Storage at 4 °C: A total of 171 volatile compounds were identified across eight storage times. These included 25 alcohols, 19 aldehydes, 18 acids, 30 hydrocarbons, 15 ketones, one sulfur-containing compound, six nitrogen-containing compounds, 28 esters, three phenols, seven ethers, and 19 other compounds.
Storage at 12 °C: Across eight storage times, 173 volatile compounds were detected. This included 23 alcohols, 29 aldehydes, 12 acids, 45 hydrocarbons, 10 ketones, five nitrogen-containing compounds, 25 esters, three phenols, two ethers, and 19 other compounds.
Storage at 20 °C: A total of 137 volatile compounds were identified across seven storage times. These included 23 alcohols, 19 aldehydes, 13 acids, 33 hydrocarbons, nine ketones, five nitrogen-containing compounds, 16 esters, two phenols, two ethers, and 15 other compounds.
Storage at 28 °C: Across seven storage times, 161 volatile compounds were identified. These included 36 alcohols, 22 aldehydes, 17 acids, 25 hydrocarbons, 11 ketones, five nitrogen-containing compounds, 25 esters, and 19 other compounds.
The results indicate significant differences in both the types and concentrations of volatile substances in oysters, depending on the storage temperature and duration.
3.2.1. Contents and Types of Volatile Substances in Oysters with Different Freshness
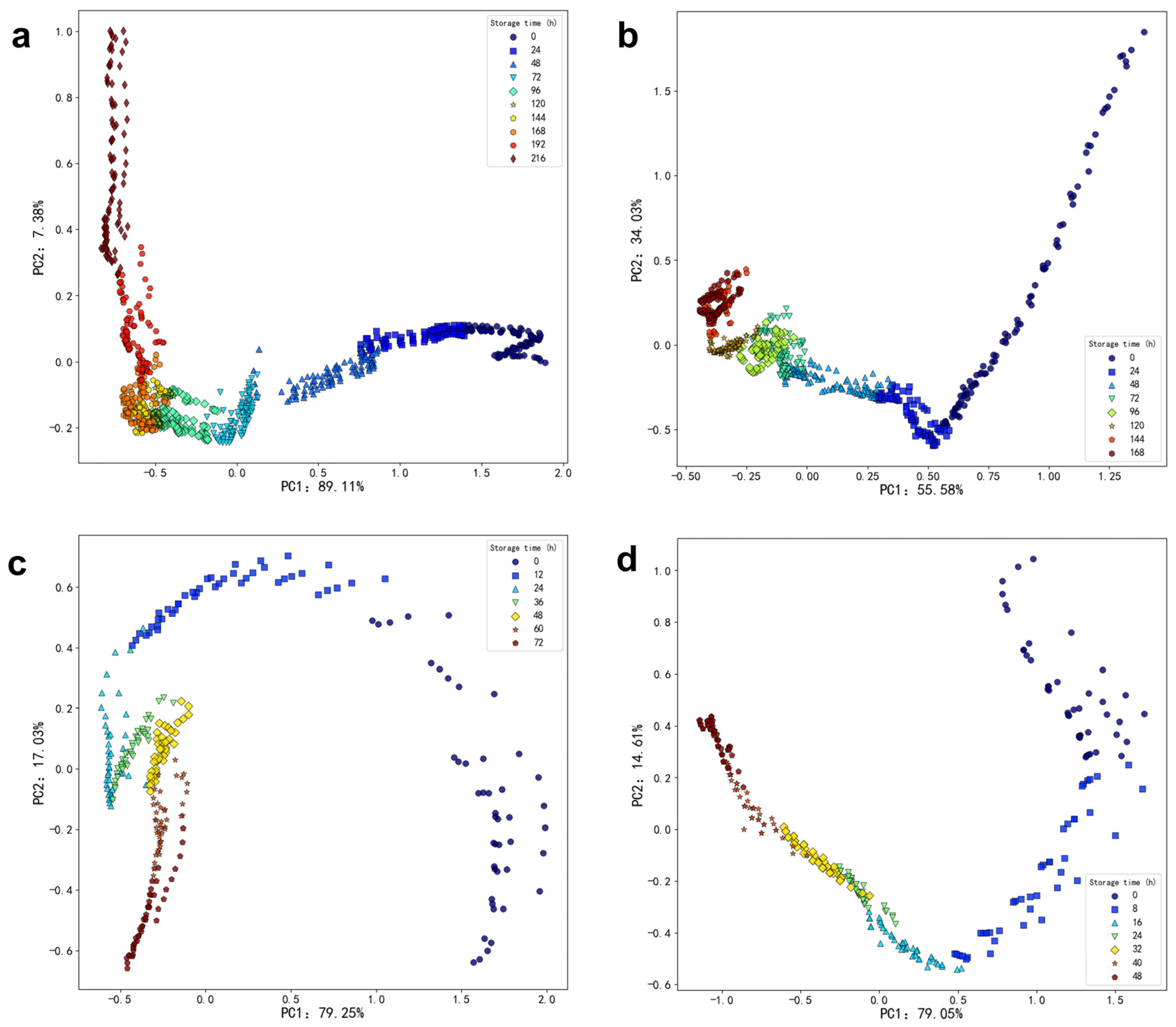
Figure 7 and
Table 6 illustrate the relative contents of volatile compounds in oysters stored at various temperatures over time. As depicted in
