Characterization of a Novel Acid-Stable Chitosanase from Lentinula edodes Suitable for Chitooligosaccharide Preparation
Abstract
1. Introduction
2. Materials and Methods
2.1. Polysaccharides
2.2. Strains and Plasmids
2.3. Sequence Analysis
2.4. RNA Extraction and cDNA Synthesis
2.5. Cloning, Expression and Purification of LeCho1
2.6. Hydrolytic Activity Assay
2.7. Biochemical Characterization of Recombinant LeCho1
2.8. Analysis of Hydrolysis Products
2.9. Statistical Analysis
3. Results
3.1. Sequence Analysis of LeCho1
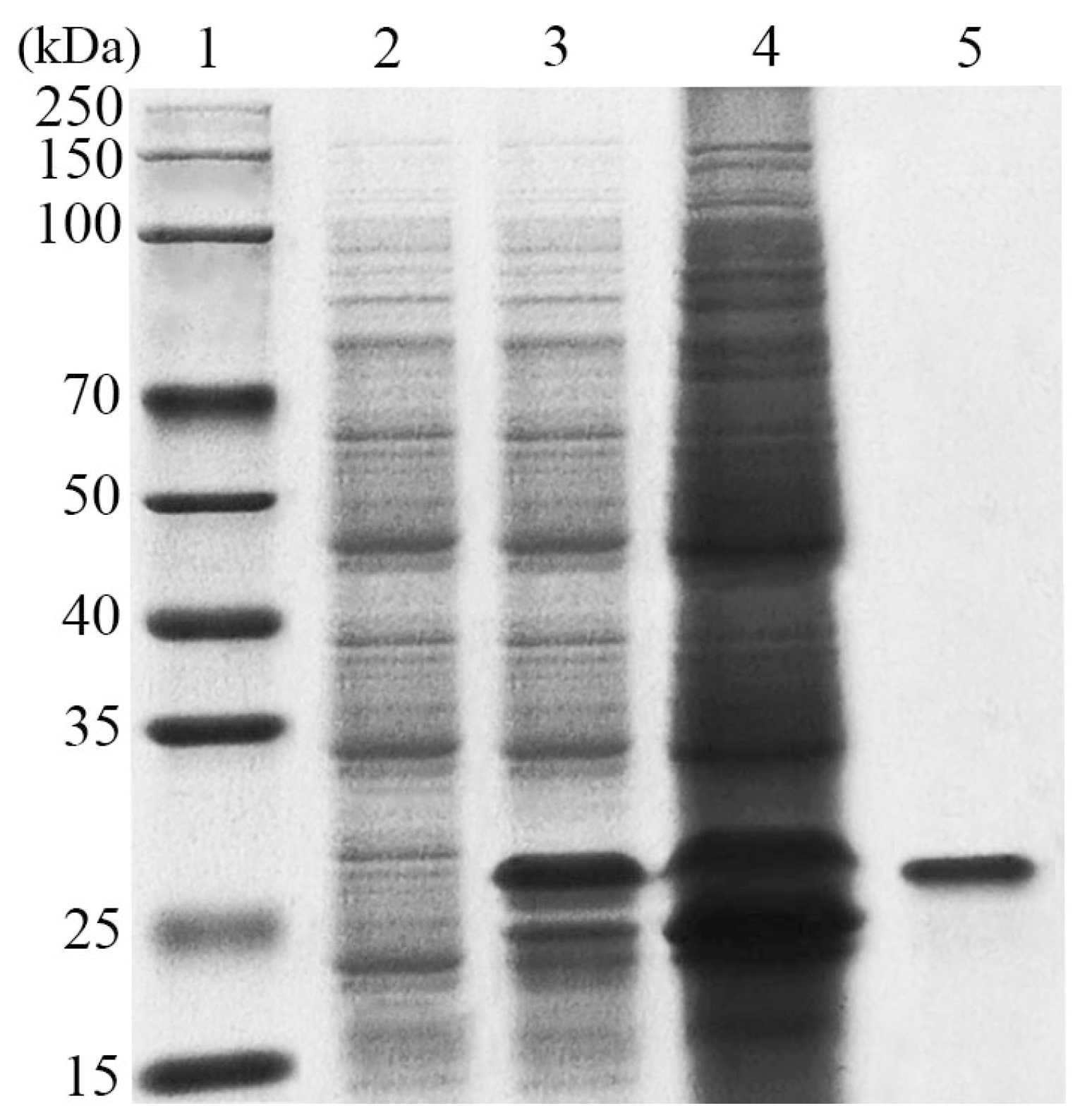
3.2. Expression and Purification of LeCho1
3.3. Substrate Specificity of the Purified Protein
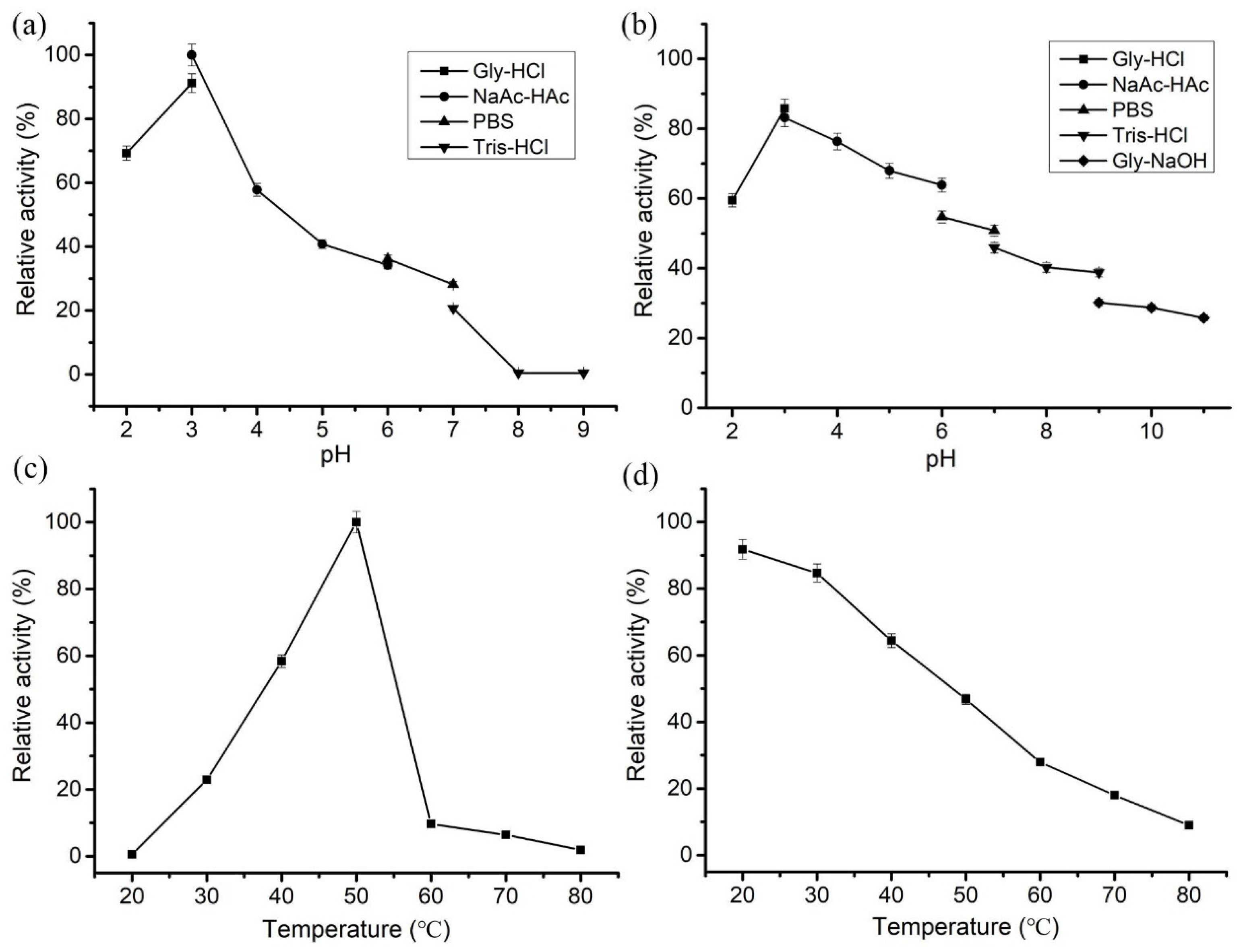
3.4. Enzymological Characterization of LeCho1
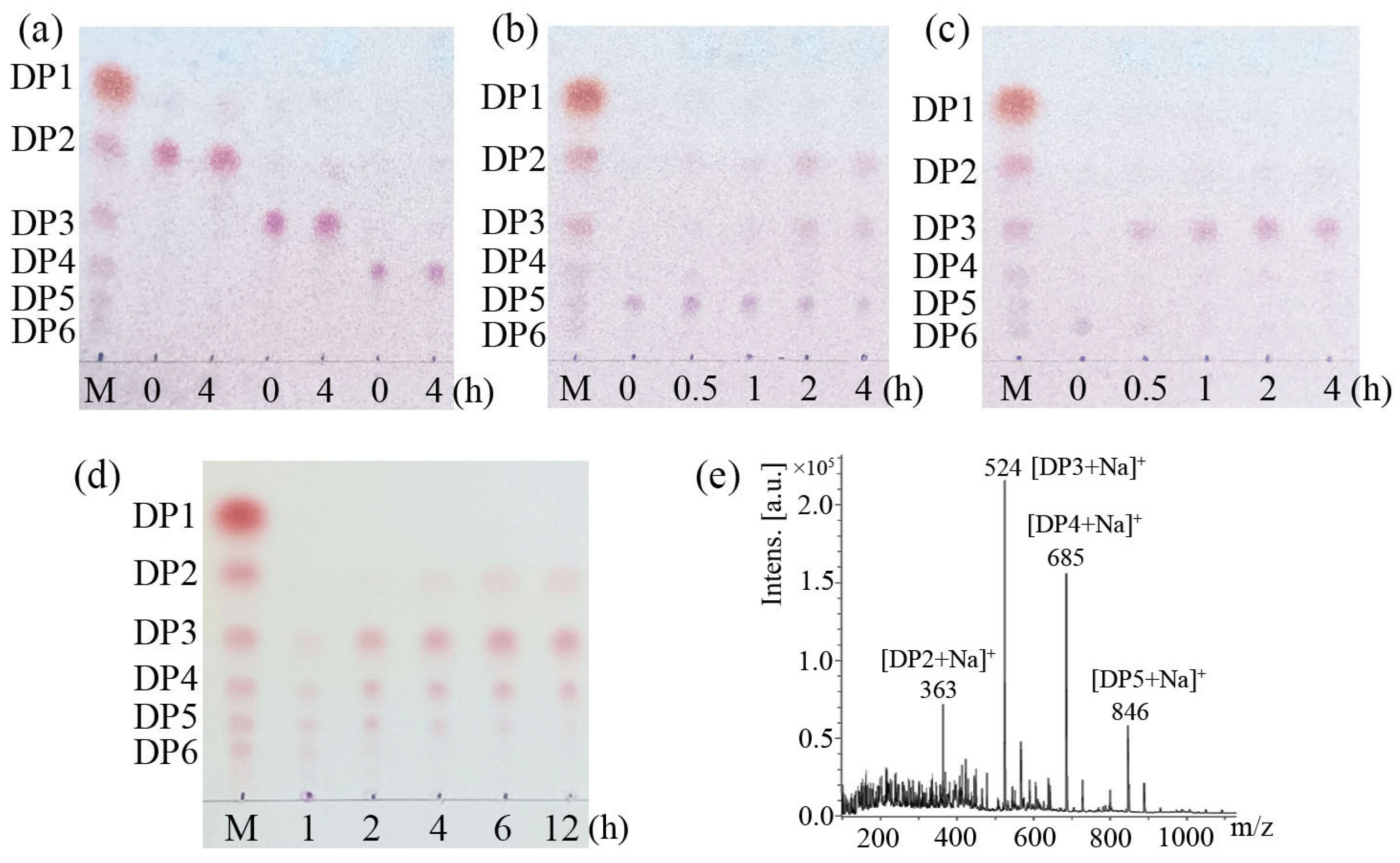
3.5. LeCho1 Is an Endo-Chitosanase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukarram, M.; Ali, J.; Dadkhah-Aghdash, H.; Kurjak, D.; Kačík, F.; Ďurkovič, J. Chitosan-induced biotic stress tolerance and crosstalk with phytohormones, antioxidants, and other signalling molecules. Front. Plant Sci. 2023, 14, 1217822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wen, B.; Xie, H.; Zhang, C.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Advances in the preparation and assessment of the biological activities of chitosan oligosaccharides with different structural characteristics. Food Funct. 2021, 12, 926–951. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, G.; Kim, Y.; Hwang, J.; Kim, S.; Jeon, Y.; Je, J.; Ahn, C.; Moon, S.; Jeon, B.; Park, P. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Tech. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Huda, N.; Xu, C.; Wu, P. Preparation and characterization of squid pen chitooligosaccharide–epigallocatechin gallate conjugates and their antioxidant and antimicrobial activities. RSC Adv. 2020, 10, 33196–33204. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Benjakul, S.; Prodpran, T. Chitooligosaccharides from squid pen prepared using different enzymes: Characteristics and the effect on quality of surimi gel during refrigerated storage. Food Prod. Process. Nu. 2019, 1, 5. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Wang, T.; Guo, X.; Luan, J.; Sun, Y.; Li, X. The use of chitooligosaccharide in beer brewing for protection against beer-spoilage bacteria and its influence on beer performance. Biotechnol. Lett. 2016, 38, 629–635. [Google Scholar] [CrossRef]
- Chen, H.; Guo, X.; Zhu, K. The effect of chitosan oligosaccharides on the shelf-life and quality of fresh wet noodles. Carbohyd. Polym. 2023, 309, 120704. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Singh, A.; Aluko, R.E.; Benjakul, S. Pacific white shrimp (Litopenaeus vannamei) shell chitosan and the conjugate with epigallocatechin gallate: Antioxidative and antimicrobial activities. J. Food Biochem. 2021, 45, e13569. [Google Scholar] [CrossRef] [PubMed]
- Nilsuwan, K.; Guerrero, P.; de la Caba, K.; Benjakul, S.; Prodpran, T. Fish gelatin films laminated with emulsified gelatin film or poly (lactic) acid film: Properties and their use as bags for storage of fried salmon skin. Food Hydrocolloid. 2021, 111, 106199. [Google Scholar] [CrossRef]
- Je, J.; Kim, S. Chitooligosaccharides as potential nutraceuticals: Production and bioactivities. Adv. Food Nutr. Res. 2012, 65, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, Y.; Li, K.; Yu, H.; Liu, S.; Yang, Y.; Chen, X.; Li, P. Monomer composition of chitooligosaccharides obtained by different degradation methods and their effects on immunomodulatory activities. Carbohyd. Polym. 2017, 157, 1288–1297. [Google Scholar] [CrossRef]
- Hoell, I.A.; Vaaje-Kolstad, G.; Eijsink, V.G. Structure and function of enzymes acting on chitin and chitosan. Biotechnol. Genet. Eng. 2010, 27, 331–366. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yang, J.; Lu, B.; Shen, H. Efficient preparation of chitooligosaccharide with a potential chitosanase Csn-SH and its application for fungi disease protection. Front. Microbiol. 