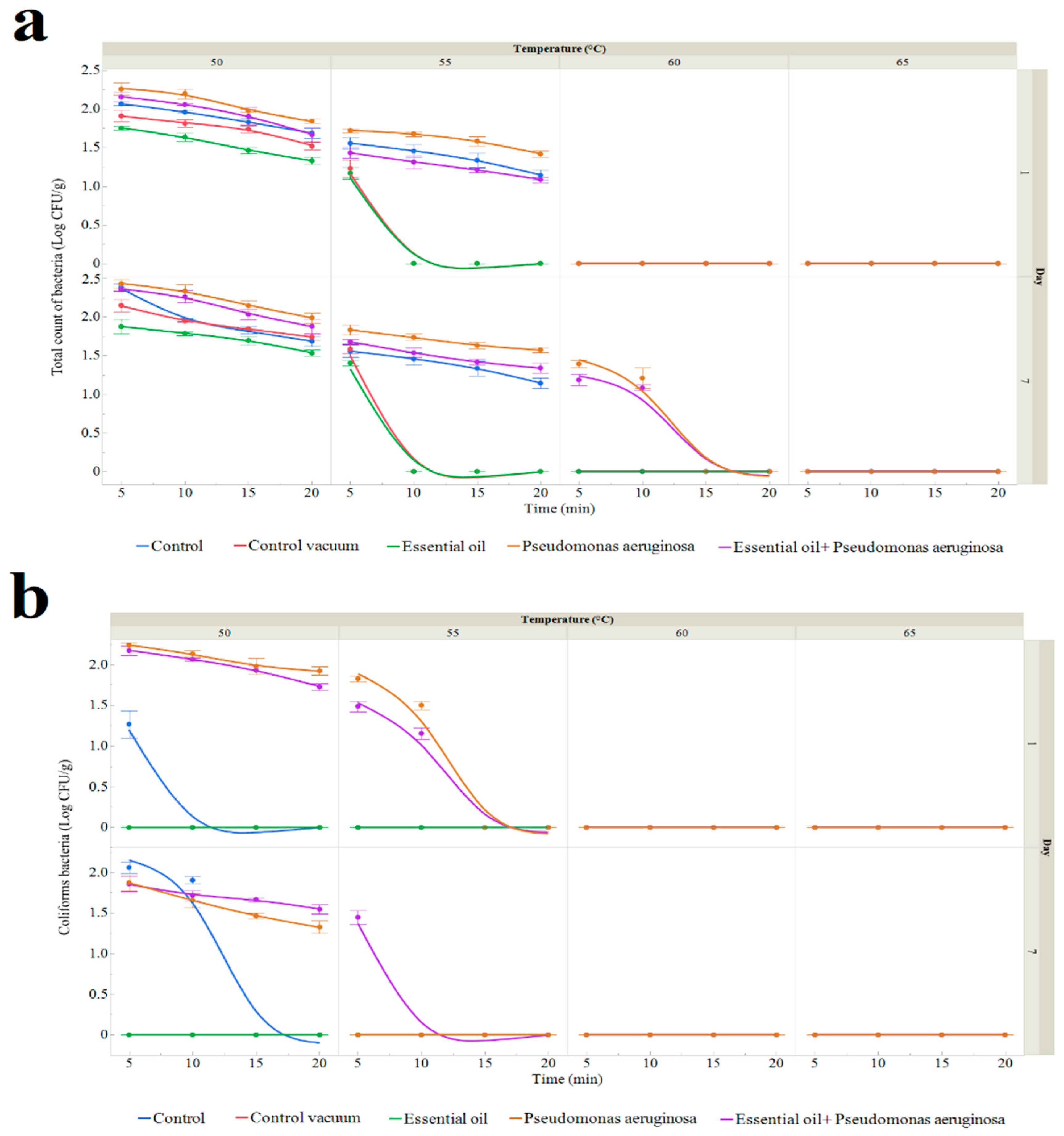
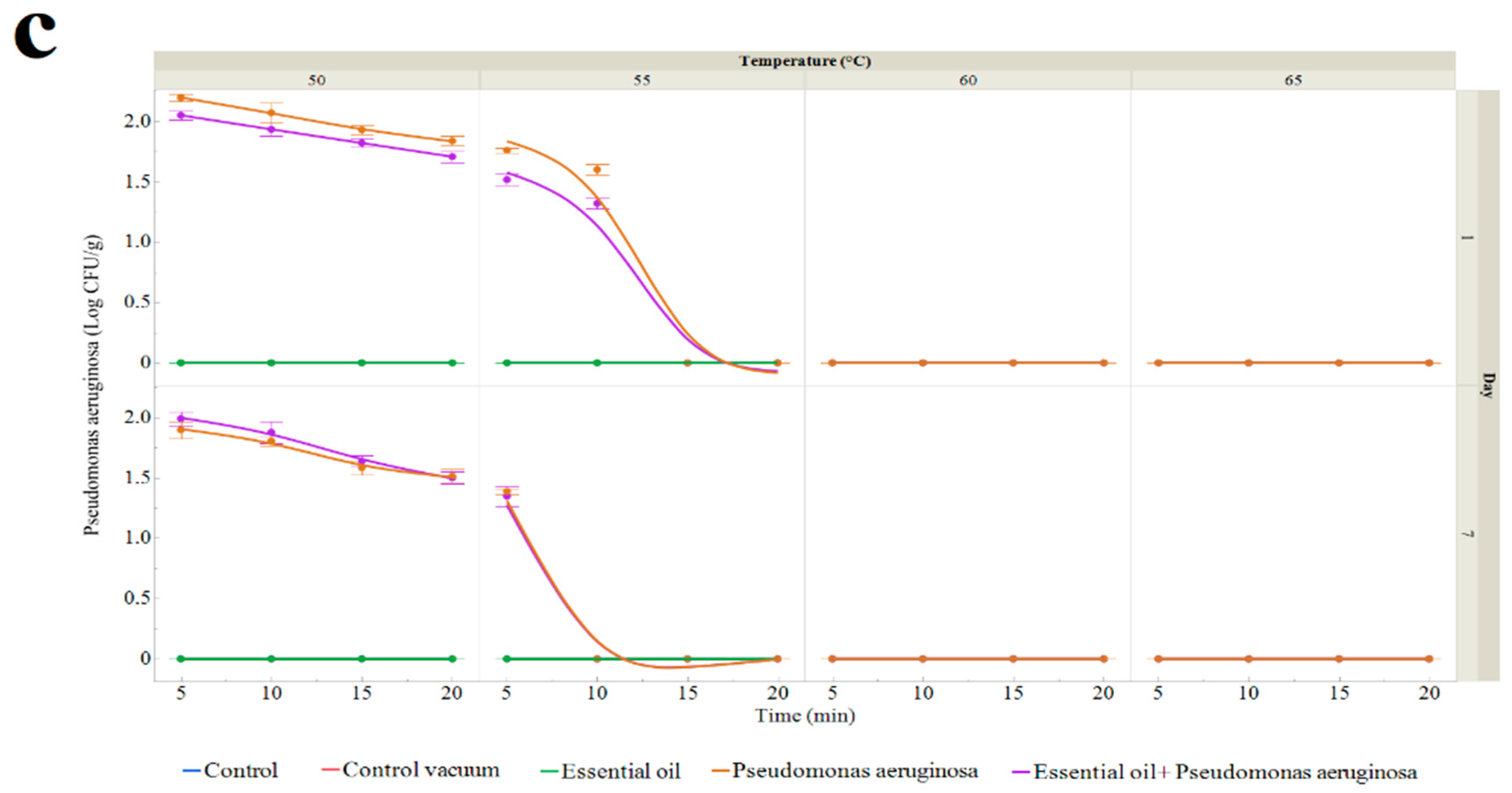
2.5. Shelf-Life Extension of Sous Vide Deer Meat
Deer Meat Preparation
- After being kept in polyethylene bags at 4 °C, fresh deer meat was cooked for 5 to 25 min at temperatures between 50 and 65 °C.
- Control vacuum: Deer meat was cooked in a water bath for 5 to 25 min at temperatures between 50 and 65 °C after being vacuum-sealed in polyethylene bags at 4 °C.
- The deer meat was treated with essential oil (1% TSEO solution), vacuum-packed, and stored at 4 °C. It was then cooked in a water bath at 50 to 65 °C for 5 to 25 min.
- Contamination with P. aeruginosa: Deer meat that was injected with the bacteria, vacuum-packed, and kept at 4 °C until exposed was cooked in a water bath for 5 to 25 min at temperatures between 50 and 65 °C.
- Treatment with P. aeruginosa and essential oil: Deer meat was treated with both Pseudomonas aeruginosa and a 1% TSEO solution. It was then vacuum-packed, kept at 4 °C, and cooked in a water bath for 5 to 25 min at temperatures between 50 and 65 °C.
