Microbial Growth Inhibition Effect, Polyphenolic Profile, and Antioxidative Capacity of Plant Powders in Minced Pork and Beef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minced Meat
2.2. Plant Material and Preparation of Powders
2.3. Determination of Antioxidative Capacity of Plant Powders
2.4. Determination of Total Phenolic Content
2.5. Preparation of Minced Meats with Plant Powders
2.6. Enumeration of Microorganisms
2.7. Estimation of pH and Water Activity
2.8. Chromatographic Analyses
2.9. Statistical Analyses
3. Results
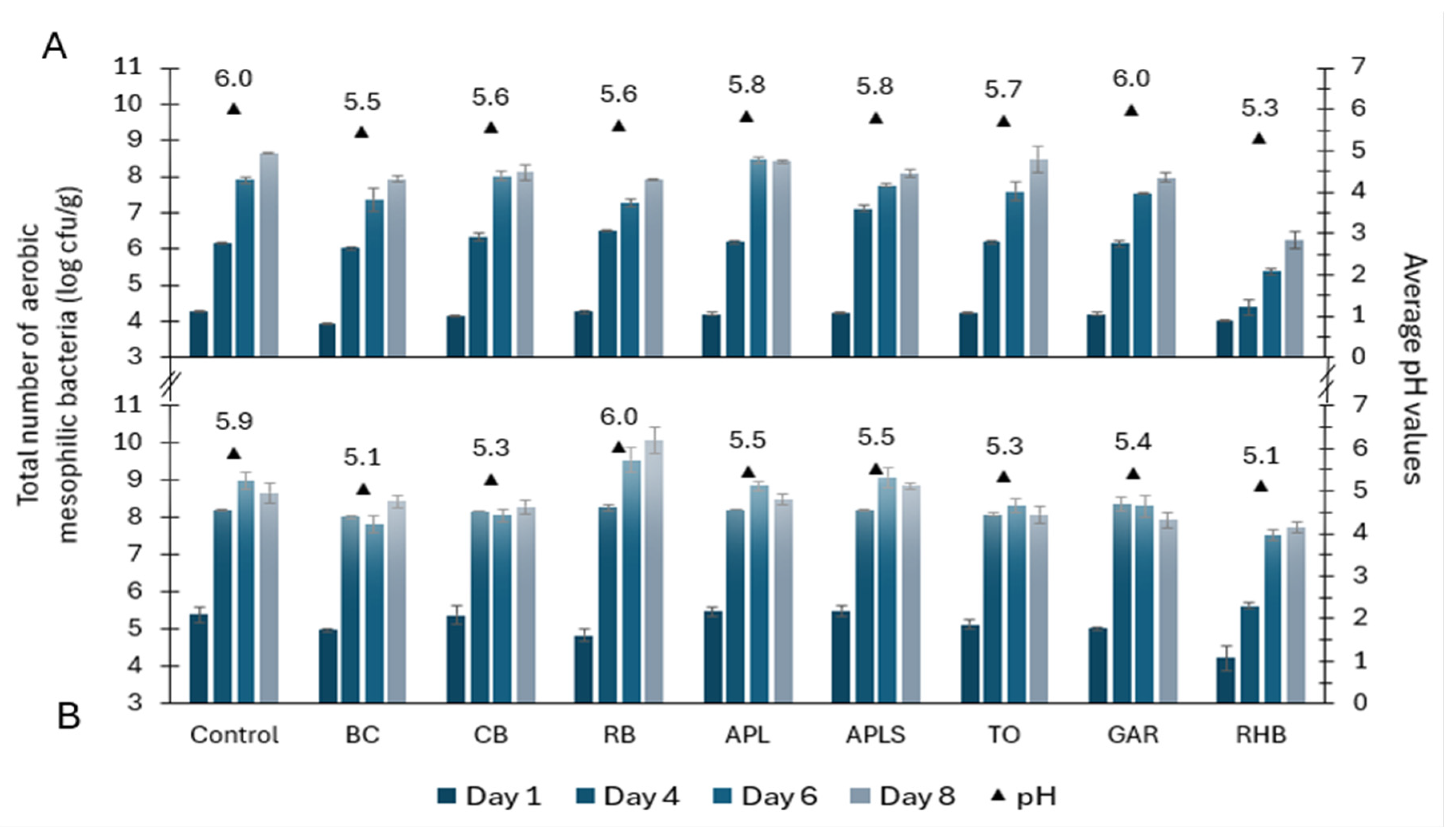
3.1. Total Microbial Counts in Minced Pork and Minced Beef
3.2. Counts of Pseudomonas spp. in Minced Pork and Minced Beef
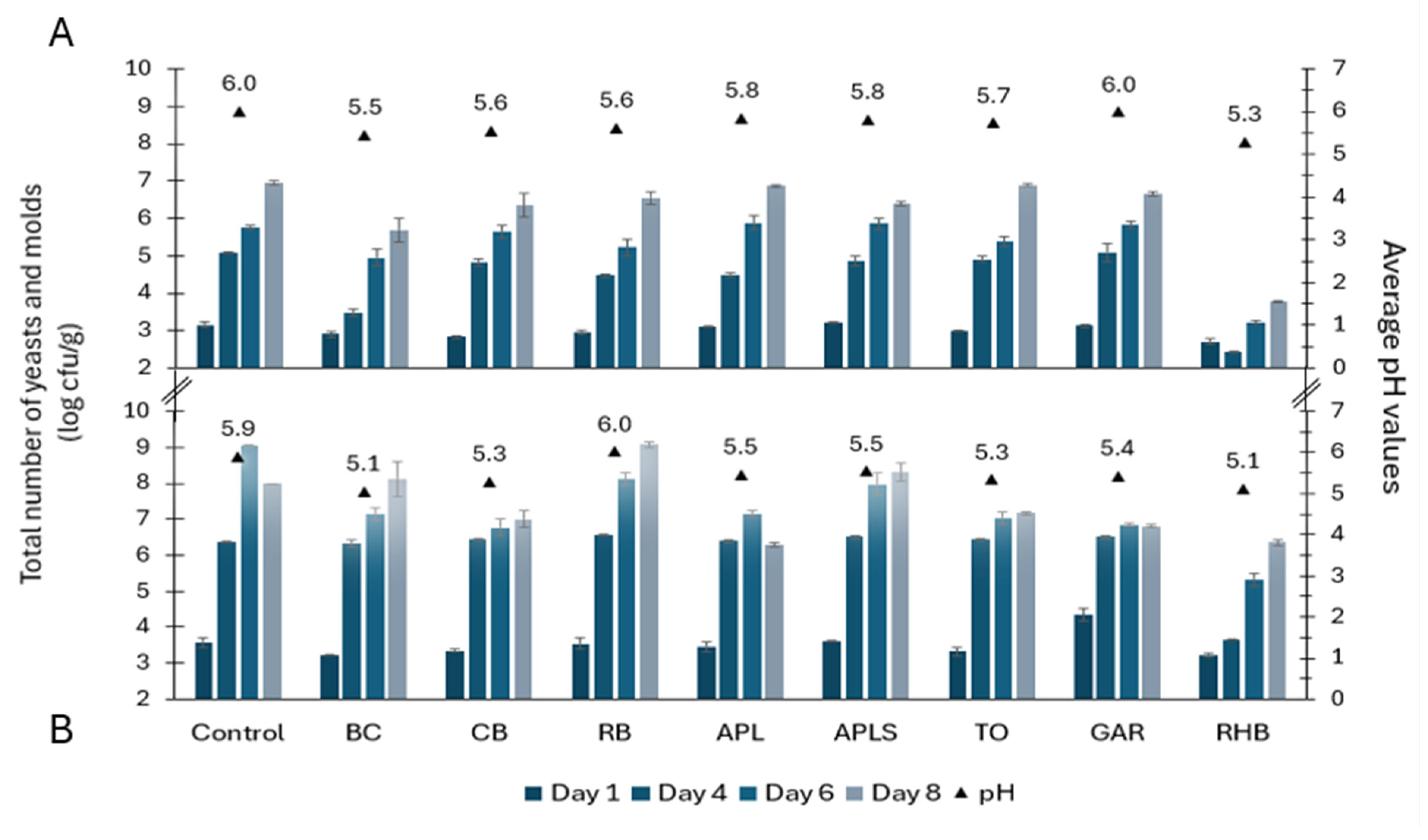
3.3. Yeasts and Molds Counts in Minced Pork and Minced Beef
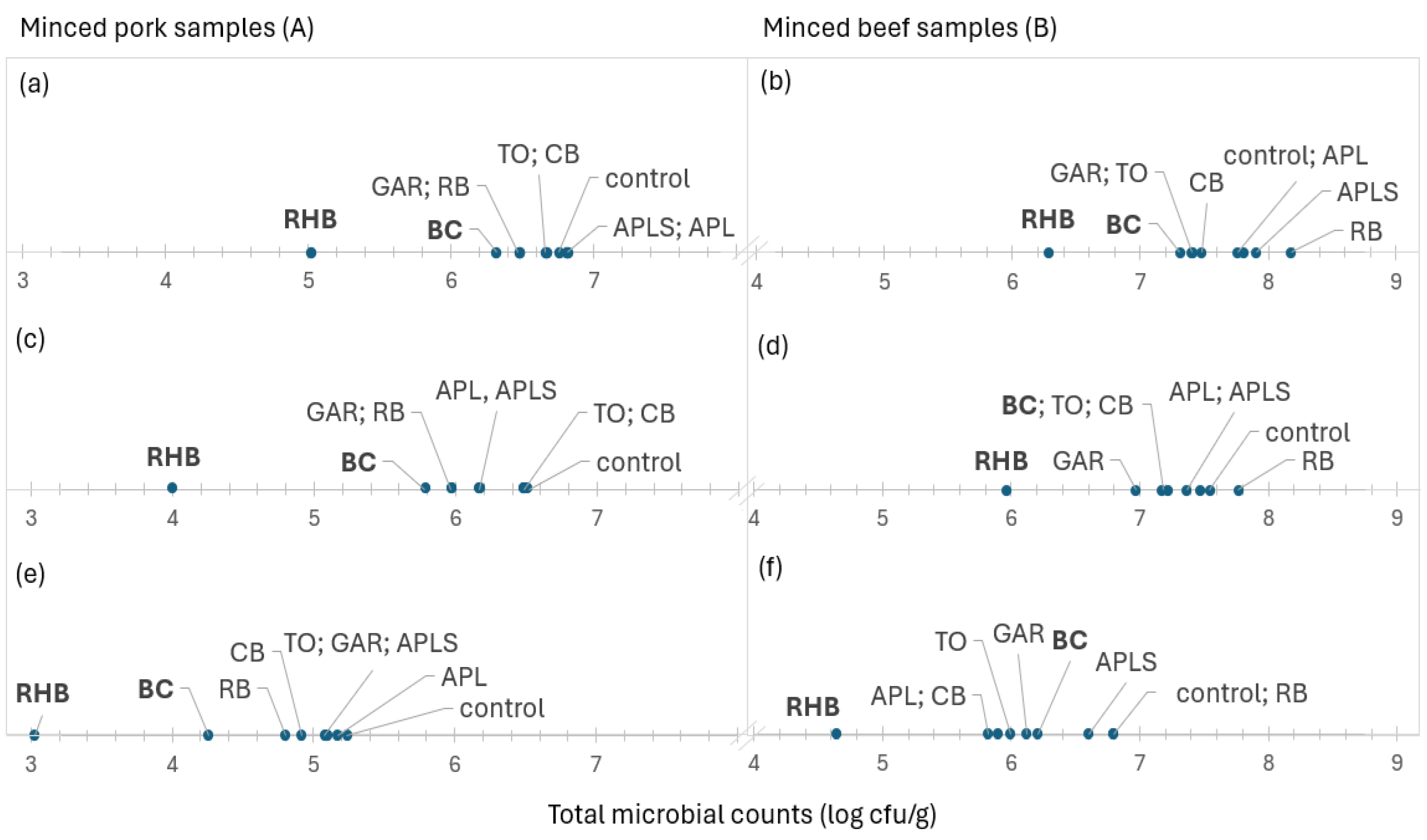
3.4. Ranking of Meat Samples Based on Microbial Counts
3.5. pH and Water Activity in Minced Pork and Minced Beef
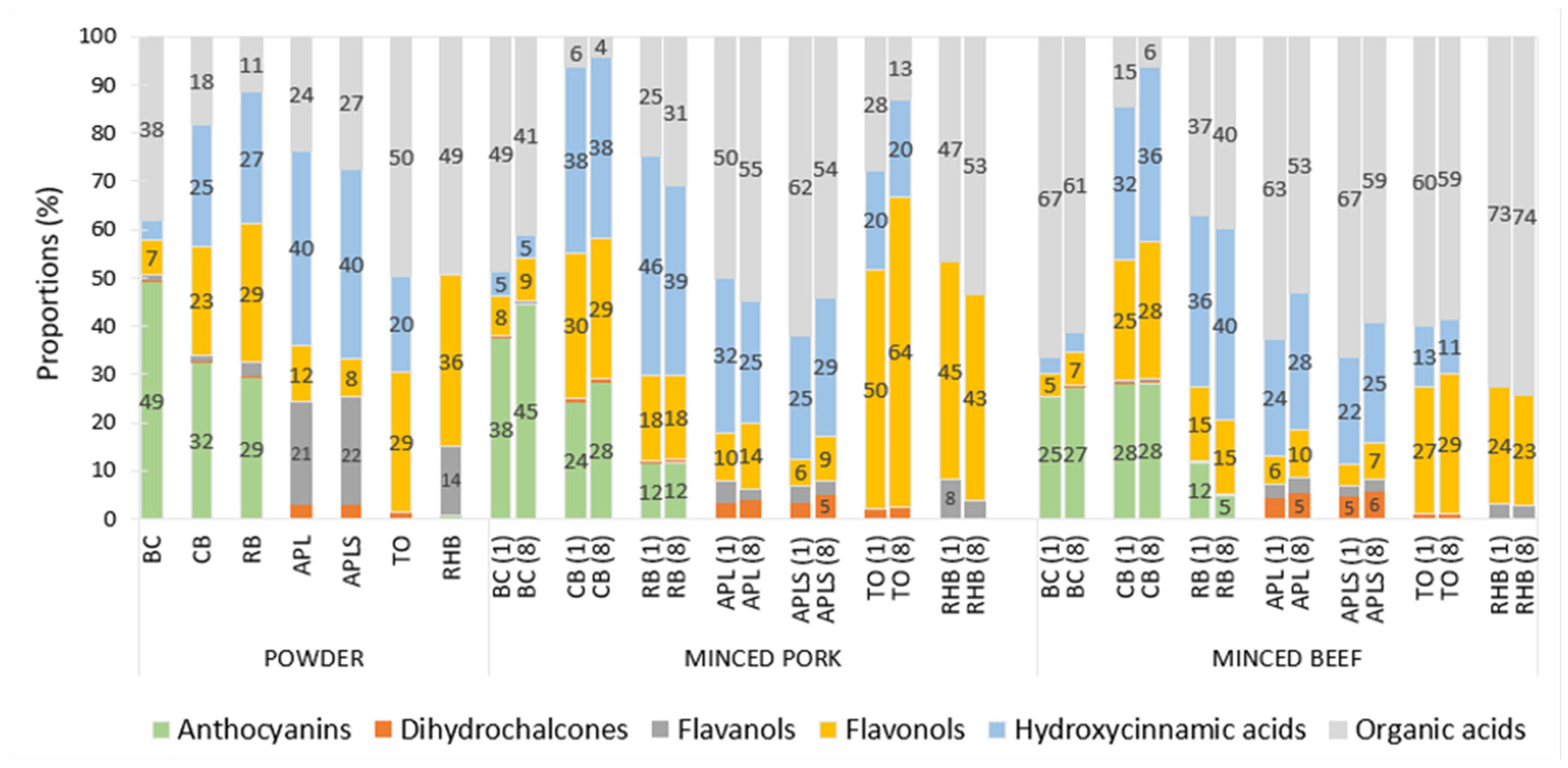
3.6. Polyphenolic Profiles of the Plant Powders and Supplemented Minced Meat Samples
3.7. Antioxidative Capacity of Plant Powders
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, P.M.; Vicente, A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Rani, Z.T.; Mhlongo, L.C.; Hugo, A. Microbial profiles of meat at different stages of the distribution chain from the abattoir to retail outlets. Int. J. Environ. Res. Public Health 2023, 20, 1986. [Google Scholar] [CrossRef] [PubMed]
- Alexa, E.A.; Papadochristopoulos, A.; O’Brien, T.; Burgess, C.M. Chapter 1—Microbial contamination of food. In Food Packaging and Preservation; Jaiswal, A.K., Shankar, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 3–19. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Kalaba, V.; Ilić, T.; Golić, B. Microbiological quality of minced meat and meat preparations. Vet. J. Repub. Srp. 2021, 1–2, 178–186. [Google Scholar] [CrossRef]