2021, 12, 682829. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Liu, M.; Xia, C.; Fan, Q.; Li, X.; Lan, Z.; Shi, G.; Dong, W.; Li, Z. Preparation of active chitooligosaccharides with a novel chitosanase AqCoA and their application in fungal disease protection. J. Agric. Food Chem. 2021, 69, 3351–3361. [Google Scholar] [CrossRef]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Zhu, B.; Yao, Z.; Jiang, L. Purification and biochemical characterization of a novel chitosanase cloned from the gene of Kitasatospora setae KM-6054 and its application in the production of chitooligosaccharides. World J. Microb. Biot. 2023, 39, 111. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Z.; Xiao, Y.; Zhang, J.; Zhou, Y.; Li, X.; Li, S.; Yu, H. Cloning and characterization of a cold-adapted chitosanase from marine bacterium Bacillus sp. BY01. Molecules 2019, 24, 3915. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Gao, W.; Wang, X.; Gao, X.; Man, Z.; Cai, Z.; Qing, Q. Gene cloning, functional expression, and characterization of a novel GH46 chitosanase from Streptomyces avermitilis (SaCsn46A). Appl. Biochem. Biotech. 2022, 194, 813–826. [Google Scholar] [CrossRef]
- Somashekar, D.; Joseph, R. Chitosanases—Properties and applications: A review. Bioresour. Technol. 1996, 55, 35–45. [Google Scholar] [CrossRef]
- Liang, D.C.; Liu, W.G.; Zuo, A.J.; Sun, S.J.; Cheng, N.; Guo, G.; Zhang, J.Y.; De Yao, K. Pre-deliver chitosanase to cells: A novel strategy to improve gene expression by endocellular degradation-induced vector unpacking. Int. J. Pharmaceut. 2006, 314, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Chung, Y.; Chang, C.; Sung, H. Purification and characterization of two chitosanase isoforms from the sheaths of bamboo shoots. J. Agr. Food Chem. 2012, 60, 649–657. [Google Scholar] [CrossRef]
- Doan, C.; Tran, T.; Tran, T.; Nguyen, T.; Nguyen, H.; Tran, T.; Vu, B.; Trinh, T.; Nguyen, A.; Wang, S. Chitosanase production from the liquid fermentation of squid pens waste by Paenibacillus elgii. Polymers 2023, 15, 3724. [Google Scholar] [CrossRef]