2.6. Microbiological Evaluation of Deer Meat
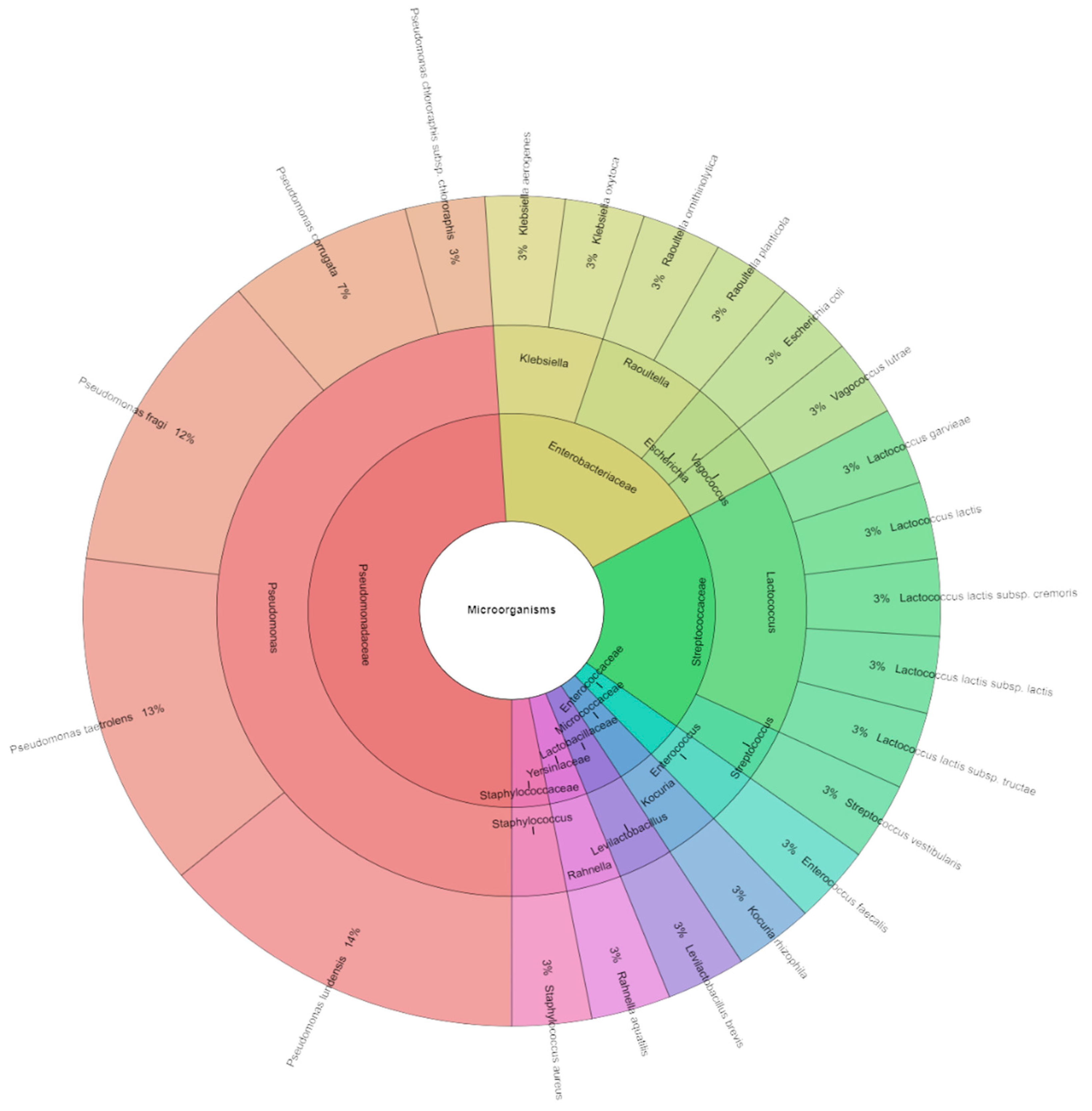
2.7. Bacteriota Identification by MALDI-TOF MS Biotyper
2.8. Statistical Analyses
3. Results
3.1. Antimicrobial Activity
3.2. Antibiofilm Activity
3.3. Microbiological Quality of Sous Vide Deer Meat Samples