Figure 7a, at 4 °C, there is no significant difference in the relative content of alcohol, nitrogen-containing, and phenolic compounds as storage time increases. Additionally, the relative content of aldehyde compounds shows a decreasing trend, while the relative content of acid compounds exhibits an initial decrease followed by an increase. Additionally, the relative content of hydrocarbon, ketone, and ester compounds demonstrates an initial increase followed by a decrease. In
Figure 7b, at 12 °C, it can be observed that the relative content of alcohol compounds tends to decrease with increasing storage time. Aldehyde and hydrocarbon compounds display a trend of initially decreasing followed by an increase in relative content during storage. The relative content of acid compounds shows an initial increase followed by a decrease. There are no significant differences in the relative content of ketone, nitrogen-containing, and ester compounds. In
Figure 7c, at 20 °C, it is evident that the relative content of alcohol substances decreases initially and then increases during the storage process. The relative content of ketone substances increases initially and then decreases over time. The trends for acid substances and nitrogen-containing compounds also show an initial increase followed by a decrease during storage. No significant differences are observed in the relative content of aldehyde, hydrocarbon, and ester substances. In
Figure 7d, at 28 °C, the relative content of aldehyde substances initially decreases before increasing again during the storage process. In contrast, hydrocarbon substances exhibit an initial increase followed by a decline. The relative content of ester substances shows a significant increase, whereas alcohol substances, ketone substances, and nitrogen-containing compounds demonstrate a decreasing trend throughout storage. Notably, no significant