- Kouzai, Y.; Mochizuki, S.; Saito, A.; Ando, A.; Minami, E.; Nishizawa, Y. Expression of a bacterial chitosanase in rice plants improves disease resistance to the rice blast fungus Magnaporthe oryzae. Plant Cell Rep. 2012, 31, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Qin, Z.; Chen, Q.; Fan, L.; Jiang, L.; Zhao, L. High level production of a Bacillus amlyoliquefaciens chitosanase in Pichia pastoris suitable for chitooligosaccharides preparation. Int. J. Biol. Macromol. 2020, 149, 1034–1041. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Nakade, K.; Sato, T. Characterization of the post-harvest changes in gene transcription in the gill of the Lentinula edodes fruiting body. Curr. Genet. 2009, 55, 409–423. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Nakade, K.; Sato, S.; Yoshida, K.; Miyazaki, K.; Natsume, S.; Konno, N. Lentinula edodes genome survey and postharvest transcriptome analysis. Appl. Environ. Microb. 2017, 83, e02990-16. [Google Scholar] [CrossRef]
- Buger, N. The bradford method for protein quantitation. Methods Vol. Biol. 1994, 32, 9–15. [Google Scholar] [CrossRef]
- Wood, I.; Elliston, A.; Ryden, P.; Bancroft, I.; Roberts, I.; Waldron, K. Rapid quantification of reducing sugars in biomass hydrolysates: Improving the speed and precision of the dinitrosalicylic acid assay. Biomass Bioenerg. 2012, 44, 117–121. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Li, Z.; Cui, Z.; Ye, X. Functional analysis of a novel endo-β-1,6-glucanase MoGlu16 and its application in detecting cell wall β-1,6-glucan of Magnaporthe oryzae. Front. Microbiol. 2024, 15, 1429065. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chang, C.; Wu, Y.; Li, Y. Exploration of glycosyl hydrolase family 75, a chitosanase from Aspergillus fumigatus. J. Biol. Chem. 2006, 281, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shimono, K.; Ochiai, N.; Shigeru, K.; Kurita, M.; Ohta, Y.; Tanaka, K.; Matsuda, H.; Kawamukai, M. Purification, characterization, and gene analysis of a chitosanase (ChoA) from Matsuebacter chitosanotabidus 3001. J. Bacteriol. 