- Wickramasinghe, N.N.; Ravensdale, J.; Coorey, R.; Chandry, S.P.; Dykes, G.A. The predominance of psychrotrophic pseudomonads on aerobically stored chilled red meat. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1622–1635. [Google Scholar] [CrossRef]
- Yu, H.H.; Chin, Y.W.; Paik, H.D. Application of natural preservatives for meat and meat products against food-borne pathogens and spoilage bacteria: A review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef]
- Koskar, J.; Meremäe, K.; Püssa, T.; Anton, D.; Elias, T.; Rätsep, R.; Mäesaar, M.; Kapp, K.; Roasto, M. Microbial growth dynamics in minced meat enriched with plant powders. Appl. Sci. 2022, 12, 11292. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Kapp, K.; Kalder, K.; Kikas, A.; Univer, T.; Püssa, T.; Raal, A. Polyphenolic compounds in apple (Malus domestica Borkh.) cultivars grown in Estonia. Proc. Est. Acad. Sci. 2023, 72, 154–166. [Google Scholar] [CrossRef]
- Raudsepp, P.; Koskar, J.; Anton, D.; Meremäe, K.; Kapp, K.; Laurson, P.; Bleive, U.; Kaldmäe, H.; Roasto, M.; Püssa, T. Antibacterial and antioxidative properties of different parts of garden rhubarb, blackcurrant, chokeberry and blue honeysuckle. J. Sci. Food Agric. 2019, 99, 2311–2320. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimia, R.; Westerlund-Wikstrom, B.; Mcdougall, G.; Stewart, D.; Heinonen, M. Rowanberry phenolics: Compositional analysis and bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef] [PubMed]
- Tamkutė, L.; Vaicekauskaitė, R.; Gil, B.M.; Carballido, J.R.; Venskutonis, P.R. Black chokeberry (Aronia melanocarpa L.) pomace extracts inhibit food pathogenic and spoilage bacteria and increase the microbiological safety of pork products. J. Food Process. Preserv. 2021, 45, e15220. [Google Scholar] [CrossRef]
