From Waste to Value: Fish Protein Hydrolysates as a Technological and Functional Ingredient in Human Nutrition
Abstract
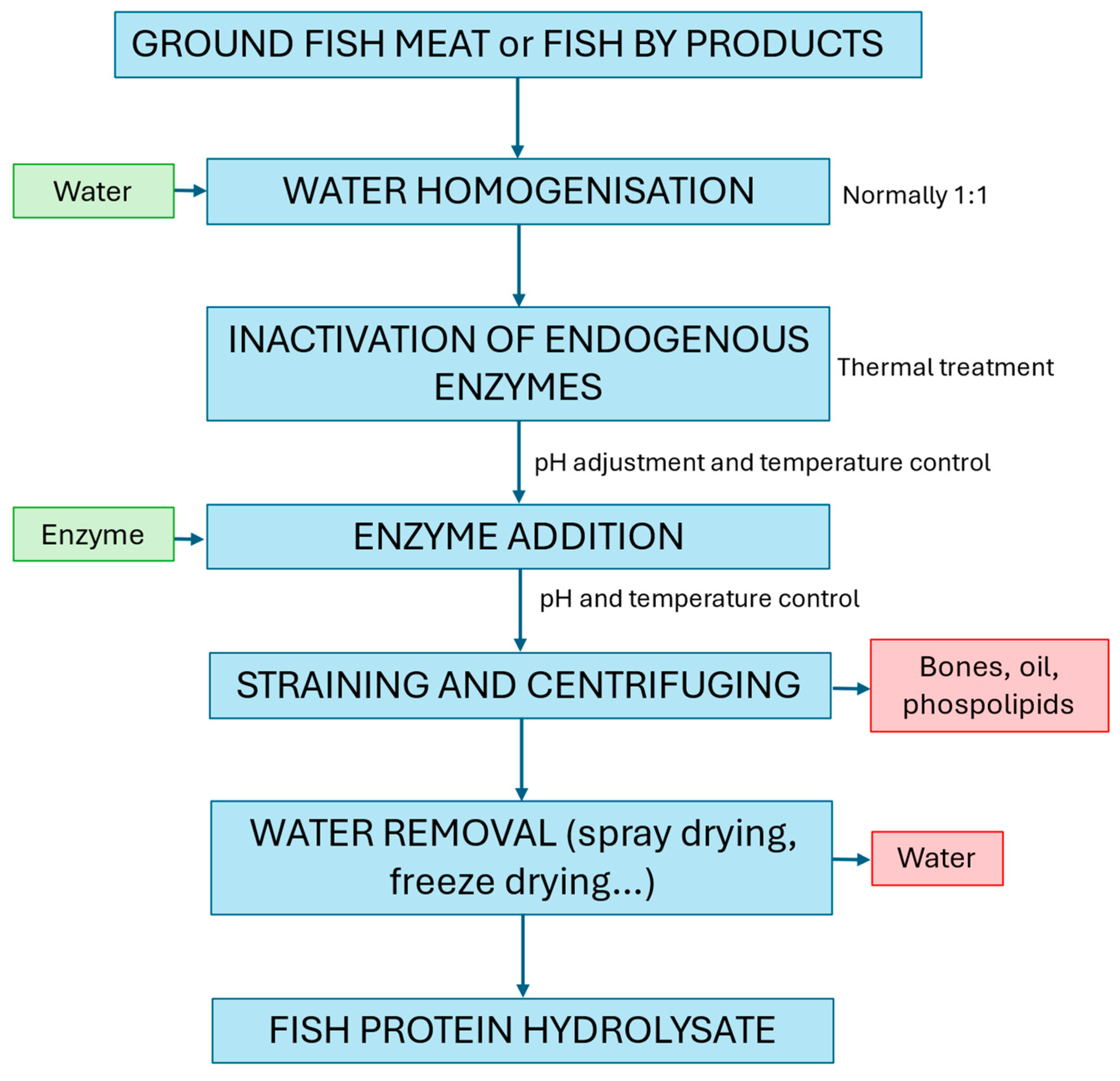
:1. Introduction
2. Materials and Methods
3. Findings to Date
3.1. Sensory and Color Characterization of FPHs
3.2. Proximate Composition
3.3. Food Safety
3.4. Bioactive Properties
3.4.1. Antioxidant Activity
3.4.2. Antihypertensive Activity
3.4.3. Antimicrobial Activity
3.4.4. Anticancer Activity
3.4.5. Antidiabetic Activity
3.5. Technological Properties
3.5.1. Solubility
3.5.2. FPHs Foaming Capacity
3.5.3. Emulsification Capacity
3.5.4. Water- and Oil-Holding Capacity
3.6. FPH Applications in the Food Industry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Wang, M.; Peng, X.; Zhong, L.; Liu, X.; Shi, Y.; Li, Y.; Chen, Y.; Tang, S. Fish Consumption in Multiple Health Outcomes: An Umbrella Review of Meta-Analyses of Observational and Clinical Studies. Ann. Transl. Med. 2023, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- AESAN. Recomendaciones de Consumo de Pescado Por Presencia de Mercuriorecomendaciones de Consumo de Pescado Por Presencia de Mercurio; AESAN: Madrid, Spain, 2019. [Google Scholar]
- FAO. El Estado Mundial de La Pesca y La Acuicultura 2022; FAO: Rome, Italy, 2022; ISBN 978-92-5-136464-2. [Google Scholar]
- APROMAR. La Acuicultura En España 2022; APROMAR: Chiclana de la Frontera, Spain, 2023. [Google Scholar]
- Arza, G.; Bardón, R.; de la Cruz, M.; Fúster, F.; Gómez, J.V.; Iglesias, N.; Marino, E.; Mendizábal, Á.; Pérez, F.; Ribes, M.Á.; et al. Guía de Los Principales Pescados, Moluscos y Crustáceos Comercializados En La Comunidad de Madrid. Identificación, Diferenciación y Tallas Mínimas; Conselleria de Sanidad: Madrid, Spain, 2013. [Google Scholar]
- Borges, S.; Odila, J.; Voss, G.; Martins, R.; Rosa, A.; Couto, J.A.; Almeida, A.; Pintado, M. Fish By-Products: A Source of Enzymes to Generate Circular Bioactive Hydrolysates. Molecules 2023, 28, 1155. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Rehman, A.; Shah, H.; Aadil, R.M.; Ali, A.; Shehzad, Q.; Ashraf, W.; Yang, F.; Karim, A.; Khaliq, A.; et al. Fish Protein and Its Derivatives: The Novel Applications, Bioactivities, and Their Functional Significance in Food Products. Food Rev. Int. 2020, 38, 1607–1634. [Google Scholar] [CrossRef]
- Bui, X.D.; Vo, C.T.; Bui, V.C.; Pham, T.M.; Bui, T.T.H.; Nguyen-Sy, T.; Nguyen, T.D.P.; Chew, K.W.; Mukatova, M.D.; Show, P.L. Optimization of Production Parameters of Fish Protein Hydrolysate from Sarda Orientalis Black Muscle (by-Product) Using Protease Enzyme. Clean. Technol. Environ. Policy 2021, 23, 31–40. [Google Scholar] [CrossRef]
- Rana, S.; Singh, A.; Surasani, V.K.R.; Kapoor, S.; Desai, A.; Kumar, S. Fish Processing Waste: A Novel Source of non-conventional Functional Proteins. Int. J. Food Sci. Technol. 2023, 58, 2637–2644. [Google Scholar] [CrossRef]
- Prihanto, A.A.; Nurdiani, R.; Bagus, A.D. Production and Characteristics of Fish Protein Hydrolysate from Parrotfish (Chlorurus sordidus) Head. PeerJ 2019, 7, e8297. [Google Scholar] [CrossRef]
- Araujo, J.; Sica, P.; Costa, C.; Márquez, M.C. Enzymatic Hydrolysis of Fish Waste as an Alternative to Produce High Value-Added Products. Waste Biomass Valorization 2021, 12, 847–855. [Google Scholar] [CrossRef]
- Idowu, A.T.; Igiehon, O.O.; Idowu, S.; Olatunde, O.O.; Benjakul, S. Bioactivity Potentials and General Applications of Fish Protein Hydrolysates. Int. J. Pept. Res. Ther. 2021, 27, 109–118. [Google Scholar] [CrossRef]
- Hassan, M.A.; Deepitha, R.P.; Xavier, K.A.M.; Gupta, S.; Nayak, B.B.; Balange, A.K. Evaluation of the Properties of Spray Dried Visceral Protein Hydrolysate from Pangasianodon Hypophthalmus (Sauvage, 1978) Extracted by Enzymatic and Chemical Methods. Waste Biomass Valorization 2019, 10, 2547–2558. [Google Scholar] [CrossRef]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Pongsetkul, J.; Sae-Leaw, T.; Sookchoo, P. Whole Wheat Cracker Fortified with Biocalcium and Protein Hydrolysate Powders from Salmon Frame: Characteristics and Nutritional Value. Food Qual. Saf. 2019, 3, 191–199. [Google Scholar] [CrossRef]
