Comparison of Ultra-High-Pressure and Conventional Cold Brew Coffee at Different Roasting Degrees: Physicochemical Characteristics and Volatile and Non-Volatile Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Sample Preparation
2.2. Total Dissolved Solids and Extraction Yield
2.3. Total Titratable Acidity, Total Sugars, and Total Phenolic Compounds
2.4. Antioxidant Capacity and Melanoidins
2.5. Caffeine, Trigonelline, and CGAs
2.6. Volatile Compounds
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics
3.2. Non-Volatile Components
3.3. Antioxidant Capacity
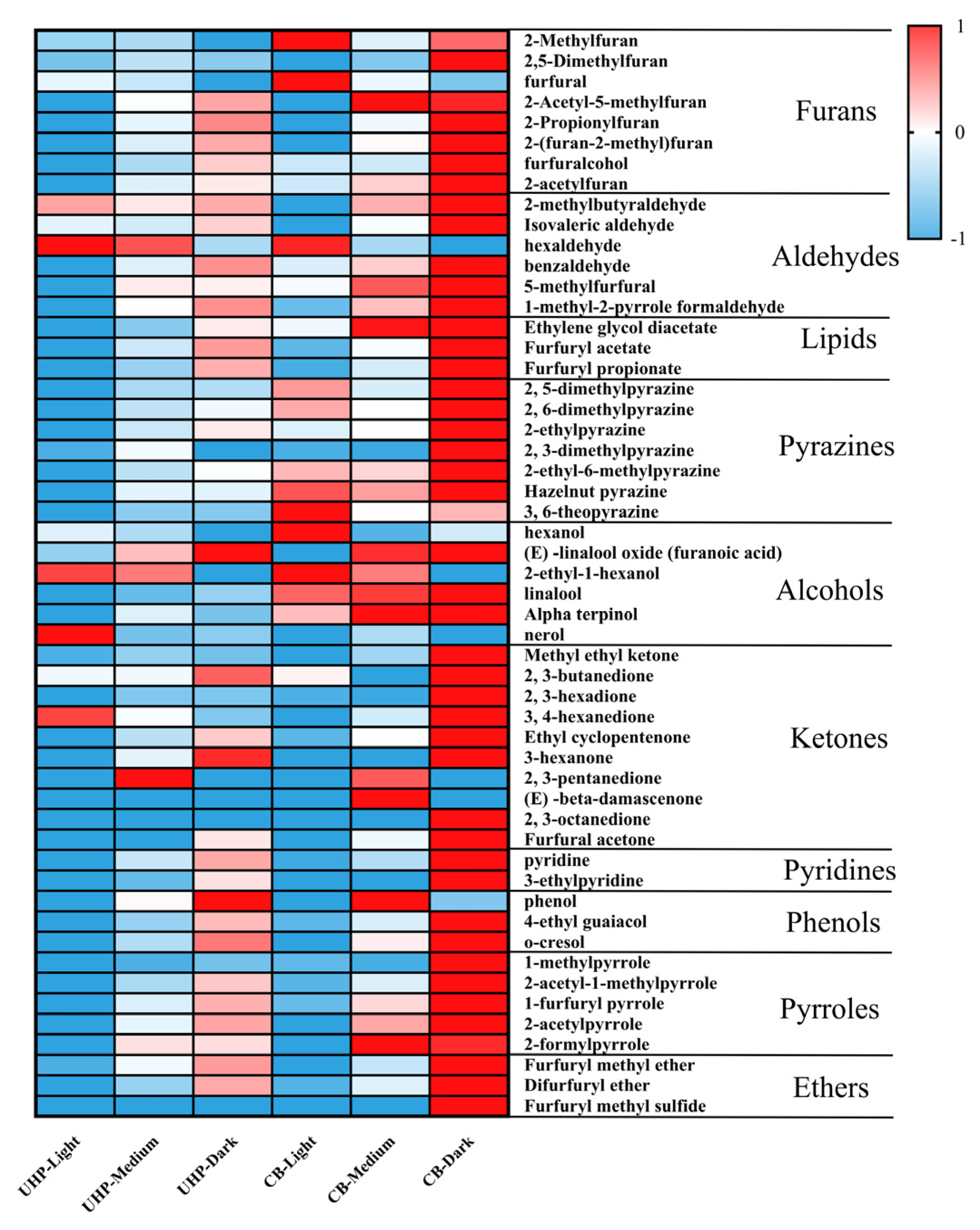
3.4. Volatile Composition
3.5. Sensory Analysis