- Anton, D.; Koskar, J.; Raudsepp, P.; Meremäe, K.; Kaart, T.; Püssa, T.; Roasto, M. Antimicrobial and antioxidative effects of plant powders in raw and cooked minced pork. Foods 2019, 8, 661. [Google Scholar] [CrossRef] [PubMed]
- Klavins, L.; Kviesis, J.; Nakurte, I.; Klavins, M. Berry press residues as a valuable source of polyphenolics: Extraction optimisation and analysis. LWT–Food Sci. Technol. 2018, 93, 583–591. [Google Scholar] [CrossRef]
- Roasto, M.; Mäesaar, M.; Püssa, T.; Anton, D.; Rätsep, R.; Elias, T.; Jortikka, S.; Pärna, M.; Kapp, K.; Tepper, M.; et al. The effect of fruit and berry pomaces on the growth dynamics of microorganisms and sensory properties of marinated rainbow trout. Microorganisms 2023, 11, 2960. [Google Scholar] [CrossRef]
- Gniewosz, M.; Stobnicka, A. Bioactive components content, antimicrobial activity, and foodborne pathogen control in minced pork by cranberry pomace extracts. J. Food Saf. 2017, 38, e12398. [Google Scholar] [CrossRef]
- Meremäe, K.; Raudsepp, P.; Rusalepp, L.; Anton, D.; Bleive, U.; Roasto, M. In vitro antibacterial and antioxidative activity and polyphenolic profile of the extracts of chokeberry, blackcurrant, and rowan berries and their pomaces. Foods 2024, 13, 421. [Google Scholar] [CrossRef]
- Garzón, G.A.; Soto, C.Y.; López-R, M.; Riedl, K.M.; Browmiller, C.R.; Howard, L. Phenolic profile, in vitro antimicrobial activity and antioxidant capacity of Vaccinium meridionale Swartz pomace. Heliyon 2020, 6, e03845. [Google Scholar] [CrossRef] [PubMed]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2018, 99, 2068–2077. [Google Scholar] [CrossRef]