- Pinela, J.; de la Fuente, B.; Rodrigues, M.; Pires, T.C.S.P.; Mandim, F.; Almeida, A.; Dias, M.I.; Caleja, C.; Barros, L. Upcycling Fish By-Products into Bioactive Fish Oil: The Suitability of Microwave-Assisted Extraction. Biomolecules 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- AZTI. Guía de Valorización de Subproductos de La Acuicultura; AZTI: Derio, Spain, 2018. [Google Scholar]
- CECOPESCA. Guía Para El Aprovechamiento de Los Subproductos de Pescado Para La Obtención de Productos Funcionales y Bioactivos; CECOPESCA: Vigo, Spain, 2012. [Google Scholar]
- Rodríguez-Hernández, L.M.; Reyes, Y.C.; Pino, J.A.; Aragüez-Fortes, Y. Microencapsulación de Aceites de Pescado/Encapsulation of Fish Oils. Cienc. Tecnol. Aliment. 2021, 31, 79–89. [Google Scholar]
- Honrado, A.; Rubio, S.; Beltrán, J.A.; Calanche, J. Fish By-Product Valorization as Source of Bioactive Compounds for Food Enrichment: Characterization, Suitability and Shelf Life. Foods 2022, 11, 3656. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; Di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L.; et al. Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and In Vivo Biological Activities. Mar. Drugs 2020, 18, 86. [Google Scholar] [CrossRef]
- Baco, N.; Oslan, S.N.H.; Shapawi, R.; Mohhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Antibacterial Activity of Functional Bioactive Peptides Derived from Fish Protein Hydrolysate. IOP Conf. Ser. Earth Environ. Sci. 2022, 967, 012019. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bao, H.N.D.; Dang, H.T.T.; Tómasson, T.; Arason, S.; Gudjónsdóttir, M. Protein Characteristics and Bioactivity of Fish Protein Hydrolysates from Tra Catfish (Pangasius hypophthalmus) Side Stream Isolates. Foods 2022, 11, 4102. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicrob. Proteins 2019, 11, 1015–1022. [Google Scholar] [CrossRef]
- Phadke, G.G.; Rathod, N.B.; Ozogul, F.; Elavarasan, K.; Karthikeyan, M.; Shin, K.-H.; Kim, S.-K. Exploiting of Secondary Raw Materials from Fish Processing Industry as a Source of Bioactive Peptide-Rich Protein Hydrolysates. Mar. Drugs 2021, 19, 480. [Google Scholar] [CrossRef]
- Guo, Y.; Michael, N.; Fonseca Madrigal, J.; Sosa Aguirre, C.; Jauregi, P. Protein Hydrolysate from Pterygoplichthys Disjunctivus, Armoured Catfish, with High Antioxidant Activity. Molecules 2019, 24, 1628. [Google Scholar] [CrossRef]
- Pereira, N.Á.; Fangio, M.F.; Rodríguez, Y.E.; Garbari, M.D.; Fernández-Gimenez, A.V. Obtención de Hidrolizados Proteicos a Partir de Desechos de La Industria Pesquera. In Oceanografía: Desvelando la Belleza, los Misterios y los Desafíos del Mar; Pinheirom, M.S.S., Ed.; Artemis: Curitiba, Brazil, 2020; pp. 99–110. [Google Scholar]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, Bioactive Properties, and Potential Applications of Fish Protein Hydrolysates: Developments and Challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic Fish Protein Hydrolysates in Finfish Aquaculture: A Review. Rev. Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Wu, Y.-H.S.; Chen, Y.-C. Trends and Applications of Food Protein-Origin Hydrolysates and Bioactive Peptides. J. Food Drug Anal. 2022, 30, 172–184. [Google Scholar] [CrossRef]
- Saidi, S.; Saoudi, M.; Ben Amar, R. Valorisation of Tuna Processing Waste Biomass: Isolation, Purification and Characterisation of Four Novel Antioxidant Peptides from Tuna by-Product Hydrolysate. Environ. Sci. Pollut. Res. 2018, 25, 17383–17392. [Google Scholar] [CrossRef] [PubMed]
- Karoud, W.; Sila, A.; Krichen, F.; Martinez-Alvarez, O.; Bougatef, A. Characterization, Surface Properties and Biological Activities of Protein Hydrolysates Obtained from Hake (Merluccius merluccius) Heads. Waste Biomass Valorization 2019, 10, 287–297. [Google Scholar] [CrossRef]
- Alahmad, K.; Noman, A.; Xia, W.; Jiang, Q.; Xu, Y. Influence of the Enzymatic Hydrolysis Using Flavourzyme Enzyme on Functional, Secondary Structure, and Antioxidant Characteristics of Protein Hydrolysates Produced from Bighead Carp (Hypophthalmichthys nobilis). Molecules 2023, 28, 519. [Google Scholar] [CrossRef]
