Efficacy Evaluation of Chlorine Dioxide and Hypochlorous Acid as Sanitisers on Quality and Shelf Life of Atlantic Salmon (Salmo salar) Fillets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Treatments and Study Setting
2.3. Physical Analyses
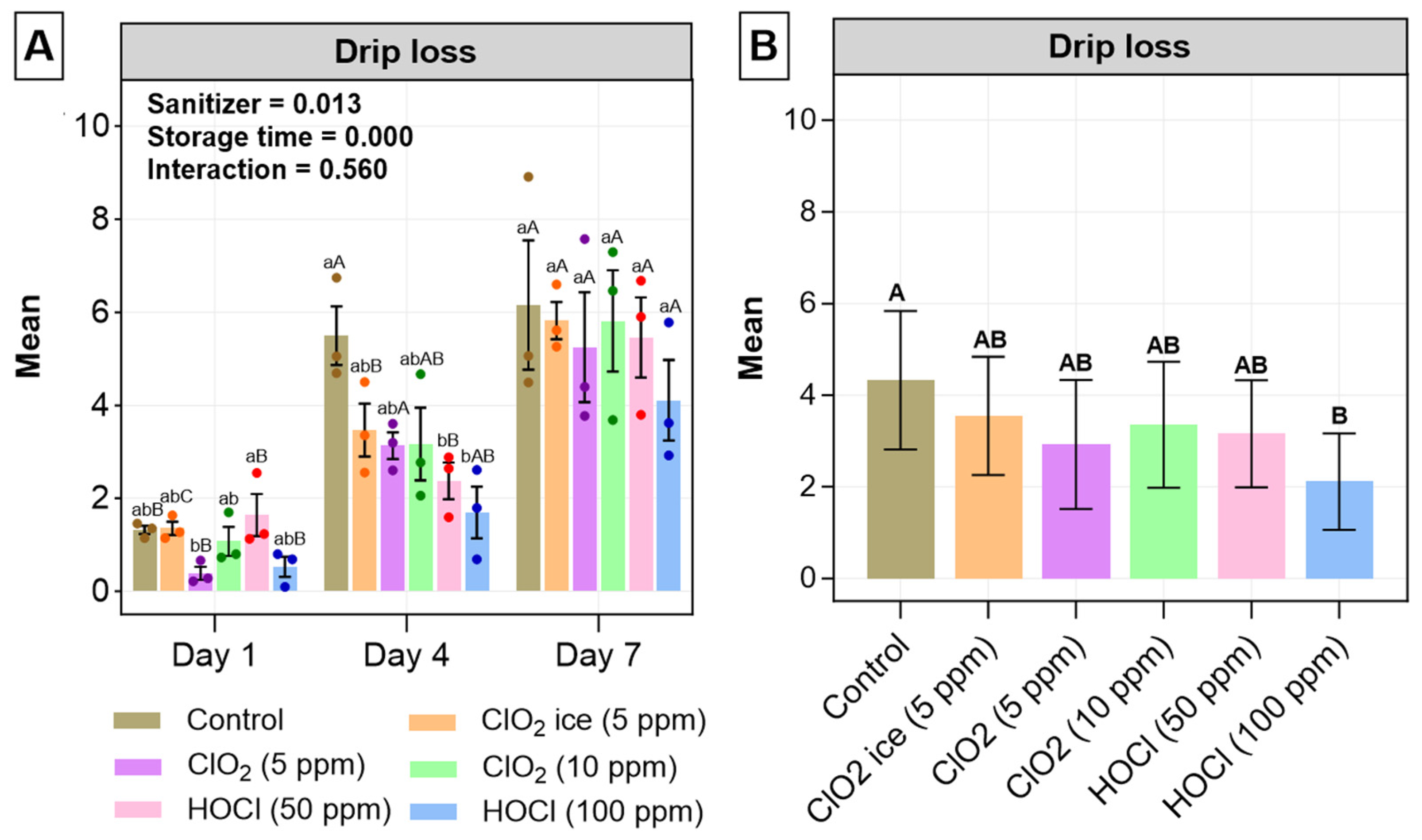
2.3.1. Drip Loss
2.3.2. Quality Index Method (QIM)
2.3.3. Texture Profile Analysis (TPA)
2.3.4. Colour
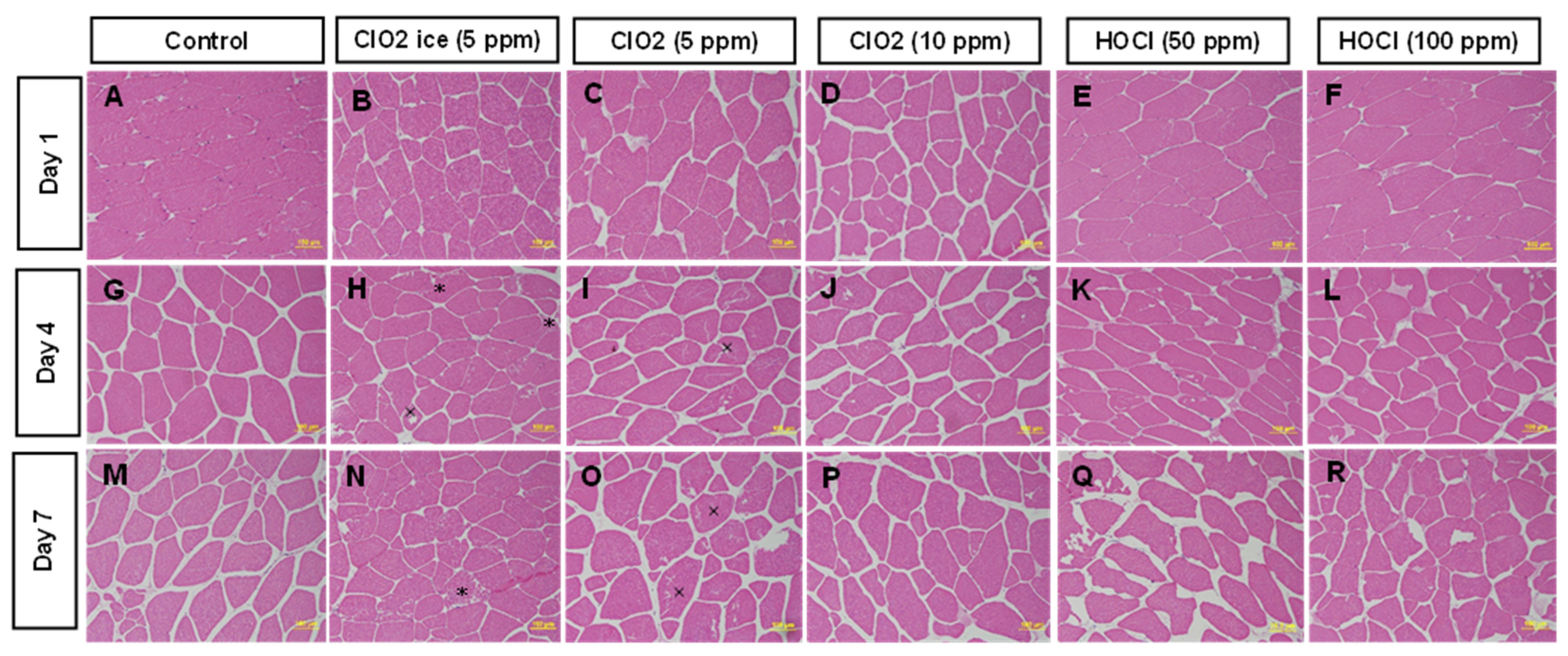
2.3.5. Histology
2.4. Chemical Analyses
2.4.1. Thiobarbituric Acid Reactive Substance (TBARS)