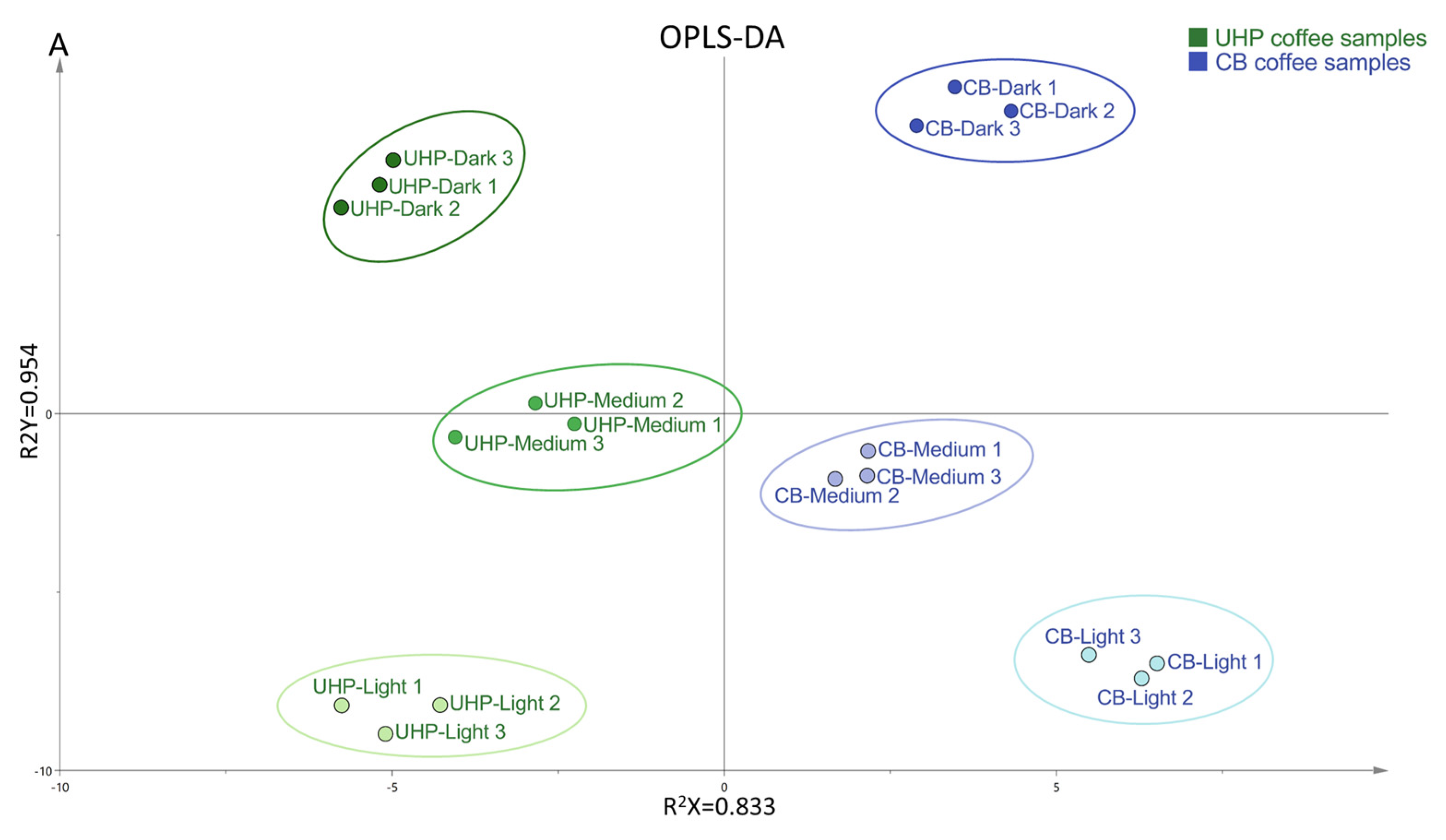
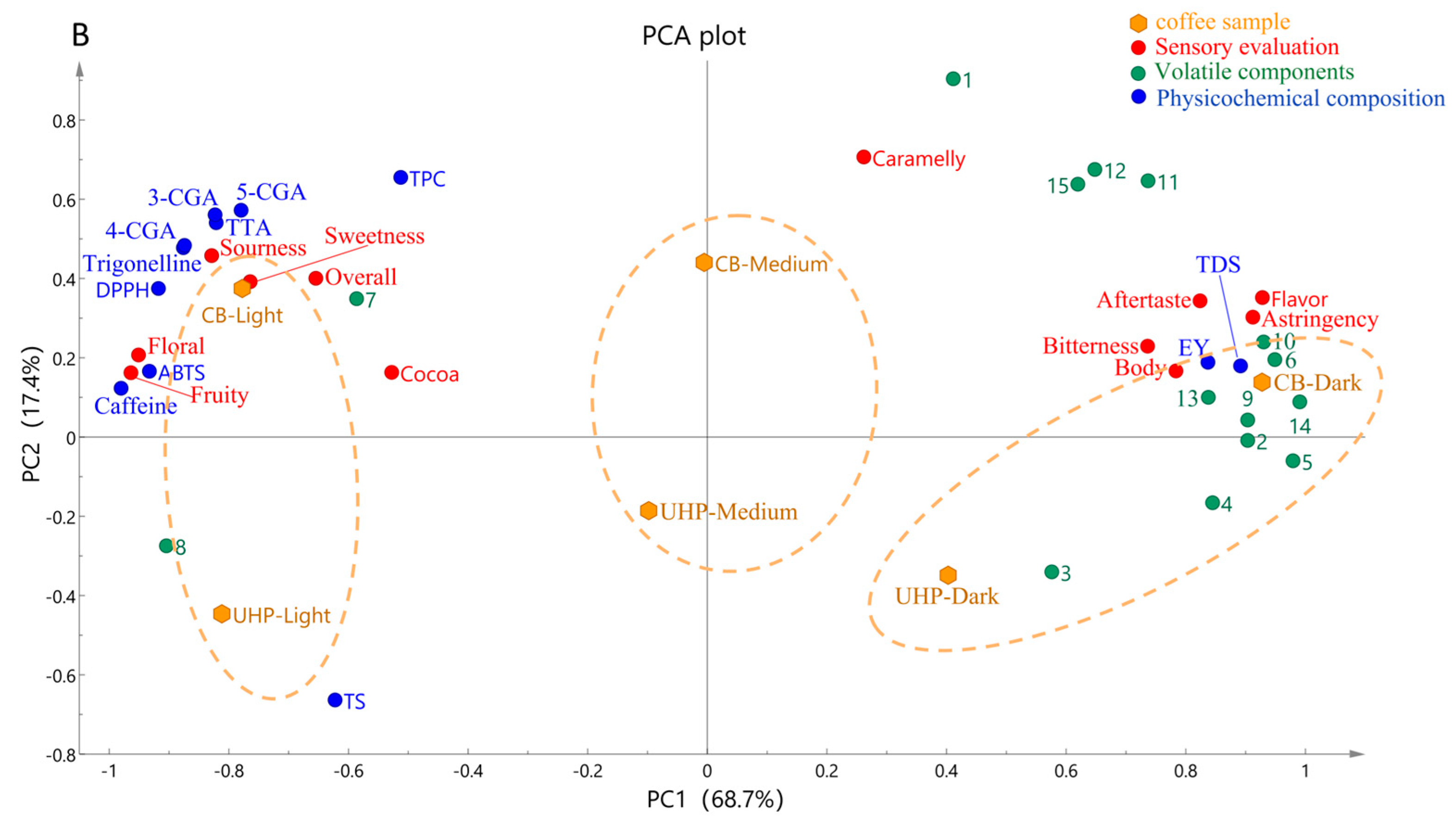
3.6. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ridder, M. Cold Brew Coffee Market Value in the U.S. 2015–2025. 2022. Available online: https://www.statista.com/statistics/659724/cold-brew-coffee-sales-us/ (accessed on 13 January 2022).