- Gan, R.; He, Y.; Li, Y. Structural Characteristics of Taste Active Peptides in Protein Hydrolysates from Tilapia By-Products. J. Food Meas. Charact. 2022, 16, 1674–1687. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Idowu, A.T.; Benjakul, S.; Prodpran, T.; Yesilsu, A.F.; Kishimura, H. Effect of Proteases and Alcohols Used for Debittering on Characteristics and Antioxidative Activity of Protein Hydrolysate from Salmon Frames. J. Food Sci. Technol. 2020, 57, 473–483. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Kizhakkethil, B.P.; George, J.C.; Aliyamveetil Abubacker, Z.; Ninan, G.; Chandragiri Nagarajarao, R. Antioxidant Peptides from Dark Meat of Yellowfin Tuna (Thunnus albacares): Process Optimization and Characterization. Waste Biomass Valorization 2021, 12, 1845–1860. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Evaluation of the Bioactive Potential of Foods Fortified with Fish Protein Hydrolysates. Food Res. Int. 2020, 137, 109572. [Google Scholar] [CrossRef]
- Lima, D.A.S.; Santos, M.M.F.; Duvale, R.L.F.; Bezerra, T.K.A.; Araújo, Í.B.d.S.; Madruga, M.S.; da Silva, F.A.P. Technological Properties of Protein Hydrolysate from the Cutting Byproduct of Serra Spanish Mackerel (Scomberomorus brasiliensis). J. Food Sci. Technol. 2021, 58, 2952–2962. [Google Scholar] [CrossRef]
- Witono, Y.; Fauziah, R.R.; Windrati, W.S.; Taruna, I.; Azkiyah, L.; Wijayanti, R.P. Formulation of Flavor Enhancer from Common Barb (Rasbora Jacobsoni) Protein Hydrolysate; AIP Publishing: Melville, NY, USA, 2019; p. 060002. [Google Scholar]
- Gao, R.; Shen, Y.; Shu, W.; Bai, F.; Jin, W.; Wang, J.; Yuan, L. Optimization of Enzymatic Conditions of Sturgeon Muscles and Their Anti-Inflammatory Potential. J. Food Qual. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Honrado, A.; Ardila, P.; Leciñena, P.; Beltrán, J.A.; Calanche, J. Nutritional Enrichment of Cereal Foods: Feasibility Study and Characterisation of Biscuits Fortified with Seabass By-products. Int. J. Food Sci. Technol. 2024, 59, 5376–5388. [Google Scholar] [CrossRef]
- Nisov, A.; Kakko, T.; Alakomi, H.-L.; Lantto, R.; Honkapää, K. Comparison of Enzymatic and PH Shift Methods to Extract Protein from Whole Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus). Food Chem. 2022, 373, 131524. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Bak, K.H.; Lametsch, R. Valorisation of Protein Hydrolysates from Animal By-products: Perspectives on Bitter Taste and Debittering Methods: A Review. Int. J. Food Sci. Technol. 2019, 54, 978–986. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S.; Maqsood, S. Valorization of Fish Byproducts: Sources to End-product Applications of Bioactive Protein Hydrolysate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1803–1842. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Hong, H.; Luo, Y.; Li, B.; Tan, Y. Mastering the Art of Taming: Reducing Bitterness in Fish by-Products Derived Peptides. Food Res. Int. 2023, 173, 113241. [Google Scholar] [CrossRef]
- Steinsholm, S.; Oterhals, Å.; Thoresen, L.; Underhaug, J.; Kousoulaki, K.; Aspevik, T. Reduction in Flavor-intense Components in Fish Protein Hydrolysates by Membrane Filtration. J. Food Sci. 2021, 86, 3855–3867. [Google Scholar] [CrossRef]
- Petrova, I.; Tolstorebrov, I.; Eikevik, T.M. Production of Fish Protein Hydrolysates Step by Step: Technological Aspects, Equipment Used, Major Energy Costs and Methods of Their Minimizing. Int. Aquat. Res. 2018, 10, 223–241. [Google Scholar] [CrossRef]
- Kumari, A.; Kaushik, N.; Slizyte, R. Khushboo Production and Microencapsulation of Protein Hydrolysate of Pink Perch (Nemipterus japonicus) By-Products Obtained from Surimi Industry for Its Sustainable Utilization. Waste Biomass Valorization 2023, 14, 209–226. [Google Scholar] [CrossRef]
- Pavarthy, U.; Nizam, K.M.; Zynudheen, A.A.; Ninan, G.; Panda, S.K.; Ravishankar, C.N. Characterization of Fish Protein Hydrolysate from Red Meat of Euthynnus Affinis and Its Application as an Antioxidant in Iced Sardine. J. Sci. Ind. Res. 2018, 77, 119. [Google Scholar]