1999, 181, 6642–6649. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Jang, H.; Lee, S.; Lee, K.; Choi, S. Purification and properties of chitosanase from chitinolytic β-Proteobacterium KNU3. J. Microbiol. Biotechn. 2004, 14, 337–343. [Google Scholar]
- Zhang, J.; Cao, H.; Li, S.; Zhao, Y.; Wang, W.; Xu, Q.; Du, Y.; Yin, H. Characterization of a new family 75 chitosanase from Aspergillus sp. W-2. Int. J. Biol. Macromol. 2015, 81, 362–369. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Yuan, F.; Deng, B.; Yu, X. Biocatalysis of heterogenously-expressed chitosanase for the preparation of desirable chitosan oligosaccharides applied against phytopathogenic fungi. Acs Sustain. Chem. Eng. 2020, 8, 4781–4791. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Tong, S.; Yuan, M.; Wang, X.; Wang, J.; Fan, Y. Expression of a Beauveria bassiana chitosanase (BbCSN-1) in Pichia pastoris and enzymatic analysis of the recombinant protein. Protein Expres. Purif. 2020, 166, 105519. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Zhu, M.; Chen, W.; Yu, S.; Zhong, B. Overexpression and biochemical properties of a GH46 chitosanase from marine Streptomyces hygroscopicus R1 suitable for chitosan oligosaccharides preparation. Front. Microbiol. 2022, 12, 816845. [Google Scholar] [CrossRef]
- Peng, N.; Xu, W.; Wang, F.; Hu, J.; Ma, M.; Hu, Y.; Zhao, S.; Liang, Y.; Ge, X. Mitsuaria chitosanase with unrevealed important amino acid residues: Characterization and enhanced production in Pichia pastoris. Appl. Microbiol. Biot. 2013, 97, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; He, S.; Sun, J.; Bao, H.; Cui, L. Secretory production and characterization of a highly effective chitosanase from Streptomyces coelicolor A3 (2) M145 in Pichia pastoris. Biotechnol. J. 2024, 19, 2300402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, K.