2.4.2. Total Volatile Base Nitrogen (TVB-N)
2.4.3. pH
2.5. Microbiological Analyses
Total Viable Count (TVC)
2.6. Statistical Analyses
3. Results and Discussion
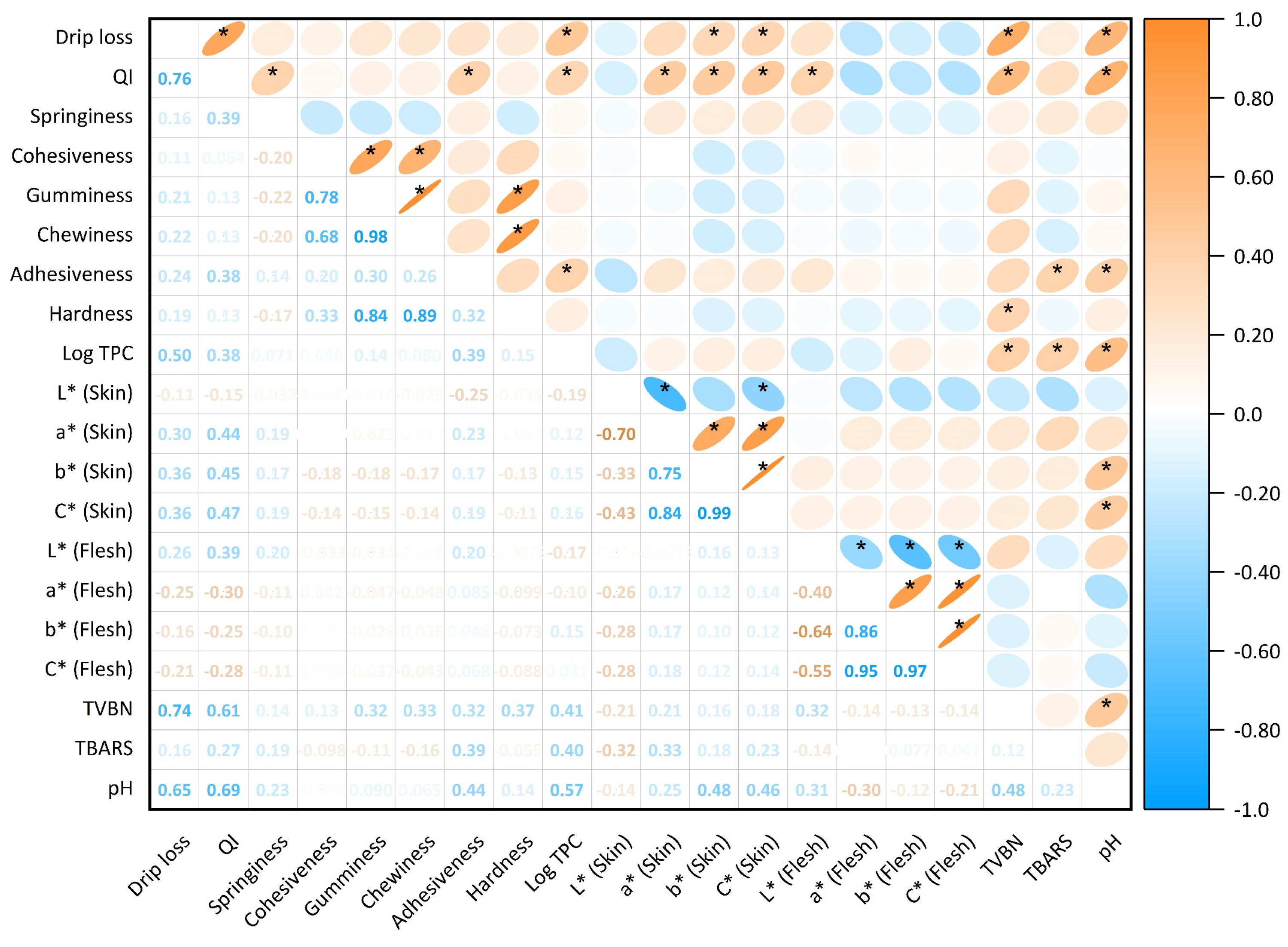
3.1. Physical Analyses
3.1.1. Drip Loss
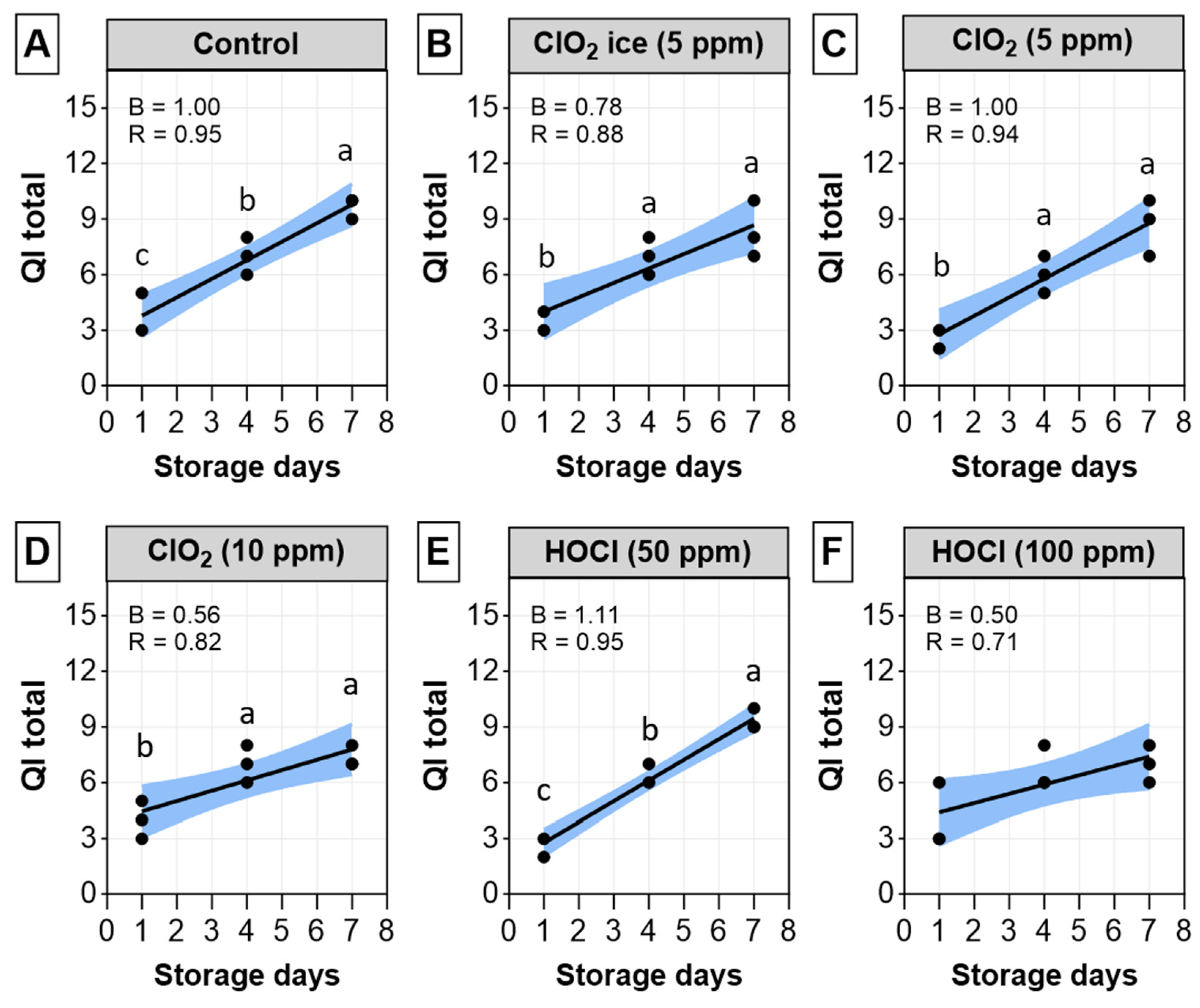
3.1.2. Quality Index Method (QIM)
3.1.3. Texture and Colour Profiles
3.1.4. Changes in Muscle Microstructure
3.2. Chemical Analyses
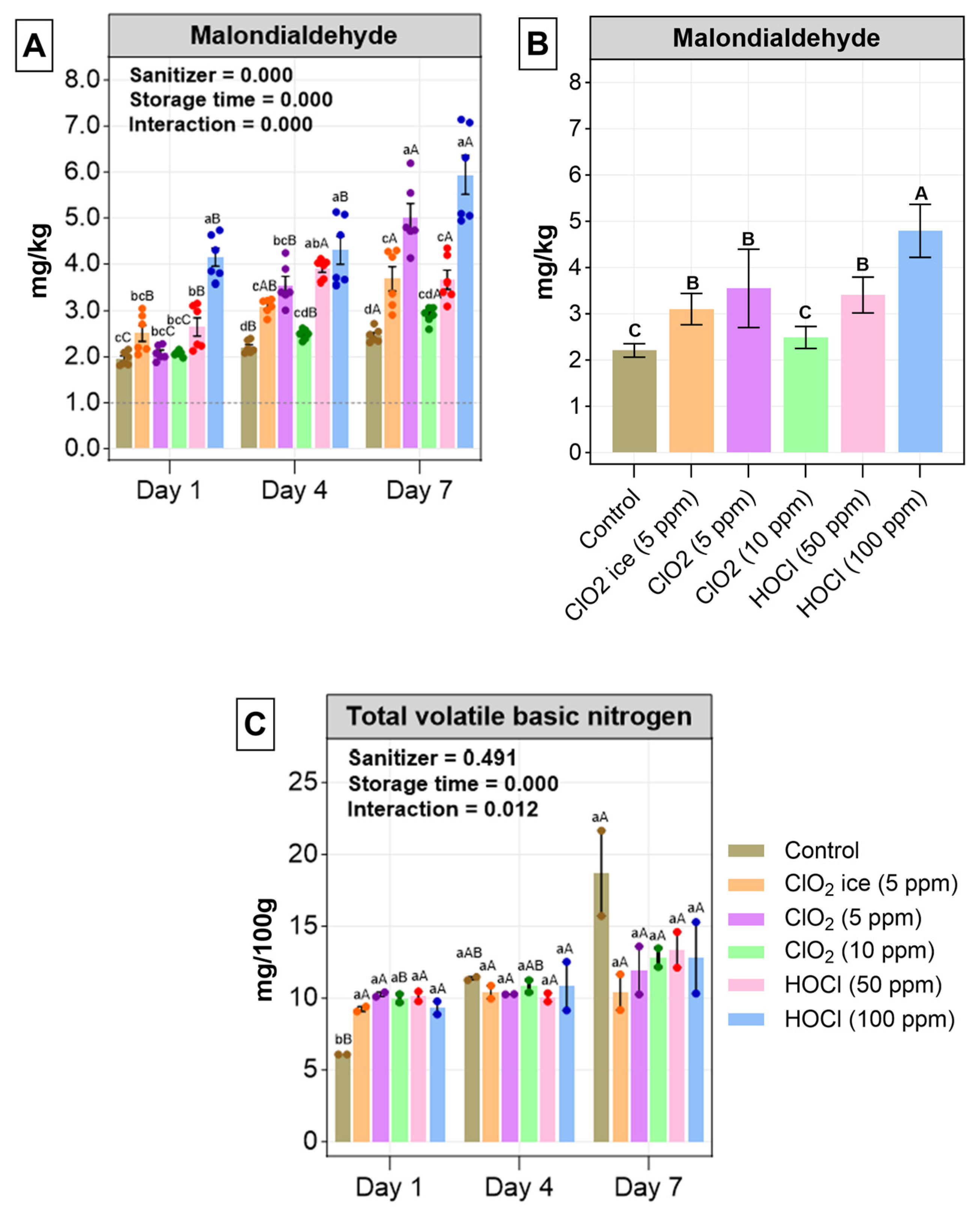
3.2.1. Thiobarbituric Acid Reactive Substance (TBARS)
3.2.2. Total Volatile Base Nitrogen (TVB-N)