- Alahmad, K.; Xia, W.; Jiang, Q.; Xu, Y. Effect of the Degree of Hydrolysis on Nutritional, Functional, and Morphological Characteristics of Protein Hydrolysate Produced from Bighead Carp (Hypophthalmichthys nobilis) Using Ficin Enzyme. Foods 2022, 11, 1320. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.B.; More, P.R.; Sonawane, S.K.; Arya, S.S. Antioxidant and Anti-Hypertensive Bioactive Peptides from Indian Mackerel Fish Waste. Int. J. Pept. Res. Ther. 2021, 27, 2671–2684. [Google Scholar] [CrossRef]
- Azizi Khesal, M.; Sharifan, A.; Hoseini, E.; Ghavami, A. Optimization of Enzymatic Hydrolysis Conditions of Caspian Kutum (Rutilus frisii kutum)” By-Product for Production of Bioactive Peptides with Antioxidative Properties. Int. J. Pept. Res. Ther. 2020, 26, 1829–1838. [Google Scholar] [CrossRef]
- Rabiei, S.; Rezaei, M.; Asgharzade, S.; Nikoo, M.; Rafieia-kopai, M. Antioxidant and Cytotoxic Properties of Protein Hydrolysates Obtained from Enzymatic Hydrolysis of Klunzinger’s Mullet (Liza klunzingeri) Muscle. Braz. J. Pharm. Sci. 2019, 55, e18304. [Google Scholar] [CrossRef]
- Suprayitno, E.; Aulanni’am, A.; Sulistiyati, T.D.; Riyadi, P.H. Chemical Characteristics and Amino Acids Profile of Protein Hydrolysates of Nile Tilapia (Oreochromis niloticus) Viscera. J. Worlds Poult. Res. 2019, 9, 324–328. [Google Scholar] [CrossRef]
- Hasani, K.; Ariaii, P.; Ahmadi, M. Antimicrobial, Antioxidant and Anti-Cancer Properties of Protein Hydrolysates from Indian Mackerel (Rastrelliger kanagurta) Waste Prepared Using Commercial Enzyme. Int. J. Pept. Res. Ther. 2022, 28, 86. [Google Scholar] [CrossRef]
- Vieira, E.F.; Van Camp, J.; Ferreira, I.M.P.L.V.O.; Grootaert, C. Protein Hydrolysate from Canned Sardine and Brewing By-Products Improves TNF-α-Induced Inflammation in an Intestinal–Endothelial Co-Culture Cell Model. Eur. J. Nutr. 2018, 57, 2275–2286. [Google Scholar] [CrossRef]
- Greyling, N.; Bordoloi, A.; Goosen, N.J. Optimising Enzymatic Conditions of Monkfish (Lophius vomerinus) Heads Hydrolysis towards Potential Waste Biomass Valorisation. Biomass Convers. Biorefinery 2021, 11, 2711–2722. [Google Scholar] [CrossRef]
- Kandyliari, A.; Mallouchos, A.; Papandroulakis, N.; Golla, J.P.; Lam, T.T.; Sakellari, A.; Karavoltsos, S.; Vasiliou, V.; Kapsokefalou, M. Nutrient Composition and Fatty Acid and Protein Profiles of Selected Fish By-Products. Foods 2020, 9, 190. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef]
- Gómez, L.J.; Zapata, J.E. Caracterización Fisicoquímica, Tecnofuncional y Calidad Biológica de Hidrolizados de Vísceras de Tilapia Roja (Oreochromis spp.). Inf. Tecnológica 2022, 33, 3–14. [Google Scholar] [CrossRef]
- Roldán, A.D.; Omote-Sibina, J.R.; Molleda, O.A. Elaboración de un hidrolizado de proteína de anchoveta (Engraulis ringens) en polvo. An. Científicos 2021, 82, 251–261. [Google Scholar] [CrossRef]
- Commission of the European Comunities. European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. [Google Scholar]
- de la Fuente, B.; Pallarés, N.; Barba, F.J.; Berrada, H. An Integrated Approach for the Valorization of Sea Bass (Dicentrarchus labrax) Side Streams: Evaluation of Contaminants and Development of Antioxidant Protein Extracts by Pressurized Liquid Extraction. Foods 2021, 10, 546. [Google Scholar] [CrossRef]
- Ramilo-Fernández, G.; Sotelo, C.G. Characterization and Potential Strategies for the Valorisation of the Southwest Atlantic Butterfish (Stromateus brasiliensis). J. Food Sci. Technol. 2020, 57, 2994–3003. [Google Scholar] [CrossRef]
- Donnarumma, D.; La Tella, R.; Vento, F.; Salerno, T.M.G.; Micalizzi, G.; Rigano, F.; Mondello, L. Evaluation of the Level of Toxic Contaminants and Essential Molecules in the Context of the Re-Use of Tuna Fishery Industry by-Products. Food Anal. Methods 2021, 14, 2161–2174. [Google Scholar] [CrossRef]
- Durante, L.; Wing, S.; Ingram, T.; Sabadel, A.; Shima, J. Changes in Trophic Structure of an Exploited Fish Community at the Centennial Scale Are Linked to Fisheries and Climate Forces. Sci. Rep. 2022, 12, 4309. [Google Scholar] [CrossRef]
- European Comission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Mangano, V.; Gervasi, T.; Rotondo, A.; De Pasquale, P.; Dugo, G.; Macrì, F.; Salvo, A. Protein Hydrolysates from Anchovy Waste: Purification and Chemical Characterization. Nat. Prod. Res. 2021, 35, 399–406. [Google Scholar] [CrossRef]