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, P. Size effects of chitooligomers on the growth and photosynthetic characteristics of wheat seedlings. Carbohyd. Polym. 2016, 138, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ma, Y.; Zou, P.; Cheng, G.; Zhou, J.; Cai, S. Effects of different oligochitosans on isoflavone metabolites, antioxidant activity, and isoflavone biosynthetic genes in soybean (Glycine max) seeds during germination. J. Agric. Food Chem. 2019, 67, 4652–4661. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.; Xing, K.; Park, H. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]


| Substrate | Specific Activity (U/mg) |
|---|---|
| Chitosan (DDA 70%) | 37.18 ± 1.28 c |
| Chitosan (DDA 80%) | 50.22 ± 1.73 b |
| Chitosan (DDA 95%) | 71.88 ± 2.48 a |
| Chitin | 0 d |
| Microcrystalline cellulose | 0 d |
| Sodium carboxymethylcellulose | 0 d |
| Metal Ions | Concentration (mM) | Relativity Activity (%) |
|---|---|---|
| No addition | 0 | 100 ± 2.11 c |
| Na+ (NaCl) | 1 | 95.93 ± 3.09 d |
| K+ (KCl) | 1 | 107.89 ± 3.48 c |
| Ba2+ (BaCl2) | 1 | 108.46 ± 3.50 c |
| Cu2+ (CuCl2) | 1 | 98.94 ± 2.72 c |
| Ca2+ (CaCl2) | 1 | 110.25 ± 3.56 b |
| Co2+ (CoCl2) | 1 | 90.32 ± 2.91 e |
| Fe2+ (FeCl2) | 1 | 107.32 ± 3.46 c |
| Mn2+ (MnCl2) | 1 | 156.63 ± 5.05 a |
| Mg2+ (MgCl2) | 1 | 118.71 ± 3.83 b |
| Ni2+ (NiCl2) | 1 | 94.87 ± 3.06 d |
| Zn2+ (ZnCl2) | 1 | 95.69 ± 3.09 d |
| Fe3+ (FeCl3) | 1 | 0.00 ± 0.00 h |
| Al3+ (AlCl3) | 1 | 85.19 ± 2.75 f |
| Cd3+ (CdCl3) | 1 | 83.32 ± 2.69 f |
| EDTA | 1 | 56.17 ± 1.78 g |
| 10 | 0.00 ± 0.00 h |
| Microorganism | Chitosanase | Family | Mw (kDa) | Optimal Temperature (°C) | Optimal pH | Metal Ions (+) | Metal Ions (−) | End Products | Reference or Source |
|---|---|---|---|---|---|---|---|---|---|
| Lentinula edodes | LeCho1 | GH75 | 27 | 50 | 3.0 | Mn2+ | Fe3+ | DP2–5 | This study |
| Aspergillus sp. W-2 | CsnW2 | GH75 | 28 | 55 | 6.0 | Mn2+, Ca2+, Mg2+ | Cu2+, Fe2+, Zn2+, Ni2+, Ge2+ | DP2–6 | [34] |