- Kerner, K.; Kazernavičiūtė, R.; Jõudu, J.; Rocchetti, G.; Lucini, L.; Tänavots, A.; Hussain, S.; Venskutonis, P.R. Evaluation of different blackcurrant seed ingredients in meatballs by using conventional quality assessment and untargeted metabolomics. Meat Sci. 2023, 200, 109160. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Chem. Biol. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- EVS-EN ISO 4833-2:2013/A1:2022; Horizontal Method for the Enumeration of Microorganisms. Part 2: Colony Count at 30 °C by the Surface Plating Technique. ISO: Geneva, Switzerland, 2022.
- EVS-EN ISO 13720:2010; Meat and Meat Products—Enumeration of Presumptive Pseudomonas spp. ISO: Geneva, Switzerland, 2010.
- EVS-ISO 21527-1:2009; Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. ISO: Geneva, Switzerland, 2009.
- JASP Team. 2024. JASP (Version 0.18.3) [Computer Software]. Available online: https://jasp-stats.org/previous-versions/ (accessed on 15 April 2024).
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20200308&from=EN (accessed on 2 April 2024).
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.E.; Broadbent, J.R. External concentration of organic acid anions and pH: Key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods. J. Food Sci. 2009, 74, 12–15. [Google Scholar] [CrossRef]
- Püssa, T.; Raudsepp, P.; Kuzina, K.; Raal, A. Polyphenolic composition of roots and petioles of Rheum rhaponticum L. Phytochem. Anal. 2009, 20, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Trajković, M.; Kitić, D.; Mihajilov-Krstev, T.; Šavikin, K.; Ranđelović, M.; Milutinović, M.; Branković, S.; Kitić, N.; Miladinović, B. Antimicrobial activity evaluation of black currant (Ribes nigrum L.) variety Čačanska crna juice and extract. Acta Fac. Med. Naiss. 2023, 40, 29. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Olschowsky, P.; Tonndorf-Martini, S.; Heyder, M.; Pfister, W.; Reise, M.; Sigusch, B. Antimicrobial effect of natural berry juices on common oral pathogenic bacteria. Antibiotics 2020, 9, 533. [Google Scholar] [CrossRef]