difference was observed in the relative content of acid substances, indicating that these compounds may not be key markers for freshness assessment in this context. Despite the numerous volatile organic compounds identified and tracked over time, the limited variation in acid compounds suggests that other pathways, such as the formation of heterocyclic compounds like cis-2-(2-Pentenyl) furan, play a more crucial role in the degradation process.
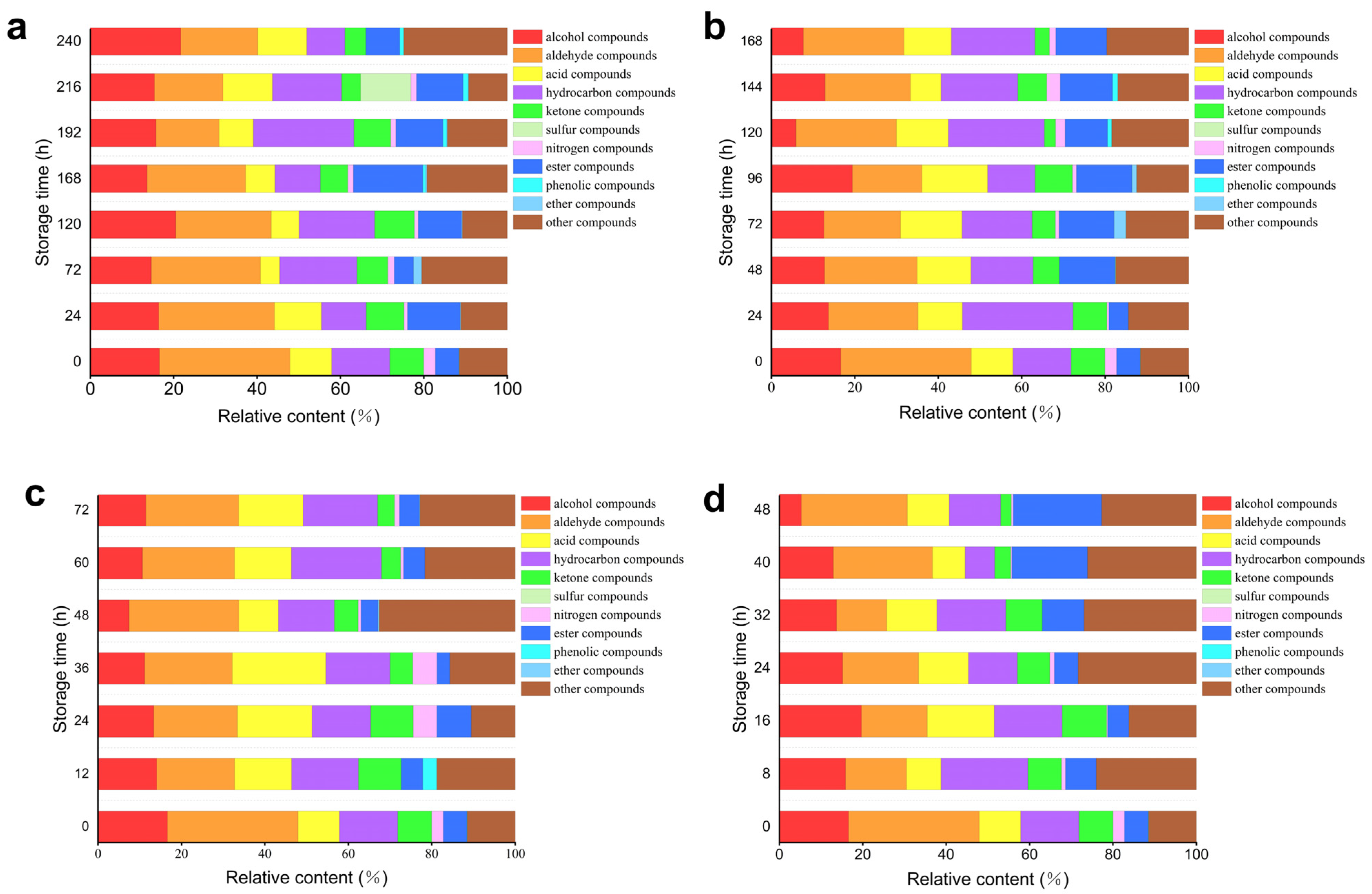
Figure 8 and
Table 7 illustrate the comparison of volatile compounds in oysters at various storage temperatures. In
Figure 8a, at 4 °C, the diagram reveals that during storage, oysters contain a substantial number of alcohols, hydrocarbons, and esters, which indicates their significant role in the volatile substances present in oysters. Conversely, the levels of phenols and ethers are relatively low. In
Figure 8b, at 12 °C, it can be observed that the storage process results in a higher concentration of alcohols, hydrocarbons, and esters, further underscoring their importance in the volatile profile of oysters. Similarly, phenolic and ether compounds remain at lower concentrations.
Figure 8c, at 20 °C, shows an increased presence of hydrocarbon compounds, along with relatively high levels of alcohols, aldehydes, and acids. In contrast, nitrogen-containing compounds, phenolic compounds, and ether compounds are found in lower quantities. Lastly,
Figure 8d, at 28 °C, indicates that the storage process leads to a notable increase in ester compounds, alongside significant amounts of hydrocarbons, alcohols, aldehydes, and acids. Conversely, nitrogen-containing and ketone compounds are present in lower quantities.