- de la Fuente, B.; Aspevik, T.; Barba, F.J.; Kousoulaki, K.; Berrada, H. Mineral Bioaccessibility and Antioxidant Capacity of Protein Hydrolysates from Salmon (Salmo salar) and Mackerel (Scomber scombrus) Backbones and Heads. Mar. Drugs 2023, 21, 294. [Google Scholar] [CrossRef]
- Wu, H.; Forghani, B.; Abdollahi, M.; Undeland, I. Five Cuts from Herring (Clupea harengus): Comparison of Nutritional and Chemical Composition between Co-Product Fractions and Fillets. Food Chem. X 2022, 16, 100488. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Lorenzo, J.M.; Mirnejad, R.; Ahmadvand, M.; Moosazadeh Moghaddam, M. Bioactivity Evaluation of Peptide Fractions from Bighead Carp (Hypophthalmichthys nobilis) Using Alcalase and Hydrolytic Enzymes Extracted from Oncorhynchus Mykiss and Their Potential to Develop the Edible Coats. Food Bioprocess Technol. 2023, 16, 1128–1148. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and Protein Hydrolysates as Food Preservatives and Bioactive Components of Edible Films and Coatings—A Review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-Product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Zhang, Y.; Dong, Y.; Hu, X. Structural Characteristics and Stability of Salmon Skin Protein Hydrolysates Obtained with Different Proteases. LWT 2022, 153, 112460. [Google Scholar] [CrossRef]
- Shahosseini, S.R.; Javadian, S.R.; Safari, R. Effects of Molecular Weights -Assisted Enzymatic Hydrolysis on Antioxidant and Anticancer Activities of Liza Abu Muscle Protein Hydrolysates. Int. J. Pept. Res. Ther. 2022, 28, 72. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Liu, M.; Li, Y.; Lv, R.; Li, X.; Wang, Q.; Ren, D.; Wu, L.; Zhou, H. Identification of Antioxidant Peptides Derived from Tilapia (Oreochromis niloticus) Skin and Their Mechanism of Action by Molecular Docking. Foods 2022, 11, 2576. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel, J. Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules 2020, 10, 310. [Google Scholar] [CrossRef]
- Qara, S.; Habibi Najafi, M.B. Bioactive Properties of Kilka (Clupeonella cultriventris caspi) Fish Protein Hydrolysates. J. Food Meas. Charact. 2018, 12, 2263–2270. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Vaidya, S.; Sheshappa, M.B. Functional Properties of Protein Hydrolyzate from Ribbon Fish (Lepturacanthus savala) as Prepared by Enzymatic Hydrolysis. Int. J. Food Prop. 2022, 25, 187–203. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Tanaaz, M.; Bhat, I.; Luckose, F.; Mamatha, B.S. Physicochemical Properties and Angiotensin-I Converting Enzyme Inhibitory Activity of Lipid-Free Ribbon Fish (Lepturacanthus savala) Protein Hydrolysate. J. Food Sci. Technol. 2023, 60, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Rezaei, M.; Tabarsa, M.; Abdollahi, M. Fish Protein Hydrolysate from Sulfated Polysaccharides Extraction Residue of Tuna Processing By-Products with Bioactive and Functional Properties. Glob. Chall. 2023, 7, 2200214. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Tian, C.; Jiang, J.; Zhang, G.-L.; Hao, H.; Hou, H.-M. Antibacterial Activity and Potential Application in Food Packaging of Peptides Derived from Turbot Viscera Hydrolysate. J. Agric. Food Chem. 2020, 68, 9968–9977. [Google Scholar] [CrossRef]
- WHO. Cancer; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Mäkinen, S.; Hiidenhovi, J.; Huang, X.; Lima, A.d.S.; Azevedo, L.; Setälä, J.; Välimaa, A.-L.; Mattila, P.; Granato, D. Production of Bioactive Peptides from Baltic Herring (Clupea harengus membras): Dipeptidyl Peptidase-4 Inhibitory, Antioxidant and Antiproliferative Properties. Molecules 2022, 27, 5816. [Google Scholar] [CrossRef]
- Yaghoubzadeh, Z.; Peyravii Ghadikolaii, F.; Kaboosi, H.; Safari, R.; Fattahi, E. Antioxidant Activity and Anticancer Effect of Bioactive Peptides from Rainbow Trout (Oncorhynchus mykiss) Skin Hydrolysate. Int. J. Pept. Res. Ther. 2020, 26, 625–632. [Google Scholar] [CrossRef]