- Cai, Y.; Xu, Z.; Pan, X.; Gao, M.; Wu, M.; Wu, J.; Lao, F. Comparative Profiling of Hot and Cold Brew Coffee Flavor Using Chromatographic and Sensory Approaches. Foods 2022, 11, 2968. [Google Scholar] [CrossRef]
- Stanek, N.; Zarębska, M.; Biłos, Ł.; Barabosz, K.; Nowakowska-Bogdan, E.; Semeniuk, I.; Błaszkiewicz, J.; Kulesza, R.; Matejuk, R.; Szkutnik, K. Influence of coffee brewing methods on the chromatographic and spectroscopic profiles, antioxidant andInfluence of coffee brewing methods on the chromatographic and spectroscopic profiles, antioxidant and sensory properties. Sci. Rep. 2021, 11, 21377. [Google Scholar] [CrossRef]
- Zhai, X.; Yang, M.; Zhang, J.; Zhang, L.; Tian, Y.; Li, C.; Bao, L.; Ma, C.; El-Aty, A.M.A. Feasibility of Ultrasound-Assisted Extraction for Accelerated Cold Brew Coffee Processing: Characterization and Comparison with Conventional Brewing Methods. Front. Nutr. 2022, 9, 849811. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Jiang, G.H.; Park, J.S.; Lee, K.C.; Seok, Y.Y.; Eun, J.B. Effects of ultrasonication, agitation and stirring extraction techniques on the physicochemical properties, health-promoting phytochemicals, and structure of cold brewed coffee. Sci. Food Agric. 2018, 99, 290–301. [Google Scholar] [CrossRef]
- Kyroglou, S.; Laskari, R.; Vareltzis, P. Optimization of sensory properties of cold brew coffee produced by reduced pressure cycles and its physicochemical characteristics. Molecules 2022, 27, 2971. [Google Scholar] [CrossRef]
- Wen, H.U.; Ting, G.U.O.; Jiang, W.J.; Guang-Li, D.O.N.G.; Da-Wei, C.H.E.N.; Shi-Lin, Y.A.N.G.; He-Ran, L.I. Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cymaroside extracted from flower buds of Lonicera japonica. Chin. J. Nat. Med. 2015, 13, 445–453. [Google Scholar]
- Nan, J.; Zou, M.; Wang, H.; Xu, C.; Zhang, J.; Wei, B.; He, L.; Xu, Y. Effect of ultra-high pressure on molecular structure and properties of bullfrog skin collagen. Int. J. Biol. Macromol. 2018, 111, 200–207. [Google Scholar] [CrossRef]
- Huang, H.-W.; Chen, B.-Y.; Wang, C.-Y. Extraction of bioactive ingredients from fruiting bodies of Antrodia cinnamomea assisted by high hydrostatic pressure. J. Food Sci. Technol. 2019, 56, 3988–3997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Manickavasagan, A.; Lim, L.-T. Extraction and Physicochemical Characteristics of High Pressure-Assisted Cold Brew Coffee. Future Foods 2022, 5, 100–113. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Y.; Tang, W.; Jiang, F.; Zhu, J.; Zhou, Y.; Ye, L. Evaluation of Physicochemical Characteristics and Sensory Properties of Cold Brew Coffees Prepared Using Ultrahigh Pressure under Different Extraction Conditions. Foods 2023, 12, 3857. [Google Scholar] [CrossRef]
- Li, T.; Deng, L.L.; Xia, R.; Luo, L.; Zhong, G. Effects of different extraction methods on physicochemical and sensory properties of coffee stock solution. Food Ferment. Ind. 2022, 48, 168–174. [Google Scholar]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical characteristics of hot and cold brew coffee chemistry: The effects of roast level and brewing temperature on compound extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Fasano, E.; De Vivo, A.; Velotto, S.; Sarghini, F.; Cirillo, T. Processing effects on acrylamide content in roasted coffee production. Food Chem. 2020, 319, 126550. [Google Scholar] [CrossRef] [PubMed]