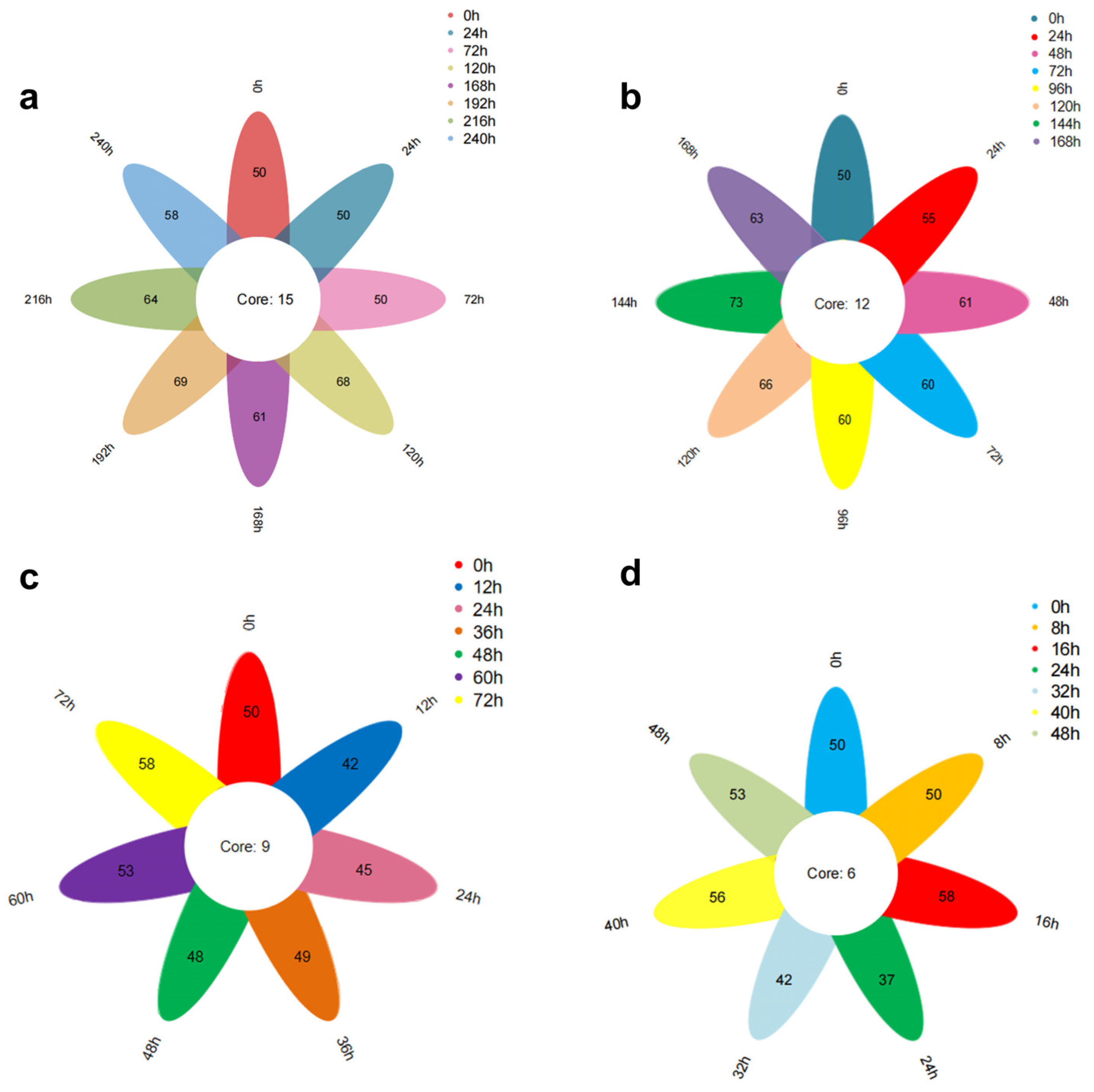
Figure 9a illustrates the Venn diagram depicting the total number of volatile substances in oysters stored at 4 °C over various periods. The data reveal that eight oyster samples with differing levels of freshness were identified to contain 50, 50, 50, 68, 61, 69, 64, and 58 types of volatile substances. Notably, these samples share a common set of fifteen volatile substances, which include cis, cis-7,10-Hexadecadienal, 2,4-Heptadienal (E,E), Benzaldehyde, 2,6-Nonadienal (E,Z), 2,4-Decadienal (E,E), 3-(Pent-1-en-1-yl)benzaldehyde, Tetradecanoic acid, n-Hexadecanoic acid, and 2-Methyl-1-nonene-3-yne.
Figure 9b presents the Venn diagram for the total number of volatile substances in oysters stored at 12 °C over different periods. The figure indicates that eight samples with varying freshness contained 50, 55, 61, 60, 60, 66, 73, and 63 types of volatile substances. These samples share a common set of twelve volatile substances, including 2,6-Cyclooctadien-1-ol, Eicosen-1-ol, and cis-9.