3.3.1. Number of Microorganisms
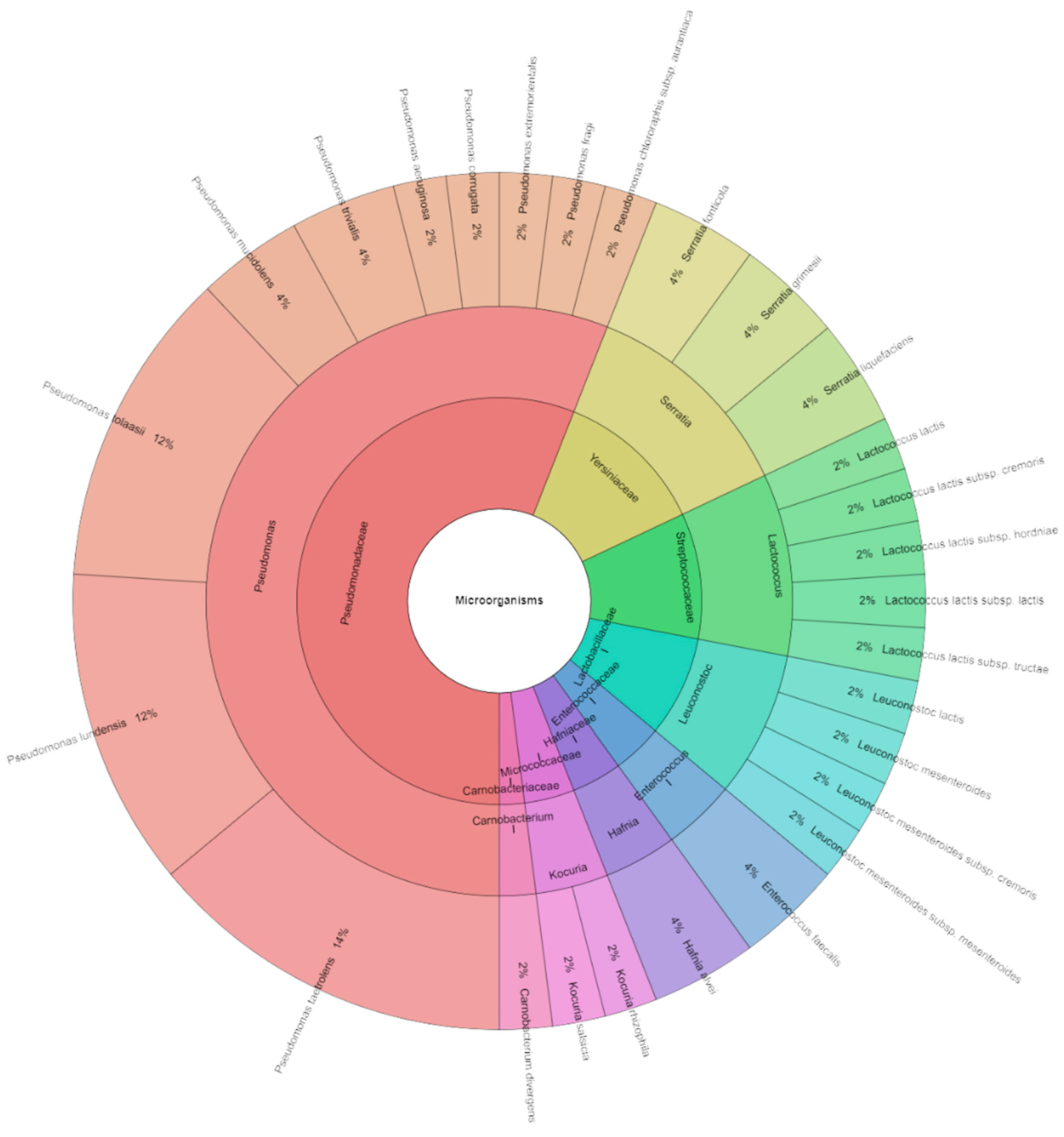
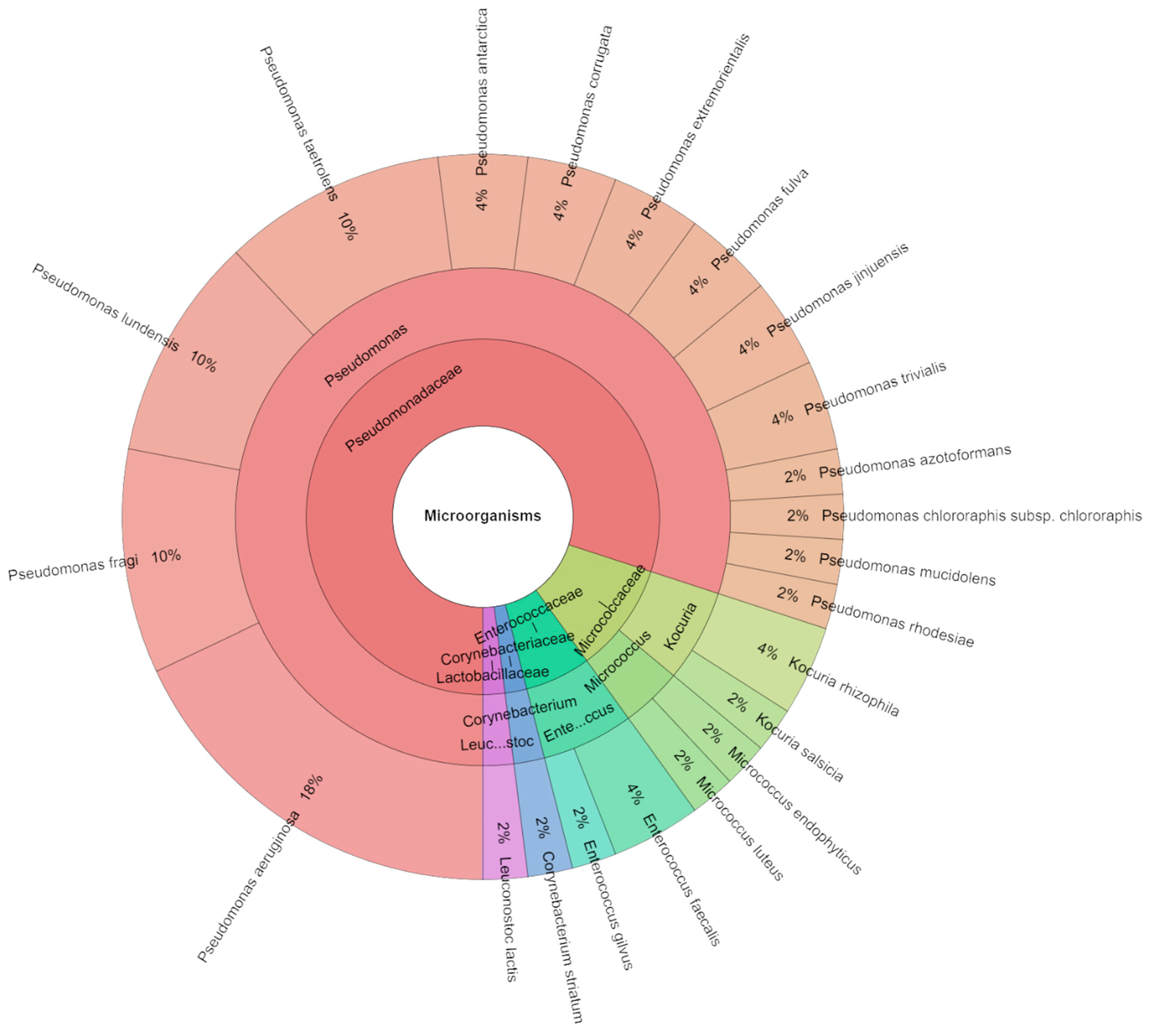
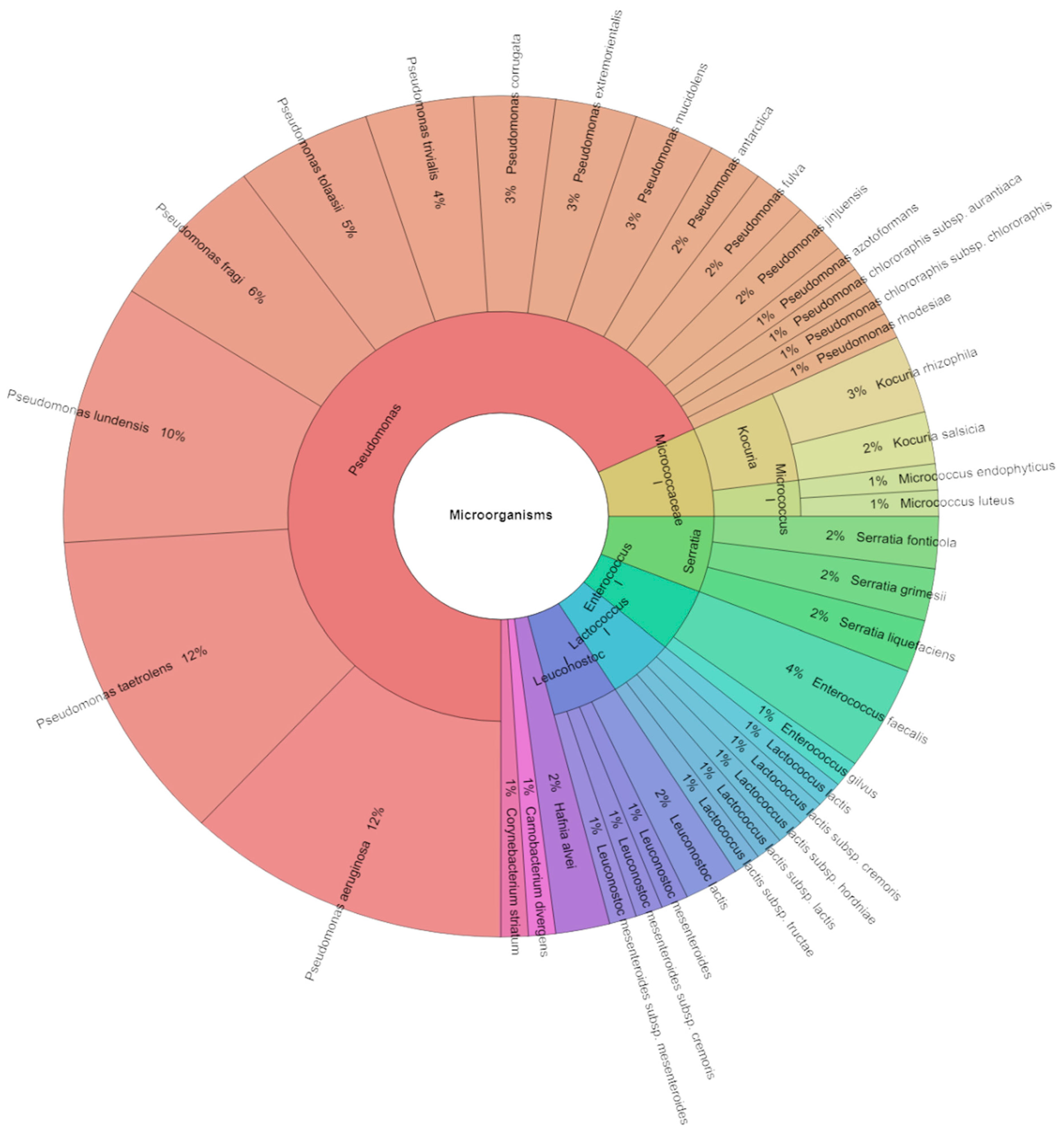
3.3.2. Identification of Microorganisms of Sous Vide Deer Meat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- González-Fandos, E.; Villarino-Rodríguez, A.; García-Linares, M.C.; García-Arias, M.T.; García-Fernández, M.C. Microbiological Safety and Sensory Characteristics of Salmon Slices Processed by the Sous Vide Method. Food Control 2005, 16, 77–85. [Google Scholar] [CrossRef]
- Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2017.