- Elmacı, I.; Gok, I. Effect of three post-harvest methods and roasting degree on sensory profile of Turkish coffee assessed by Turkish and Brazilian panelists. J. Sci. Food Agric. 2021, 101, 5368–5377. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Rao, N.Z. The Effect of Time, Roasting Temperature, and Grind Size on Caffeine and Chlorogenic Acid Concentrations in Cold Brew Coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-M.; Chu, M.; Park, H.; Park, J.; Lee, K.-G. Analysis of Volatile Compounds in Coffee Prepared by Various Brewing and Roasting Methods. Foods 2021, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The effect of brewing process parameters on antioxidant activity and caffeine content in infusions of roasted and unroasted Arabica coffee beans originated from different countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, Y.; Jiang, F.; Jiang, T.; Zhu, J.; Tang, W.; Liu, X.; Zhou, Y.; Yu, L. Comparison of Characterization of Cold Brew and Hot Brew Coffee Prepared at Various Roasting Degrees. J. Food Process. Preserv. 2023, 2023, 3175570. [Google Scholar] [CrossRef]
- Vezzulli, F.; Lambri, M.; Bertuzzi, T. Volatile compounds in green and roasted Arabica specialty coffee: Discrimination of origins, post-harvesting processes, and roasting level. Foods 2023, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Bhumiratana, N.; Adhikari, K.; Chambers, E., IV. Evolution of sensory aroma attributes from coffee beans to brewed coffee. LWT-Food Sci. Technol. 2011, 44, 2185–2192. [Google Scholar] [CrossRef]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D‘ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of Nine Common Coffee Extraction Methods: Instrumental and Sensory Analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef]
- Wang, X.; William, J.; Fu, Y.; Lim, L.T. Effects of Capsule Parameters on Coffee Extraction in Single-Serve Brewer. Food Res. Int. 2016, 89, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.S.; Landhausser, S.M. A Method for Routine Measurements of Total Sugar and Starch Content in Woody Plant Tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Bilge, G. Investigating the Effects of Geographical Origin, Roasting Degree, Particle Size and Brewing Method on the Physicochemical and Spectral Properties of Arabica Coffee by Pca Analysis. J. Food Sci. Technol. 2020, 57, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.L.B.; Viegas, M.C.; Ferrão, M.A.G.; Fonseca, A.F.; Ferrão, R.G.; Benassi, M.T. Coffee Brews Composition from Coffea Canephora Cultivars with Different Fruit-Ripening Seasons. Br. Food J. 2020, 122, 827–840. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. Effect of Different Drying Techniques on Bioactive Components, Fatty Acid Composition, and Volatile Profile of Robusta Coffee Beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef]
- Gorecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its in Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; Velásquez, S.; Fernandez-Alduenda, M.; Ruiz-Pardo, Y. Specialty and regular coffee bean quality for cold and hot brewing: Evaluation of sensory profile and physicochemical characteristics. LWT 2021, 145, 111363. [Google Scholar] [CrossRef]