- Cho, J.; Kim, H.-J.; Kwon, J.-S.; Kim, H.-J.; Jang, A. Effect of marination with black currant juice on the formation of biogenic amines in pork belly during refrigerated storage. Food Sci. Anim. Resour. 2021, 41, 763–778. [Google Scholar] [CrossRef]
- Anttonen, M.J.; Karjalainen, R.O. High-performance liquid chromatography analysis of black currant (Ribes nigrum L.) fruit phenolics grown either conventionally or organically. J. Agric. Food Chem. 2006, 54, 7530–7538. [Google Scholar] [CrossRef]
- Badarinath, A.V.; Rao, K.M.; Chetty, C.M.S.; Ramkanth, S.T.V.S.R.; Rajan, T.V.S.; Gnanaprakash, K. A review on in-vitro antioxidant methods: Comparisions, correlations and considerations. Int. J. PharmTech Res. 2010, 2, 1276–1285. [Google Scholar]
- Burri, S.C.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Kashmiri, Z.N.; Mankar, S.A. Free radicals and oxidative stress in bacteria. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 34–40. [Google Scholar]
- Wyżga, B.; Skóra, M.; Wybraniec, S.; Hąc-Wydro, K. Study on the effect of blackcurrant extract—Based preservative on model membranes and pathogenic bacteria. Arch. Biochem. Biophys. 2023, 750, 109806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, Y.; Deng, H.; Meng, Y. Antibacterial mechanism of apple phloretin on physiological and morphological properties of Listeria monocytogenes. Food Sci. Technol. 2022, 42, e55120. [Google Scholar] [CrossRef]