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents—How P. Aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Z.; Wang, D.; Liu, Y.; Zhang, Z.; Wozniak, D.J. Regulation of Biofilm Exopolysaccharide Biosynthesis and Degradation in Pseudomonas aeruginosa. Annu. Rev. Microbiol. 2022, 76, 413–433. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Soteyome, T.; Hua, J.; Zhang, L.; Yuan, L.; Ye, Y.; Cai, Z.; Yang, L.; Chen, L.; et al. Impact of pmrA on Cronobacter sakazakii Planktonic and Biofilm Cells: A Comprehensive Transcriptomic Study. Food Microbiol. 2021, 98, 103785. [Google Scholar] [CrossRef]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa Displays Multiple Phenotypes during Development as a Biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Wiklund, E. Game and Venison—Meat for the Modern Consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef]
- Wongprawmas, R.; Mora, C.; Pellegrini, N.; Guiné, R.P.F.; Carini, E.; Sogari, G.; Vittadini, E. Food Choice Determinants and Perceptions of a Healthy Diet among Italian Consumers. Foods 2021, 10, 318. [Google Scholar] [CrossRef]
- Korkmaz, B.; Reich, F.; Alter, T.; Steinhoff-Wagner, J.; Maaz, D.; Gremse, C.; Haase, A.; Mader, A.; Schafft, H.A.; Bandick, N.; et al. Microbial Load of Rinsed and Unrinsed Body Cavities of Roe Deer (Capreolus capreolus) on the Killing Day and after Cold Storage: A Preliminary Investigation. Food Control 2022, 141, 109141. [Google Scholar] [CrossRef]
- Avagnina, A.; Nucera, D.; Grassi, M.A.; Ferroglio, E.; Dalmasso, A.; Civera, T. The Microbiological Conditions of Carcasses from Large Game Animals in Italy. Meat Sci. 2012, 91, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Bonke, R.; Drees, N.; Heurich, M.; Märtlbauer, E.; Gareis, M. Shiga Toxin-Producing Escherichia coli (STEC) Shedding in a Wild Roe Deer Population. Vet. Microbiol. 2019, 239, 108479. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Nieto, G.; Garrido, M.D.; Bañón, S. Microbial, Physical–Chemical and Sensory Spoilage during the Refrigerated Storage of Cooked Pork Loin Processed by the Sous Vide Method. Meat Sci. 2008, 80, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zavadlav, S.; Blažić, M.; Van De Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.E. Sous Vide Cooking: A Review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Karyotis, D.; Skandamis, P.N.; Juneja, V.K. Thermal Inactivation of Listeria monocytogenes and Salmonella spp. in Sous-Vide Processed Marinated Chicken Breast. Food Res. Int. 2017, 100, 894–898. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Badoni, M.; Zawadski, S.; McLeod, B.; Holman, D.; Uttaro, B. Effects of a Novel Three-Step Sous-Vide Cooking and Subsequent Chilled Storage on the Microbiota of Beef Steaks. Meat Sci. 2020, 159, 107938. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Abdallah, E.M.; Alanazi, N.A.; Ed-Dra, A.; Jamal, A.; Idriss, H.; Alshammari, A.S.; Shommo, S.A.M. Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential. Pharmaceuticals 2023, 16, 1451. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; De Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in Membrane Fatty Acids Composition of Microbial Cells Induced by Addiction of Thymol, Carvacrol, Limonene, Cinnamaldehyde, and Eugenol in the Growing Media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Galovičová, L.; Valková, V.; Ďuranová, H.; Borotová, P.; Štefániková, J.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Felsöciová, S.; et al. Chemical Composition and Biological Activity of Salvia officinalis Essential Oil. Acta Hortic. Regiot. 2021, 24, 81–88. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2023, 24, 5179. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Garzoli, S.; Ben Hsouna, A.; Ban, Z.; Elizondo-Luevano, J.H.; Kluz, M.I.; Ben Saad, R.; Haščík, P.; Čmiková, N.; Waskiewicz-Robak, B.; et al. Enhancing Deer Sous Vide Meat Shelf Life and Safety with Eugenia caryophyllus Essential Oil against Salmonella enterica. Foods 2024, 13, 2512. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Čmiková, N.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Vukic, M.D. Citrus limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot. Plants 2024, 13, 524. [Google Scholar] [CrossRef]