Figure 9c illustrates the Venn diagram depicting the total number of volatile substances in oysters stored at 20 °C over various periods. The figure reveals that seven distinct freshness samples of oysters were identified, containing 50, 42, 45, 49, 48, 53, and 58 types of volatile substances. Notably, these samples shared a common set of nine volatile substances: Ethanol, 2-(dodecyloxy); Eicosen-1-ol, cis-9-; 2,6-Cyclooctadien-1-ol; 2,4-Heptadienal, (E,E)-; Tetradecanoic acid; n-Hexadecanoic acid; 1,5-Cyclooctadiene, 3-(1-methyl-2-propenyl)-; and 3,5-Octadien-2-one.
Figure 9d presents the Venn diagram for oysters stored at 28 °C over various periods. From this figure, it can be observed that seven different freshness samples of oysters were identified, with 50, 50, 58, 37, 42, 56, and 53 types of volatile substances. These samples shared a common set of six volatile substances: Eicosen-1-ol, cis-9-; Tetradecanoic acid; n-Hexadecanoic acid; Dodecanoic acid; and cis-2-(2-Pentenyl) furan. The microbial influence during storage leads to fat oxidation and protein hydrolysis, resulting in the loss of older substances and the generation of new ones. Consequently, the number of common volatile substances in oysters with varying freshness levels is relatively small at each temperature.
Figure 9 illustrates the impact of storage time on the total volatile compounds under different temperature conditions (28 °C, 20 °C, 12 °C, 4 °C). The results indicate that the quantities of total volatile compounds at each temperature are 6, 9, 12, and 15, respectively, following a clear arithmetic sequence. The data reveal a decreasing trend in total volatile compounds as storage temperature increases.
This indicates that as the temperature increases, the biochemical reactions of volatile compounds become more extensive and complex.
3.2.2. Analysis of Volatile Substances in Oysters with Different Freshness
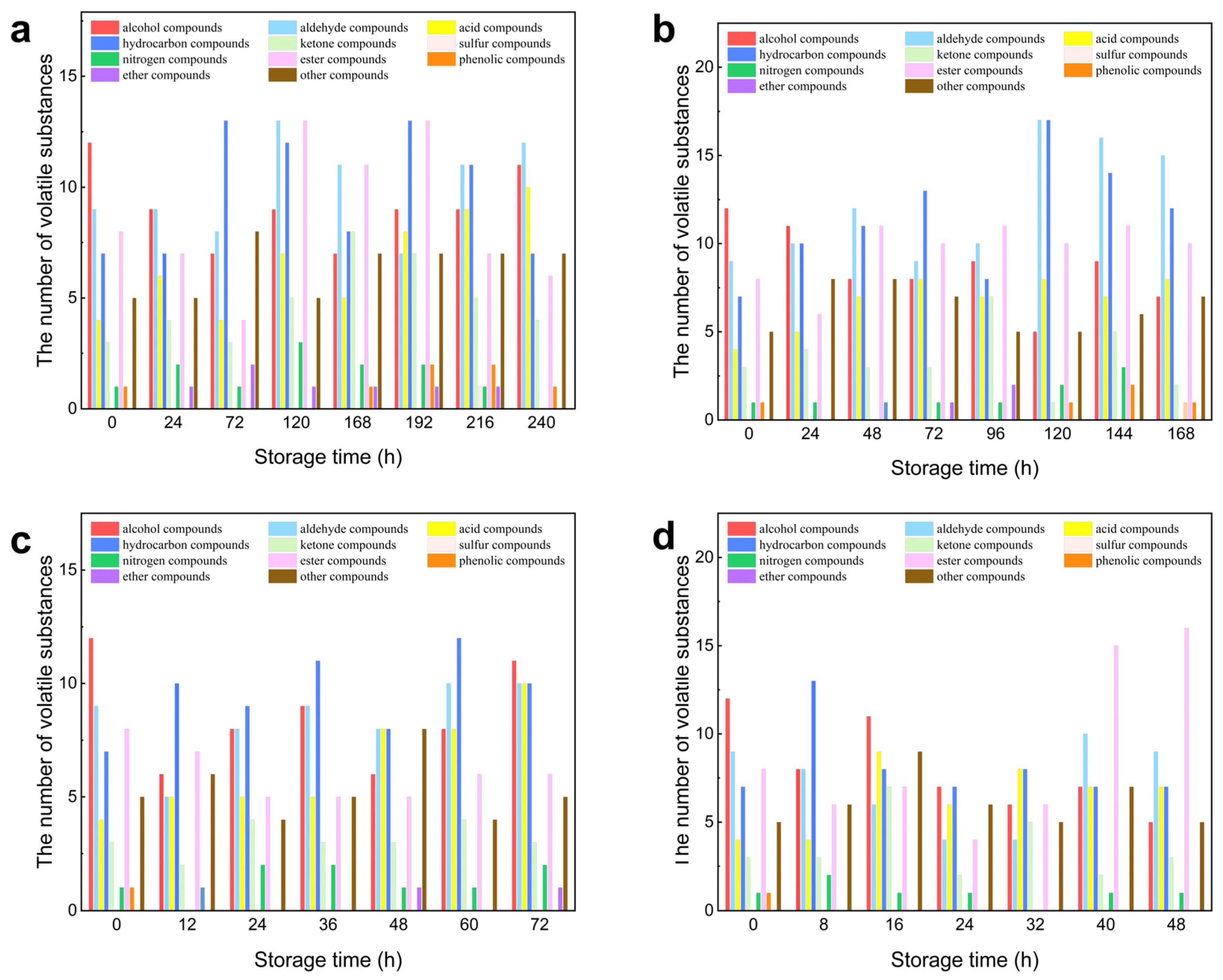
Alcohols are the most representative volatile organic compounds produced during the storage of oysters. The types and relative percentages of alcohols in oysters vary at different stages of spoilage. According to GC-MS analysis of oysters stored at various temperatures (4, 12, 20, and 28 °C), 11, 20, 20, and 34 different alcohols were detected, respectively. During storage, the content of alcohols remained relatively high. Among these alcohols, Styrene, 1-Hexadecanol, Ethanol, 