- Abrankó, L.; Clifford, M.N. An unambiguous nomenclature for the acylquinic acids commonly known as chlorogenic acids. J. Agric. Food Chem. 2017, 65, 3602–3608. [Google Scholar] [CrossRef]
- ISO8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007. Available online: https://www.iso.org/standard/36385.html (accessed on 1 June 2009).
- Ginz, M.; Balzer, H.H.; Bradbury, A.G.W.; Maier, H.G. Formation of aliphatic acids by carbohydrate degradation during roasting of coffee. Eur. Food Res. Technol. 2000, 211, 404–410. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.G.; Shibamoto, T. Antioxidative activities of fractions obtained from brewed coffee. J. Agric. Food Chem. 2004, 52, 592–596. [Google Scholar] [CrossRef]
- Wei, F. Chemical Changes in the Components of Coffee Beans during Roasting. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 83–91. [Google Scholar]
- Crozier, T.W.M.; Stalmach, A.; Lean, M.E.J.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Herawati, D.; Giriwono, P.E.; Dewi, F.N.A.; Kashiwagi, T.; Andarwulan, N. Critical roasting level determines bioactive content and antioxidant activity of Robusta coffee beans. Food Sci. Biotechnol. 2019, 28, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Trugo, L.C.; Macrae, R. A study of the effect of roasting on the chlorogenic acid composition of coffee using HPLC. Food Chem. 1984, 15, 219–227. [Google Scholar] [CrossRef]
- Vorotnikov, V.; Mpourmpakis, G.; Vlachos, D.G. DFT study of furfural conversion to furan, furfuryl alcohol, and 2-methylfuran on Pd (111). ACS Catal. 2012, 2, 2496–2504. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. J. Agric. Food Chem. 2009, 57, 5823–5831. [Google Scholar] [CrossRef]
- Poisson, L.; Auzanneau, N.; Mestdagh, F.; Blank, I.; Davidek, T. New insight into the role of sucrose in the generation of α-diketones upon coffee roasting. J. Agric. Food Chem. 2016, 66, 2422–2431. [Google Scholar] [CrossRef]
- Pickard, S.; Becker, I.; Merz, K.-H.; Richling, E. Determination of the alkylpyrazine composition of coffee using stable isotope dilution–gas chromatography–mass spectrometry (SIDA-GC-MS). J. Agric. Food Chem. 2013, 61, 6274–6281. [Google Scholar] [CrossRef]
- Münchow, M.; Alstrup, J.; Steen, I.; Giacalone, D. Roasting conditions and coffee flavor: A multi-study empirical investigation. Beverages 2020, 6, 29. [Google Scholar] [CrossRef]
- Seninde, D.R.; Chambers, E., IV; Chambers, D. Determining the impact of roasting degree, coffee to water ratio and brewing method on the sensory characteristics of cold brew Ugandan coffee. Food Res. Int. 2020, 137, 109667. [Google Scholar] [CrossRef]
| Extraction Conditions | Roast Level | Total Dissolved Solids/% | Extraction Yield/% | Titratable Acidity/(mL 0.1 mol/L NaOH) | Total Phenol Content/(mg/mL) | Total Sugar/(mg/mL) | Melanoidin/(mg/mL) |
|---|---|---|---|---|---|---|---|
| UHP (300 MPa 20 min) | Light | 1.10 ± 0.02 c | 16.08 ± 0.25 c | 0.34 ± 0.01 ab | 4.33 ± 0.22 ab | 0.84 ± 0.02 a | 4.21 ± 0.19 d |
| Medium | 1.18 ± 0.02 b | 17.11 ± 0.32 b | 0.32 ± 0.01 b | 3.40 ± 0.19 c | 0.81 ± 0.01 a | 5.23 ± 0.33 d | |