- Vale, J.P.C.D.; Ribeiro, L.H.D.F.; Vasconcelos, M.A.D.; Sá-Firmino, N.C.; Pereira, A.L.; Nascimento, M.F.D.; Rodrigues, T.H.S.; Silva, P.T.D.; Sousa, K.C.D.; Silva, R.B.D.; et al. Chemical Composition, Antioxidant, Antimicrobial and Antibiofilm Activities of Vitex gardneriana Schauer Leaves’s Essential Oil. Microbial Pathog. 2019, 135, 103608. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Roselló, J.; Llorens-Molina, J.A.; Larran, S.; Sempere-Ferre, F.; Santamarina, M.P. Biofilm Containing the Thymus serpyllum Essential Oil for Rice and Cherry Tomato Conservation. Front. Plant Sci. 2024, 15, 1362569. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hosseini, S.E.; Sharifan, A. Effect of Edible Chitosan Film Enriched with Anise (Pimpinella anisum L.) Essential Oil on Shelf Life and Quality of the Chicken Burger. Food Sci. Nutr. 2018, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In Vitro Antioxidant and Antifungal Properties of Essential Oils Obtained from Aromatic Herbs Endemic to the Southeast of Spain. J. Food Prot. 2013, 76, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Yakoubi, S.; Cherrat, A.; Diouri, M.; El Hilali, F.; Zair, T. Chemical Composition and Antibacterial Activity of Thymus zygis Subsp. Gracilis (Boiss.) R. Morales Essential Oils from Morocco. Mediterr. J. Chem. 2014, 3, 746–758. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Moukhles, A.; Ellaghdach, A.; Driss, A.B.; Amrani, M.A.E.; Aghmiz, A.; Mansour, A.I. Chemical Profile and In Vitro Antibacterial Potential of Essential Oils and Hydrolat Extracts from Aerial Parts of Three Wild Species of Moroccan Thymus. Sci. Afr. 2022, 18, e01434. [Google Scholar] [CrossRef]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. Int. J. Mol. Sci. 2023, 24, 6936. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical Composition, Antimicrobial, Antioxidant and Antitumor Activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. Essential Oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Ćirić, A.; Kataranovski, D.; Marin, P.D.; Vukojević, J.; Brkić, D. Antifungal Activity of the Essential Oil of Thymus vulgaris L. and Thymol on Experimentally Induced Dermatomycoses. Drug Dev. Ind. Pharm. 2008, 34, 1388–1393. [Google Scholar] [CrossRef]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Van Griensven, L.J.L.D. Chemical Composition of Essential Oilsof Thymus and Mentha Speciesand Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Bakó, C.; Balázs, V.L.; Kerekes, E.; Kocsis, B.; Nagy, D.U.; Szabó, P.; Micalizzi, G.; Mondello, L.; Krisch, J.; Pethő, D.; et al. Flowering Phenophases Influence the Antibacterial and Anti-Biofilm Effects of Thymus vulgaris L. Essential Oil. BMC Complement Med. Ther. 2023, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Althunibat, O.Y.; Qaralleh, H.; Al-Dalin, S.Y.A.; Abboud, M.; Khleifat, K.; Majali, I.S.; Aldal’in, H.K.; Rayyan, W.A.; Jaafraa, A. Effect of Thymol and Carvacrol, the Major Components of Thymus capitatus on the Growth of Pseudomonas aeruginosa. J. Pure Appl. Microbiol. 2016, 10, 367–374. [Google Scholar]
- Schmidt, E.; Wanner, J.; Höferl, M.; Jirovetz, L.; Buchbauer, G.; Gochev, V.; Girova, T.; Stoyanova, A.; Geissler, M. Chemical Composition, Olfactory Analysis and Antibacterial Activity of Thymus vulgaris Chemotypes Geraniol, 4-Thujanol/Terpinen-4-Ol, Thymol and Linalool Cultivated in Southern France. Nat. Prod. Commun. 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Antibacterial and Antibiofilm Efficacy of Thyme (Thymus vulgaris L.) Essential Oil against Foodborne Illness Pathogens, Salmonella enterica Subsp. Enterica Serovar Typhimurium and Bacillus cereus. Antibiotics 2023, 12, 485. [Google Scholar] [CrossRef]
- Božik, M.; Cejnar, P.; Šašková, M.; Nový, P.; Maršík, P.; Klouček, P. Stress Response of Escherichia coli to Essential Oil Components—Insights on Low-Molecular-Weight Proteins from MALDI-TOF. Sci. Rep. 2018, 8, 13042. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential Oils as Potential Antimicrobial Agents in Meat and Meat Products: A Review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Sodium Nitrite in Processed Meat and Poultry Meats: A Review of Curing and Examining the Risk/Benefit of Its Use. Am. Meat Sci. Assoc. 2011, 3, 1–14. [Google Scholar]
- El-Wahab, H.M.F.A.; Moram, G.S.E.-D. Toxic Effects of Some Synthetic Food Colorants and/or Flavor Additives on Male Rats. Toxicol. Ind. Health 2013, 29, 224–232. [Google Scholar] [CrossRef]