2-(tetradecyloxy)-, 1-Octadecanol, and methyl ether were only present in the early stages of storage. In contrast, 1-Ethynylcyclododecanol, Cyclohexanol, 5-methyl-2-(1-methylethenyl)-, 6-Pentyltetrahydro-2H-pyran-2-ol, and others were formed in the later stages of storage. Myrtenol, which has a grass, wood, and camphor-like aroma, exhibited particularly noticeable changes at 4 °C, with both its absolute and relative contents increasing significantly. At 12 °C, the absolute content of Myrtenol also increased markedly; however, due to the rise in other more volatile substances, the relative content of Myrtenol stabilized. Alcohols are generally formed through the reduction of aldehydes and ketones, as well as the oxidation of lipids, with a typically high threshold. Among these, unsaturated alcohols, such as 1-octene-3-ol, contribute to the fishy taste commonly associated with aquatic products. An increasing body of research indicates that unsaturated alcohols are produced through the oxidation of unsaturated fatty acids, imparting a mushroom-like flavor to fresh seafood [
26]. The odor threshold of unsaturated alcohols is generally lower than that of saturated alcohols, significantly influencing the odor properties of oyster meat.
Aldehydes and ketones exhibit relatively high concentrations and low thresholds during storage, which significantly contributes to flavor [
27]. Aldehydes are primarily formed [
28] from unsaturated fatty acid hydroperoxides through the action of lipoxygenase, a crucial enzyme in food production that generates a variety of oxidative flavors. These aldehydes possess strong aromas reminiscent of grass, fat, and fruit [
29]. Ketones, on the other hand, are unstable intermediate compounds typically produced by the degradation of amino acids and the thermal oxidation of unsaturated fatty acids. They can subsequently be oxidized or reduced into corresponding alcohols, which impart unique fruit flavors [
30]. At storage temperatures of 4, 12, 20, and 28 °C, a total of 19, 29, 16, and 23 types of aldehydes, as well as 15, 10, 8, and 11 types of ketones, were detected in oysters at different storage durations. Notably, compounds such as benzaldehyde, 4-ethyl-, 2, 4-decadienal (E,Z)-, bicyclo [6.1.0]non-4-ene-9-carbaldehyde, 1-cyclohexene-1-acetaldehyde, and α,2-dimethyl- were identified exclusively in the later stages of storage, along with ketones like 2-tridecanone and 2-nonanone.