| Dark | 1.28 ± 0.03 a | 18.12 ± 0.38 a | 0.27 ± 0.01 c | 3.02 ± 0.13 d | 0.73 ± 0.02 b | 6.35 ± 0.28 a | |
| CB (0.1 MPa 12 h) | Light | 1.11 ± 0.01 c | 16.10 ± 0.23 c | 0.37 ± 0.02 a | 4.82 ± 0.35 a | 0.73 ± 0.01 b | 4.33 ± 0.19 d |
| Medium | 1.21 ± 0.02 b | 17.39 ± 0.29 b | 0.35 ± 0.01 ab | 4.49 ± 0.24 ab | 0.73 ± 0.02 b | 5.17 ± 0.25 c | |
| Dark | 1.30 ± 0.02 a | 18.31 ± 0.33 a | 0.30 ± 0.01 c | 4.23 ± 0.25 b | 0.70 ± 0.01 b | 5.92 ± 0.25 b |
| Extraction Conditions | Roast Level | Caffeine/(mg/mL) | Trigonelline/(mg/mL) | 3-CGA/(mg/mL) | 4-CGA/(mg/mL) | 5-CGA/(mg/mL) | Total CGA/(mg/mL) |
|---|---|---|---|---|---|---|---|
| UHP (300 MPa 20 min) | Light | 1.46 ± 0.03 a | 0.84 ± 0.07 a | 1.03 ± 0.06 bc | 0.48 ± 0.03 b | 0.42 ± 0.03 b | 1.93 ± 0.03 c |
| Medium | 1.36 ± 0.04 b | 0.67 ± 0.03 c | 0.92 ± 0.04 c | 0.42 ± 0.02 c | 0.36 ± 0.02 c | 1.70 ± 0.03 d | |
| Dark | 1.22 ± 0.01 c | 0.52 ± 0.03 d | 0.68 ± 0.02 e | 0.29 ± 0.01 d | 0.27 ± 0.02 d | 1.24 ± 0.02 f | |
| CB (0.1 MPa 12 h) | Light | 1.48 ± 0.02 a | 0.89 ± 0.05 a | 1.27 ± 0.04 a | 0.61 ± 0.02 a | 0.57 ± 0.03 a | 2.45 ± 0.06 a |
| Medium | 1.35 ± 0.04 b | 0.79 ± 0.05 b | 1.09 ± 0.03 b | 0.50 ± 0.03 b | 0.43 ± 0.02 b | 2.02 ± 0.03 b | |
| Dark | 1.24 ± 0.02 c | 0.57 ± 0.02 d | 0.81 ± 0.02 d | 0.32 ± 0.01 d | 0.34 ± 0.01 c | 1.47 ± 0.02 e |
| Extraction Conditions | Roast Level | DPPH Antioxidant Capacity/(Trolox/(mmol/L)) | ABTS Antioxidant Capacity/(Trolox/(mmol/L)) |
|---|---|---|---|
| UHP (300 MPa 20 min) | Light | 5.84 ± 0.38 b | 4.20 ± 0.23 b |
| Medium | 5.57 ± 0.46 b | 2.64 ± 0.28 cd | |
| Dark | 4.26 ± 0.22 c | 1.53 ± 0.19 e | |
| CB (0.1 MPa 12 h) | Light | 6.52 ± 0.32 a | 4.77 ± 0.35 a |
| Medium | 5.87 ± 0.33 b | 2.89 ± 0.33 c | |
| Dark | 4.55 ± 0.29 c | 2.20 ± 0.21 d |
| Compounds | Odor Description | Retention Index | Reference Retention Index | m/z | Threshold Value (ug/kg) | OAVs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UHP-Light | UHP-Medium | UHP-Dark | CB-Light | CB-Medium | CB-Dark | ||||||
| Hazelnut pyrazine | Nutty, Meaty, Roasted, Hazelnut | 1489 | 1494 | 150, 135, 149 | 0.084 | 3660.26 | 5911.55 | 5913.94 | 8061.77 | 8563.89 | 8886.86 |
| Linalool | Floral, Sweet, Rose, Woody, Blueberry | 1048 | 1098 | 71, 93, 55 | 0.22 | 1511.96 | 2628.95 | 2746.82 | 746.49 | 3522.20 | 3734.97 |
| Butane-2,3-dione | Strong, Butter, Sweet, Creamy, Pungent | 613 | 589 | 41, 68 | 0.059 | 2765.71 | 2780.95 | 4069.59 | 1071.06 | 2992.19 | 4736.33 |
| 3-methylbutanal | Ethereal, Aldehydic, Chocolate, Peach | 632 | 654 | 44, 43, 41 | 1.1 | 657.62 | 619.01 | 815.10 | 226.50 | 708.74 | 1221.61 |
| Furfuryl methyl sulfide | Onion, Garlic, Pungent, Vegetable, Horseradish | 984 | 979 | 81, 53, 128 | 0.4 | 251.22 | 584.52 | 774.63 | 187.91 | 496.11 | 1029.28 |
| 1-Methylpyrrole-2-carboxaldehyde; | Roasted, Nutty | 991 | 971 | 109, 53, 80 | 37 | 8.81 | 27.57 | 35.57 | 14.58 | 32.02 | 45.96 |
| Hexan-1-ol | Ethereal, Oil, Fruity, Alcoholic, Sweet | 859 | 864 | 56, 43, 41 | 5.6 | 16.48 | 15.11 | 10.97 | 23.96 | 12.26 | 6.12 |
| Hexanal | Fruity, Fatty, Leafy, Sweaty | 807 | 832 | 44, 56, 41 | 5 | 26.21 | 22.15 | 8.12 | 25.00 | 7.82 | 3.70 |
| 2-methylbutanal | Cocoa, Coffee, Fermented, Alcoholic | 704 | 695 | 41, 29, 57 | 84.3 | 7.66 | 9.00 | 10.95 | 4.92 | 10.76 | 16.48 |