- Sweis, I.E.; Cressey, B.C. Potential Role of the Common Food Additive Manufactured Citric Acid in Eliciting Significant Inflammatory Reactions Contributing to Serious Disease States: A Series of Four Case Reports. Toxicol. Rep. 2018, 5, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical Composition and in Vitro Antibacterial Properties of Essential Oils of Four Thymus Species from Organic Growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Nalbone, L.; Filali, F.R.; Trabelsi, N.; El Majdoub, Y.O.; Bouchrif, B.; Giarratana, F.; Giuffrida, A. Comprehensive Evaluation on the Use of Thymus vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella enterica. Sustainability 2021, 13, 4594. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Mizan, M.F.R.; Kim, H.-S.; Yang, S.; Ha, S.-D. Activity of Thyme and Tea Tree Essential Oils against Selected Foodborne Pathogens in Biofilms on Abiotic Surfaces. LWT 2018, 89, 134–139. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening Essential Oils for Their Antimicrobial Activities against the Foodborne Pathogenic Bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Rafiq, R.; Hayek, S.; Anyanwu, U.; Hardy, B.; Giddings, V.; Ibrahim, S.; Tahergorabi, R.; Kang, H. Antibacterial and Antioxidant Activities of Essential Oils from Artemisia Herba-Alba Asso., Pelargonium capitatum × Radens and Laurus nobilis L. Foods 2016, 5, 28. [Google Scholar] [CrossRef]
- Alves Coelho Trevisan, D.; Aline Zanetti Campanerut-Sá, P.; Da Silva, A.F.; Farias Pereira Batista, A.; Seixas, F.A.V.; Peralta, R.M.; De Sá-Nakanishi, A.B.; De Abreu Filho, B.A.; Machinski Junior, M.; Graton Mikcha, J.M. Action of Carvacrol in Salmonella Typhimurium Biofilm: A Proteomic Study. J. Appl. Biomed. 2020, 18, 106–114. [Google Scholar] [CrossRef]
- Basavegowda, N.; Patra, J.K.; Baek, K.-H. Essential Oils and Mono/Bi/Tri-Metallic Nanocomposites as Alternative Sources of Antimicrobial Agents to Combat Multidrug-Resistant Pathogenic Microorganisms: An Overview. Molecules 2020, 25, 1058. [Google Scholar] [CrossRef]
- Guillín, Y.; Cáceres, M.; Torres, R.; Stashenko, E.; Ortiz, C. Effect of Essential Oils on the Inhibition of Biofilm and Quorum Sensing in Salmonella Enteritidis 13076 and Salmonella Typhimurium 14028. Antibiotics 2021, 10, 1191. [Google Scholar] [CrossRef]
- Heir, E.; Holck, A.L.; Omer, M.K.; Alvseike, O.; Måge, I.; Høy, M.; Rode, T.M.; Sidhu, M.S.; Axelsson, L. Effects of Post-Processing Treatments on Sensory Quality and Shiga Toxigenic Escherichia Coli Reductions in Dry-Fermented Sausages. Meat Sci. 2013, 94, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.-J.E. Spoilage Microbiota Associated to the Storage of Raw Meat in Different Conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Álvarez-González, T.; Alonso-Calleja, C. Effect of Several Packaging Conditions on the Microbiological, Physicochemical and Sensory Properties of Ostrich Steaks during Refrigerated Storage. Food Microbiol. 2018, 72, 146–156. [Google Scholar] [CrossRef]
- Kunová, S.; Sendra, E.; Haščík, P.; Vuković, N.L.; Vukić, M.D.; Hsouna, A.B.; Mnif, W.; Kačániová, M. Microbiological Quality of Deer Meat Treated with Essential Oil Litsea cubeba. Animals 2022, 12, 2315. [Google Scholar] [CrossRef] [PubMed]



| Inhibition Zone (mm) | TSEO | Ciprofloxacin |
|---|---|---|
| Pseudomonas aeruginosa | 17.33 ± 0.58 | 33.33 ± 0.56 |
| Minimal inhibition concentration (mg/mL) | MIC50 | MIC90 |
| Pseudomonas aeruginosa | 0.134 ± 0.09 | 0.146 ± 0.05 |
| Minimal biofilm inhibition concentration (mg/mL) | MBIC50 | MBIC90 |
| Pseudomonas aeruginosa | 0.227 ± 0.07 | 0.236 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kačániová, M.; Garzoli, S.; Ben Hsouna, A.; Bianchi, A.; Kluz, M.I.; Elizondo-Luevano, J.H.; Ban, Z.; Saad, R.B.; Mnif, W.; Haščík, P. The Potential of Thymus serpyllum Essential Oil as an Antibacterial Agent against Pseudomonas aeruginosa in the Preservation of Sous Vide Red Deer Meat. Foods 2024, 13, 3107. https://doi.org/10.3390/foods13193107
Kačániová M, Garzoli S, Ben Hsouna A, Bianchi A, Kluz MI, Elizondo-Luevano JH, Ban Z, Saad RB, Mnif W, Haščík P. The Potential of Thymus serpyllum Essential Oil as an Antibacterial Agent against Pseudomonas aeruginosa in the Preservation of Sous Vide Red Deer Meat. Foods. 2024; 13(19):3107. https://doi.org/10.3390/foods13193107
Chicago/Turabian StyleKačániová, Miroslava, Stefania Garzoli, Anis Ben Hsouna, Alessandro Bianchi, Maciej Ireneusz Kluz, Joel Horacio Elizondo-Luevano, Zhaojun Ban, Rania Ben Saad, Wissem Mnif, and Peter Haščík. 2024. "The Potential of Thymus serpyllum Essential Oil as an Antibacterial Agent against Pseudomonas aeruginosa in the Preservation of Sous Vide Red Deer Meat" Foods 13, no. 19: 3107. https://doi.org/10.3390/foods13193107