- WHO. Diabetes; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Wan, P.; Cai, B.; Chen, H.; Chen, D.; Zhao, X.; Yuan, H.; Huang, J.; Chen, X.; Luo, L.; Pan, J. Antidiabetic Effects of Protein Hydrolysates from Trachinotus Ovatus and Identification and Screening of Peptides with α-Amylase and DPP-IV Inhibitory Activities. Curr. Res. Food Sci. 2023, 6, 100446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chai, L.; Wu, Q.; Wang, Y.; Li, S.; Chen, J. Anti-Diabetic Properties of Bioactive Components from Fish and Milk. J. Funct. Foods 2021, 85, 104669. [Google Scholar] [CrossRef]
- Sharkey, S.J.; Harnedy-Rothwell, P.A.; Allsopp, P.J.; Hollywood, L.E.; FitzGerald, R.J.; O’Harte, F.P.M. A Narrative Review of the Anti-Hyperglycemic and Satiating Effects of Fish Protein Hydrolysates and Their Bioactive Peptides. Mol. Nutr. Food Res. 2020, 64. [Google Scholar] [CrossRef]
- Harnedy-Rothwell, P.A.; Khatib, N.; Sharkey, S.; Lafferty, R.A.; Gite, S.; Whooley, J.; O’Harte, F.P.; FitzGerald, R.J. Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistiuspoutassou) Protein Hydrolysates. Mar. Drugs 2021, 19, 383. [Google Scholar] [CrossRef]
- Henriques, A.; Vázquez, J.A.; Valcarcel, J.; Mendes, R.; Bandarra, N.M.; Pires, C. Characterization of Protein Hydrolysates from Fish Discards and By-Products from the North-West Spain Fishing Fleet as Potential Sources of Bioactive Peptides. Mar. Drugs 2021, 19, 338. [Google Scholar] [CrossRef]
- Wangkheirakpam, M.R.; Mahanand, S.S.; Majumdar, R.K.; Sharma, S.; Hidangmayum, D.D.; Netam, S. Fish Waste Utilization with Reference to Fish Protein Hydrolysate—A Review. Fish. Technol. 2019, 56, 169–178. [Google Scholar]
- Ozel, O.T.; Cakmak, E.; Ozturk, E. Effects of Alternative Oil Sources on Growth Performance, Lipid Metabolism and mRNA Level of Some Genes in Juvenile Black Sea Trout (Salmo trutta labrax Pallas,1811). Turk. J. Fish. Aquat. Sci. 2018, 18, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Dinakarkumar, Y.; Krishnamoorthy, S.; Margavelu, G.; Ramakrishnan, G.; Chandran, M. Production and Characterization of Fish Protein Hydrolysate: Effective Utilization of Trawl by-Catch. Food Chem. Adv. 2022, 1, 100138. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S. Protein Hydrolysates Prepared from the Viscera of Skipjack Tuna (Katsuwonus pelmamis): Antioxidative Activity and Functional Properties. Turk. J. Fish. Aquat. Sci. 2018, 18, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Halim, N.R.A.; Mhd Sarbon, N. Characterization of Asian Swamp Eel (Monopterus sp.) Protein Hydrolysate Functional Properties Prepared Using Alcalase® Enzyme. Food Res. 2019, 4, 207–215. [Google Scholar] [CrossRef]
- Mohanty, U.; Majumdar, R.K.; Mohanty, B.; Mehta, N.K.; Parhi, J. Influence of the Extent of Enzymatic Hydrolysis on the Functional Properties of Protein Hydrolysates from Visceral Waste of Labeo Rohita. J. Food Sci. Technol. 2021, 58, 4349–4358. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Puthenveetil Kizhakkethil, B.; Anant Jadhav, M.; Sivam, V.; Ashraf, P.M.; Ninan, G.; Aliyamveetil Abubacker, Z. Protein Hydrolysate from Yellowfin Tuna Red Meat as Fortifying and Stabilizing Agent in Mayonnaise. J. Food Sci. Technol. 2020, 57, 413–425. [Google Scholar] [CrossRef]
- Lima, K.O.; da Rocha, M.; Alemán, A.; López-Caballero, M.E.; Tovar, C.A.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Yogurt Fortification by the Addition of Microencapsulated Stripped Weakfish (Cynoscion guatucupa) Protein Hydrolysate. Antioxidants 2021, 10, 1567. [Google Scholar] [CrossRef]
- Khodaei, D.; Forde, A.; Noci, F.; Ryan, L. Physicochemical and Sensory Characteristics of Pasta Enriched with Blue Whiting (Micromesistius poutassou) Fish Protein Hydrolysate. Int. J. Food Sci. Technol. 2023, 58, 2782–2789. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, J.; Lee, M.-Y.; Cho, H.-Y.; Choi, M.-J. Effects of Hydrolyzed Animal Protein on the Enhancement of Saltiness and Quality Characteristics of White Pan Bread. Food Bioprocess Technol. 2019, 12, 1832–1841. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Huda, N. Characteristics and Nutritional Value of Biscuits Fortified with Debittered Salmon (Salmo Salar) Frame Hydrolysate. Int. J. Food Sci. Technol. 2020, 55, 3553–3562. [Google Scholar] [CrossRef]
- Singh, S. Fish Protein Hydrolysate Market. Available online: https://www.precedenceresearch.com/fish-protein-hydrolysate-market (accessed on 20 June 2024).