Fatty acids are often characterized by their aromatic and sweet notes; however, their impact on overall flavor is minimal due to their higher thresholds [
31]. The primary substances identified during storage include acetic acid, caprylic acid, dodecanoic acid, tetradecanoic acid, pentadecanoic acid, and palmitic acid, among others. Acetic acid is the principal contributor to rancidity, which becomes particularly pronounced when its concentration exceeds 1%. Octanoic acid imparts a greasy and creamy taste, detectable at all storage temperatures, and is typically produced during the later stages of storage.
Composed of saturated fatty alcohols and lower levels of saturated fatty acids, most esters emit fruity and floral notes that effectively mask the bitter taste of amino acids and the pungent odor of fatty acids. A total of 28 esters were detected at 4 °C, with the relative content of esters being highest after 168 h of storage. At 12 °C and 20 °C, 21 and 13 types of esters were detected, respectively, while at 28 °C, a total of 27 esters were identified. At 12 °C, the absolute content of esters exhibited an increasing trend, although the relative content began to decline after the fifth day due to the production of additional substances. Conversely, at 20 °C and 28 °C, the absolute content of esters remained low, and the trends in relative content were not clearly defined, likely due to interference from microorganisms and changes in pH.
At all temperatures, the types and relative contents of phenolic and ether compounds are minimal. At 28 °C, no phenols or ethers were detected.
Various kinds of hydrocarbons are present during the storage process, but their relative content remains low. At temperatures of 4, 12, 20, and 28 °C, 30, 46, 31, and 34 types of hydrocarbons were detected, respectively. At 4 °C and 20 °C, the content of hydrocarbon compounds initially increased and then decreased, reaching peak levels at 192 h and 60 h, respectively. At 12 °C, the content of hydrocarbons showed a trend of first decreasing and then increasing. Conversely, at 28 °C, the content of hydrocarbons fluctuated significantly and the trend is irregular. Among these hydrocarbons, compounds such as Styrene, 1,3-Cyclooctadiene, and Allylidenecyclohexane were only detected in the early stages of storage. In contrast, substances like Pentadecane, 1H-Indene, 1-ethylideneoctahydro-, trans-Cyclohexene, and 3-ethyl appeared in the later stages of storage. Due to their higher odor threshold, hydrocarbons exert less influence on food odor [
32]. However, under certain conditions, hydrocarbons can be converted into aldehydes and ketones, which may be responsible for the fishy odor observed in aquatic products [
33].
Under all temperature conditions, the relative content of nitrogen-containing compounds remains low, while sulfur-containing compounds are only detected at 4 °C. At temperatures of 4, 12, 20, and 28 °C, a total of 6, 4, 5, and 7 types of nitrogen-containing compounds were identified, respectively. The primary nitrogen-containing compounds include oxime-, methoxy-phenyl-, and 2-(E)-hexen-1-OL, as well as (4S)-4-amino-5-methyl-. The decomposition of proteins predominantly generates nitrogen-containing compounds through the action of microorganisms and enzymes. Notably, only one sulfur-containing compound was detected in the volatile substances of oysters at 4 °C. These compounds were not present in particularly fresh oyster samples but became apparent with extended storage. Most sulfur compounds, which are characterized by aromas reminiscent of onion, cabbage, boiled sulfur, or rotten eggs, have low thresholds that significantly influence the overall flavor of the food.
The other compounds identified primarily include furan compounds, pyridine compounds, and various additional compounds. GC-MS analysis of oysters stored at different temperatures—4, 12, 20, and 28 °C—revealed the detection of 19, 17, 12, and 21 types of nitrogen compounds, respectively. Among the furan compounds, 2-ethyl-, cis-2-(2-pentenyl) furan was found to be the most abundant, characterized by a strong fleshy, burnt, and sweet aroma, as well as a very low aroma threshold, which contributes to an appealing fragrance. The Maillard reaction, which occurs between amino acids and reducing sugars, is one of the key processes responsible for the development of meat flavor. This complex reaction yields numerous important flavor compounds, including furan, pyrazine, pyrrole, and other heterocyclic compounds [
34]. Furan, 2-pentyl- is primarily generated through the oxidation and degradation of lipids during the heating process. It is commonly found in cooked meat and meat products, imparting a baked flavor along with notes of beans, fruit, green, and similar vegetable flavors [
35]. In low concentrations, furan, 2-ethyl- significantly influences the odor of oysters, contributing both burnt and sweet aromas [
36].