- Barreca, E.B.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Venskutonis, P.R. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of the extracts isolated from the pomace of rowanberry (Sorbus aucuparia L.). Food Res. Int. 2020, 136, 109310. [Google Scholar] [CrossRef]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa fruits as a rich dietary source of chlorogenic acids and anthocyanins: 1H-NMR, HPLC-DAD, and chemometric studies. Molecules 2020, 15, 3234. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. LWT 2019, 116, 108558. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Claire Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Mohammed, E.T.; Mustafa, Y.F. Coumarins from red delicious apple seeds: Extraction, phytochemical analysis, and evaluation as antimicrobial agents. Syst. Rev. Pharm. 2020, 11, 64–70. [Google Scholar] [CrossRef]
- Weiss, J.; Loeffler, M.; Terjung, N. The antimicrobial paradox: Why preservatives lose activity in foods. Curr. Opin. Food Sci. 2015, 4, 69–75. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Oulahal, N.; Dumas, E.; Thanh, N.T.T.; Bouajila, J.; Souchard, J.-P.; Degraeve1, P. Effect of interaction with food constituents on plant extracts antibacterial activity. Food Sci. Appl. Biotechnol. 2018, 1, 77–85. [Google Scholar] [CrossRef]

| Pseudomolecular Ion Mass-to-Charge Ratio (m/z) | Compound | Detected in Tested Samples * | ||||||
|---|---|---|---|---|---|---|---|---|
| BC | CB | RB | APL | APLS | TO | RHB | ||
| Anthocyanins: | ||||||||
| 417.0827; 435.0933 | Cyanidin pentosides | +●○▲∆ | +●○▲∆ | |||||
| 447.0928; 465.1038 | Cyanidin hexoside | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | + ▲∆ | |||
| 593.1506; 611.1618 | Cyanidin rutinoside | +●○ ▲∆ | + | + | ||||
| 463.0882; 481.0988 | Delphinidin glucoside | +●○ ▲∆ | ||||||
| 609.1461; 627.1567 | Delphinidin rutinoside | +●○ ▲∆ | ||||||
| Dihydrochalcones: | ||||||||