| Aspergillus fumigatus CJ22-326 | Csn75 | GH75 | 23.5 | 55–65 | 5.0–6.0 | Mn2+ | Cu2+, Mg2+ | DP2–4 | [35] |
| Beauveria bassiana | BbCSN-1 | GH75 | 33 | 30 | 5.0 | Mn2+ | Cu2+, Co2+ | DP2–3 | [36] |
| Streptomyces coelicolor A3(2) M145 | CsnA | GH46 | 26 | 65 | 6.0 | Mn2+, Co2+ | Fe3+, Al3+, Ni2+ | DP2–3 | [39] |
| Paenibacillus elgii TKU051 | chitosanase | GH46 | 28 | 55 | 6.0 | Mn2+ | Cu2+ | DP2–3 | [23] |
| Streptomyces hygroscopicus R1 | ShCsn46 | GH46 | 28 | 55 | 5.5 | Mn2+ | Cu2+, Fe2+, Al3+ | DP2–6 | [37] |
| Bacillus sp. BY01 | CsnB | GH46 | 30 | 35 | 5.0 | Mn2+, Cu2+, Mg2+ | Fe3+ | DP2–3 | [18] |
| Matsuebacter chitosanotabidus 3001 | ChoA | GH80 | 34 | 30–40 | 4.0 | ND | Ag+ | DP2–6 | [32] |
| β-Proteobacterium KNU3 | ChoK | GH80 | 34 | 70 | 6.0 | Cu2+, Co2+ | Ca2+, Zn2+, Mg2+ | ND | [33] |
| Mitsuaria sp. 141 | ChoA | GH80 | 33 | 65 | 5.5 | Fe2+ | Cu2+, Hg2+ | DP1–5 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, Y.; Li, J.; Zong, H.; Chen, Y.; Zhou, J.; Li, X.; Ye, X. Characterization of a Novel Acid-Stable Chitosanase from Lentinula edodes Suitable for Chitooligosaccharide Preparation. Foods 2024, 13, 3127. https://doi.org/10.3390/foods13193127
Wang Y, Zhao Y, Li J, Zong H, Chen Y, Zhou J, Li X, Ye X. Characterization of a Novel Acid-Stable Chitosanase from Lentinula edodes Suitable for Chitooligosaccharide Preparation. Foods. 2024; 13(19):3127. https://doi.org/10.3390/foods13193127
Chicago/Turabian StyleWang, Yanxin, Yujie Zhao, Jingchen Li, Haobo Zong, Ying Chen, Jinyu Zhou, Xinlian Li, and Xianfeng Ye. 2024. "Characterization of a Novel Acid-Stable Chitosanase from Lentinula edodes Suitable for Chitooligosaccharide Preparation" Foods 13, no. 19: 3127. https://doi.org/10.3390/foods13193127
APA StyleWang, Y., Zhao, Y., Li, J., Zong, H., Chen, Y., Zhou, J., Li, X., & Ye, X. (2024). Characterization of a Novel Acid-Stable Chitosanase from Lentinula edodes Suitable for Chitooligosaccharide Preparation. Foods, 13(19), 3127. https://doi.org/10.3390/foods13193127