- Honrado, A.; Ardila, P.; Leciñena, P.; Beltrán, J.A.; Calanche, J.B. Transforming ‘Bonito Del Norte’ Tuna By-Products into Functional Ingredients for Nutritional Enhancement of Cereal-Based Foods. Foods 2023, 12, 4437. [Google Scholar] [CrossRef] [PubMed]
- Sierra Lopera, L.M.; Sepúlveda Rincón, C.T.; Vásquez Mazo, P.; Figueroa Moreno, O.A.; Zapata Montoya, J.E. Byproducts of aquaculture processes: Development and prospective uses. Rev. Vitae 2018, 25, 128–140. [Google Scholar] [CrossRef]


| DH (Time) | Color | Enzyme | Species | Authors | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| 16.56% (1 h) | 86.89 ± 0.52 a | 1.39 ± 0.08 b | 15.81 ± 0.37 b | Flavourzyme® | Bighead Carp (Hypophthalmichthys nobilis) | [32] |
| 22.23% (3 h) | 84.06 ± 0.24 b | 1.43 ± 0.05 b | 17.42 ± 0.18 a | |||
| 25.48% (6 h) | 83.98 ± 0.16 b | 1.92 ± 0.11 a | 17.97 ± 0.21 a | |||
| 5.3% (0.25 h) | 84.33 ± 0.02 a | −0.54 ± 0.02 a | 22.13 ± 0.04 a | Savinase® | Hake (Merluccius merluccius) | [31] |
| 6.5% (0.5 h) | 84.16 ± 0.03 a | −0.48 ± 0.01 a | 21.1 ± 0.02 b | |||
| 7.7% (1 h) | 82.71 ± 1.10 a | −0.50 ± 0.04 a | 15.16 ± 0.25 c | |||
| 8.6% (2 h) | 79.34 ± 2.80 b | −0.31 ± 0.04 b | 13.47 ± 0.28 d | |||
| 31.59% (4 h) | 90.62 ± 0.05 | −0.61 ± 0.01 | 17.16 ± 0.11 | Papain | Tuna (Thunnus albacares) | [35] |
| 13.36% (1 h) | 89.23 ± 0.08 a | 0.17 ± 0.01 b | 10.96 ± 0.56 b | Ficin | Bighead Carp (Hypophthalmichthys nobilis) | [49] |
| 17.09% (3 h) | 88.82 ± 0.10 b | 0.23 ± 0.05 ab | 12.29 ± 0.10 a | |||
| 20.15% (6 h) | 86.55 ± 0.09 c | 0.31 ± 0.06 a | 12.68 ± 0.03 a | |||
| Authors | Species | RM/FPH | Moisture | Protein | Fat | Ash |
|---|---|---|---|---|---|---|
| [51] | Caspian kutum (Rutilus kutum) by-products mixture | RM | 78.88 | 15.1 | 4.73 | 2.19 |
| FPH | 7.52 | 87.38 | 1.61 | 3.95 | ||
| [48] | mackerel tuna (Euthynnus affinis) muscle | RM | - | 28.25 | - | - |
| FPH | 1.35 | 89.90 | 0.06 | 4.03 | ||
| [52] | Liza (Liza klunzingeri) muscle | RM | 73.36 | 22.46 | 2.21 | 9.52 |
| FPH | 1.87 | 87.84 | 0.77 | 2.00 |
| Authors | [53] | [54] | [54] | [55] | [34] | [34] | [56] |
|---|---|---|---|---|---|---|---|
| Species | Nile Tilapia (Oreochromis niloticus) Viscera | Indian Mackerel (Rastrelliger kanagurta) Skin and Head | Indian Mackerel (Rastrelliger kanagurta) Skin and Head | Sardine (Sardina pilchardus) by-Products Mixture | Salmon (Salmo salar) Frames | Salmon (Salmo salar) Frames | Monkfish (Lophius vomerinus) Heads |
| Origin | Freshwater | Seawater | Seawater | Seawater | Seawater | Seawater | Seawater |
| Enzyme | ALC | ALC | FLA | GI | ALC | FLA | ALC |
| Histidine | 2.04 | 3.55 | 3.29 | 5.20 | 3.40 | 3.58 | 1.40 |
| Isoleucine | 1.56 | 4.05 | 5.05 | 1.20 | 3.76 | 3.33 | 5.39 |
| Leucine | 2.19 | 7.35 | 6.99 | 4.85 | 7.06 | 6.39 | 8.47 |
| Lysine | 2.82 | 7.99 | 7.19 | 3.02 | 8.23 | 8.15 | 12.2 |
| Methionine | 0.88 | 3.15 | 3.05 | 1.52 | 3.14 | 2.83 | 3.42 |
| Phenylalanine | 1.07 | 4.25 | 4.15 | 3.02 | 3.55 | 2.99 | 4.15 |
| Tyrosine | 1.42 | 3.55 | 3.28 | 2.52 | 3.11 | 2.27 | 3.49 |
| Threonine | 1.26 | 3.95 | 4.15 | - | 4.56 | 4.13 | 4.49 |
| Tryptophan | 0.42 | - | - | 0.36 | 0.69 | 0.48 | - |
| Arginine | 1.93 | 7.55 | 8.21 | 2.90 | 6.52 | 6.56 | 5.22 |
| Valine | 2.78 | 5.25 | 5.45 | 2.58 | 4.48 | 4.17 | 5.50 |
| Asparagine + aspartate | 3.15 | 7.99 | 6.98 | - | 9.59 | 9.22 | 10.1 |
| Glutamine + glutamate | 3.85 | 12.55 | 11.79 | 11.52 | 14.13 | 14.65 | 14.0 |
| Serine | 1.19 | 4.59 | 4.69 | - | 4.61 | 4.62 | 4.98 |
| Glycine | 1.27 | 5.99 | 5.25 | 4.90 | 9.03 | 10.93 | 6.45 |
| Alanine | 1.56 | 4.98 | 4.25 | 9.52 | 6.75 | 7.19 | 5.92 |
| Proline | 0.99 | 5.29 | 4.99 | 3.10 | 5.14 | 5.53 | 4.37 |
| Cysteine | 0.32 | 0.95 | 0.99 | 2.44 | 0.01 | 0.00 | - |