| 435.1297 | Phloridzin | +●○ | +●○ | +●○ | +●○▲∆ | +●○ ▲∆ | + | +●○ |
| 597.1825 | Phloretin-di-C-hexoside | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | + | + | +●○▲∆ | |
| Flavanols: | ||||||||
| 289.0718 | Catechins | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | +●○▲∆ | +●○ ▲∆ | +●○▲∆ | |
| 305.0700 | (epi)gallocatechin | + | ||||||
| 577.1352 | Procyanidin B-type | + | + | + | +●○▲∆ | +●○ ▲∆ | + | |
| 865.1985 | Procyanidin C-type | + | + | + | + | + | ||
| Flavonols: | ||||||||
| 301.0354 | Quercetin | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | + | + | +●○ ∆ | +● ▲∆ |
| 433.0776 | Quercetin pentosides | +●○ | +●○▲∆ | +●○ | +●○▲∆ | +●○ ▲∆ | +●○ | |
| 447.0928 | Quercetin rhamnoside | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | +●○▲∆ | +●○ ▲∆ | + | +●○▲∆ |
| 463.0882 | Quercetin hexosides | + | +●○▲∆ | +●○▲∆ | + | + | + | + ▲∆ |
| 463.0882 | Aromadendrin glucuronide | + | + | + | ||||
| 595.1305 | Quercetin pentosyl-hexosides | +●○▲∆ | +●○▲∆ | +●○ | ||||
| 593.1506 | Kaempferol rutinoside | + | + | + | + | + | ||
| 609.1461 | Quercetin rhamnosyl hexoside | +●○ ▲∆ | +●○ ▲∆ | +●○ ▲∆ | +●○ | +●○ | +●○ | +●○ |
| 625.1410 | Quercetin dihexosides | + | +●○▲∆ | +●○▲∆ | + | |||
| 741.1884 | Quercetin pentosyl rutinoside | ▲∆ | +● ▲∆ | |||||
| 771.1989 | Quercetin hexosyl rutinoside | +● ▲∆ | ||||||
| Hydroxycinnamic acids: | ||||||||
| 153.0193 | Protocatechuic acid | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | +○ ▲∆ | +○ ▲∆ | +●○▲∆ | + |
| 337.0929 | Coumaroylquinic acids | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | +●○▲∆ | +●○ ▲∆ | + ▲∆ | |
| 341.0878 | Caffeic acid glucosides | +● ▲∆ | + | + | +● ▲∆ | + | ||
| 353.0873 | Chlorogenic acids | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | +●○▲∆ | +●○ ▲∆ | +●○▲∆ | |