| 1-Furfurylpyrrole | Plastic, Green, Fruity, Coffee, Vegetable | 1128 | 1170 | 81, 147, 53 | 100 | 2.76 | 7.56 | 10.24 | 4.46 | 9.33 | 14.24 |
| 5-methylfurfural | Spice, Caramel, Maple | 959 | 953 | 110, 53, 27 | 1110 | 3.74 | 6.00 | 5.98 | 5.74 | 7.23 | 7.95 |
| 2-ethyl-6-methylpyrazine | Nutty, Peanut, Musty, Corn, Raw, Earthy, Bread | 1011 | 1005 | 121, 67, 39 | 500 | 1.74 | 2.59 | 2.95 | 3.33 | 3.18 | 4.20 |
| 4-Ethyl-2-methoxyphenol | Spicy, Smoky, Bacon, Phenolic, Clove | 1257 | 1288 | 137, 152, 15 | 69.5 | <1 | 1.80 | 4.39 | <1 | 2.85 | 6.92 |
| O-cresol | Phenolic, Medicinal, Herbal, Leathery | 1048 | 1059 | 108, 79, 77 | 25 | <1 | 1.96 | 4.64 | <1 | 3.22 | 6.04 |
| 2, 6-dimethylpyrazine | Cocoa, Roasted, Nuts, Roast, Beef, Coffee | 925 | 928 | 108, 42, 40 | 718 | <1 | 1.00 | 1.08 | 1.21 | 1.10 | 1.44 |
| Specific Attributes | UHP-L | UHP-M | UHP-D | CB-L | CB-M | CB-D |
|---|---|---|---|---|---|---|
| Flavor—Nutty/Cocoa | 4.25 ± 0.28 d | 5.63 ± 0.37 c | 7.00 ± 0.52 b | 4.50 ± 0.40 d | 6.38 ± 0.33 b | 8.50 ± 0.65 a |
| Flavor—Fruity | 6.50 ± 0.40 b | 4.25 ± 0.20 c | 3.00 ± 0.18 d | 7.50 ± 0.39 a | 4.50 ± 0.52 c | 2.00 ± 0.15 c |
| Flavor—Floral | 4.50 ± 0.30 a | 2.80 ± 0.20 b | 1.00 ± 0.22 c | 4.50 ± 0.38 a | 2.50 ± 0.25 b | 1.00 ± 0.10 c |
| Flavor—Caramelly | 4.00 ± 0.22 c | 5.25 ± 0.35 b | 5.00 ± 0.30 b | 5.00 ± 0.42 b | 6.50 ± 0.48 a | 5.00 ± 0.33 b |
| Sweetness | 4.00 ± 0.32 b | 5.20 ± 0.29 a | 3.00 ± 0.20 c | 5.00 ± 0.43 a | 5.25 ± 0.37 a | 2.00 ± 0.25 d |
| Sourness | 5.50 ± 0.33 a | 4.20 ± 0.45 b | 3.00 ± 0.22 c | 5.75 ± 0.40 a | 4.50 ± 0.39 b | 2.50 ± 0.20 d |
| Astringency | 2.00 ± 0.15 e | 3.13 ± 0.28 d | 5.00 ± 0.35 b | 3.00 ± 0.20 d | 4.75 ± 0.38 c | 6.50 ± 0.42 a |
| Bitterness | 2.00 ± 0.10 e | 3.35 ± 0.30 d | 5.50 ± 0.40 b | 3.00 ± 0.28 d | 4.30 ± 0.42 c | 6.50 ± 0.42 a |
| Flavor | 4.00 ± 0.35 d | 5.33 ± 0.45 c | 7.00 ± 0.60 ab | 5.00 ± 0.47 c | 6.85 ± 0.50 b | 8.00 ± 0.65 a |
| Body | 3.50 ± 0.20 d | 4.50 ± 0.42 c | 7.00 ± 0.50 a | 5.00 ± 0.44 bc | 5.50 ± 0.30 b | 7.50 ± 0.62 a |
| Aftertaste | 4.00 ± 0.33 d | 5.50 ± 0.39 c | 7.00 ± 0.50 a | 5.50 ± 0.44 bc | 6.50 ± 0.35 b | 7.50 ± 0.40 a |
| Overall | 7.00 ± 0.35 b | 6.50 ± 0.42 b | 5.00 ± 0.33 c | 8.00 ± 0.47 a | 7.80 ± 0.67 a | 4.00 ± 0.38 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Xiao, Y.; Zhou, Y.; Tang, W.; Jiang, F.; Zhou, X.; Lu, H. Comparison of Ultra-High-Pressure and Conventional Cold Brew Coffee at Different Roasting Degrees: Physicochemical Characteristics and Volatile and Non-Volatile Components. Foods 2024, 13, 3119. https://doi.org/10.3390/foods13193119
Shi Q, Xiao Y, Zhou Y, Tang W, Jiang F, Zhou X, Lu H. Comparison of Ultra-High-Pressure and Conventional Cold Brew Coffee at Different Roasting Degrees: Physicochemical Characteristics and Volatile and Non-Volatile Components. Foods. 2024; 13(19):3119. https://doi.org/10.3390/foods13193119
Chicago/Turabian StyleShi, Qihan, Ying Xiao, Yiming Zhou, Wenxiao Tang, Feng Jiang, Xiaoli Zhou, and Hongxiu Lu. 2024. "Comparison of Ultra-High-Pressure and Conventional Cold Brew Coffee at Different Roasting Degrees: Physicochemical Characteristics and Volatile and Non-Volatile Components" Foods 13, no. 19: 3119. https://doi.org/10.3390/foods13193119