| Authors | Species | Enzymes | Fractionation | L. monocytogenes | L. innocua | S. aureus | B. cereus | E. coli | S. enterica | S. typhimurium | Pseudomonas |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [80] | skipjack tuna (Katsuwonus pelamis) | ALC | head | 3 mm | 2.17 mm | 1.17 mm | 1.67 mm | 1.83 mm | 1.83 mm | ||
| ALC | bones | 2 mm | 2.17 mm | 2.00 mm | 2.17 mm | 3.17 mm | 2.67 mm | ||||
| ALC | skin | 2.67 mm | 3.00 mm | 1.33 mm | 2.33 mm | 3.17 mm | 2.00 mm | ||||
| [23] | Yellowfin tuna (Thunnus albacores) viscera | PRO | <30 kDa | 95% | 82% | 80% | 78% | ||||
| PRO | 10–30 kDa | 60% | 46% | 50% | 50% | ||||||
| PRO | 3–10 kDa | 92% | 72% | 85% | 83% | ||||||
| PRO | <4 kDa | 100% | 97% | 95% | 95% | ||||||
| [54] | Indian mackerel (Rastrelliger kanagurta) | ALC (10 min) | Skin/head | 13–18 mm | <7 mm | ||||||
| ALC (20 min) | >18 mm | 7–13 mm | |||||||||
| ALC (30 min) | >18 mm | 13–18 mm | |||||||||
| FLA (10 min) | <7 mm | <7 mm | |||||||||
| FLA (20 min) | 13–18 mm | <7 mm | |||||||||
| FLA (30 min) | >18 mm | 7–13 mm | |||||||||
| [77] | Whole common Kilka fish (Clupeonella cultriventris caspi) | PEP (30 min) | 10–30 Da | NI | NI | NI | NI | ||||
| PEP (60 min) | NI | 32% | 23% | 34% | |||||||
| PEP (90 min) | NI | 77% | NI | NI | |||||||
| PRO (30 min) | NI | NI | NI | NI | |||||||
| PRO (60 min) | NI | NI | NI | NI | |||||||
| PRO (90 min) | NI | 56% | 18% | NI |
| Authors | Species | Enzymes | Degree of Hydrolysis (%) | pH | Main Results |
|---|---|---|---|---|---|
| [94] | Skipjack Tuna (Katsuwonus pelmamis) viscera | ALC | 20% | 3, 5, 7 and 9 | Solubility increased directly proportional to pH (91.89%, 96.39%, 97.65%, and 100% respectively). |
| [47] | Pink Perch (Nemipterus japonicus) head and viscera | 15.5% | 2, 4, 6, 8 and 10 | Solubility increased from 89.8 to 99.7 when hydrolysis pH increased. | |
| [78] | Ribbon Fish (Lepturacanthus savala) viscera | 19–41 | 2–12 | Solubility increases as pH does, but at the isoelectric point (pH 4.4–5.5), solubility decreases deeply. Solubility increased 50% when comparing the one with the least degree of hydrolysis and the highest one. | |
| [70] | Bighead Carp (Hypophthalmichthys nobilis) viscera | - | 3, 5, 7 and 9 | Solubility decreased at the isoelectric point. Fractionation allowed to see how small peptides (<3 kDa) showed higher solubility. | |
| [93] | Secutor insidiator (Pugnose ponyfish) flesh | PRK and PAP | 8 and 9 | 2, 4, 6, 7, 8 and 10 | Solubility increased 69% when comparing native protein and FPH from both enzymes. |
| [32] | Bighead Carp (Hypophthalmichthys nobilis) whole fish | FLA | 16.56–22.23 and 25.48 | 6 | The highest solubility value (97.4%) was found at the highest degree of hydrolysis (25.48%). |
| [34] | Salmon (Salmo salar) frames | ALC and FLA | 26.88 and 25.02 | 7 | Debittering with alcohols resulted in a reduction of solubility. The decrease in alkalase FPH was 3 and 5.7% using 2-butanol and iso-propanol, respectively. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honrado, A.; Miguel, M.; Ardila, P.; Beltrán, J.A.; Calanche, J.B. From Waste to Value: Fish Protein Hydrolysates as a Technological and Functional Ingredient in Human Nutrition. Foods 2024, 13, 3120. https://doi.org/10.3390/foods13193120
Honrado A, Miguel M, Ardila P, Beltrán JA, Calanche JB. From Waste to Value: Fish Protein Hydrolysates as a Technological and Functional Ingredient in Human Nutrition. Foods. 2024; 13(19):3120. https://doi.org/10.3390/foods13193120
Chicago/Turabian StyleHonrado, Adrián, Marta Miguel, Paula Ardila, José Antonio Beltrán, and Juan B. Calanche. 2024. "From Waste to Value: Fish Protein Hydrolysates as a Technological and Functional Ingredient in Human Nutrition" Foods 13, no. 19: 3120. https://doi.org/10.3390/foods13193120