| 515.1195 | Di-caffeoylquinic acids | +●○▲∆ | +●○▲∆ | +●○▲∆ | +●○ ▲∆ | +●○▲∆ | ||
| Organic acids: | ||||||||
| 147.0299 | Citramalic acid | +●○ ▲∆ | +●○ ▲∆ | |||||
| 191.0197 | Citric acid | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | +●○▲∆ | +●○ ▲∆ | +●○▲∆ | +●○▲∆ |
| 191.0556 | Quinic acid | +●○ ▲∆ | +●○▲∆ | +●○▲∆ | +●○▲∆ | +●○ ▲∆ | +●○▲∆ | + |
| Plant Material | Abbreviation | AO mM TE/DWg | TPC mg GAE/DWg |
|---|---|---|---|
| Blackcurrant | BC | 130 ± 8.7 | 24.6 ± 0.07 |
| Chokeberry | CB | 127 ± 25.9 | 55.0 ± 1.29 |
| Rowan berries | RB | 119 ± 0.6 | 19.9 ± 0.06 |
| Apple with seeds | APLS | 105 ± 4.4 | 13.3 ± 0.04 |
| Apple, seeds removed | APL | 104 ± 4.1 | 12.7 ± 0.04 |
| Rhubarb | RHB | 88 ± 9.2 | 10.5 ± 0.05 |
| Tomato | TO | 75 ± 3.2 | 16.1 ± 0.05 |
| Garlic | GAR | 72 ± 5.5 | 3.9 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meremäe, K.; Rusalepp, L.; Sünter, A.; Raudsepp, P.; Anton, D.; Mäesaar, M.; Elias, T.; Püssa, T.; Roasto, M. Microbial Growth Inhibition Effect, Polyphenolic Profile, and Antioxidative Capacity of Plant Powders in Minced Pork and Beef. Foods 2024, 13, 3117. https://doi.org/10.3390/foods13193117
Meremäe K, Rusalepp L, Sünter A, Raudsepp P, Anton D, Mäesaar M, Elias T, Püssa T, Roasto M. Microbial Growth Inhibition Effect, Polyphenolic Profile, and Antioxidative Capacity of Plant Powders in Minced Pork and Beef. Foods. 2024; 13(19):3117. https://doi.org/10.3390/foods13193117
Chicago/Turabian StyleMeremäe, Kadrin, Linda Rusalepp, Alar Sünter, Piret Raudsepp, Dea Anton, Mihkel Mäesaar, Terje Elias, Tõnu Püssa, and Mati Roasto. 2024. "Microbial Growth Inhibition Effect, Polyphenolic Profile, and Antioxidative Capacity of Plant Powders in Minced Pork and Beef" Foods 13, no. 19: 3117. https://doi.org/10.3390/foods13193117