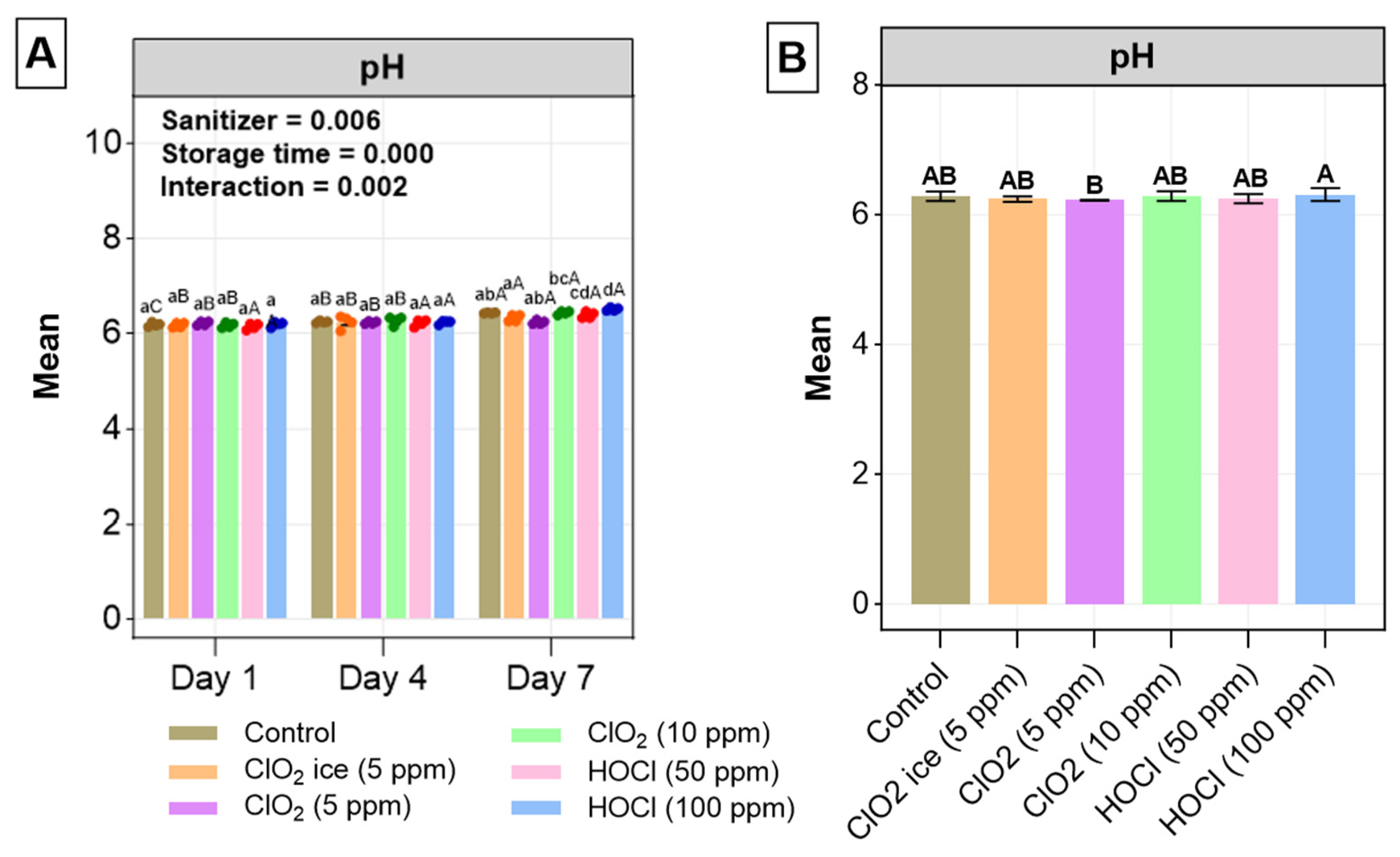
3.2.3. pH
3.3. Microbiological Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luiz, D.d.B.; Silva, C.D.F.; Campelo, S.R.; Santos, V.R.V.d.; Lima, L.K.F.d.; Chicrala, P.C.M.S.; Iwashita, M.K.P. Evaluation of the effectiveness of ozone as a sanitizer for fish experimentally contaminated with Salmonella sp. Braz. J. Food Technol. 2017, 20, e2016150. [Google Scholar] [CrossRef]
- Veasey, S.; Muriana, P.M. Evaluation of electrolytically-generated hypochlorous acid (‘electrolyzed water’) for sanitation of meat and meat-contact surfaces. Foods 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Food Safety; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- New South Wales Government. Food Poisoning; New South Wales Government: Silverwater, Australia, 2021.
- Food and Agriculture Organization. Cultured Aquatic Species Information Programme Salmo Salar; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Wu, P.-f.; He, L.-h.; Xian, H.-x. Survey on the sanitary quality raw salmon. Mod. Prev. Med. 2009, 8, 1564–1565. [Google Scholar]
- Cartwright, K.; Evans, B. Salmon as a food-poisoning vehicle-two successive Salmonella outbreaks. Epidemiol. Infect. 1988, 101, 249–257. [Google Scholar] [CrossRef]
- Bell, J.G.; Henderson, R.J.; Tocher, D.R.; Sargent, J.R. Replacement of dietary fish oil with increasing levels of linseed oil: Modification of flesh fatty acid compositions in Atlantic salmon (Salmo salar) using a fish oil finishing diet. Lipids 2004, 39, 223–232. [Google Scholar] [CrossRef]
- Soares, N.; Silva, P.; Barbosa, C.; Pinheiro, R.; Vicente, A. Comparing the effects of glazing and chitosan-based coating applied on frozen salmon on its organoleptic and physicochemical characteristics over six-months storage. J. Food Eng. 2017, 194, 79–86. [Google Scholar] [CrossRef]
- World Health Organization. Benefits and Risks of the Use of Chlorine-Containing Disinfectants in Food Production and Food Processing: Report of a Joint FAO/WHO Expert Meeting; World Health Organization: Ann Arbor, MI, USA, 2009. [Google Scholar]
- Dewi, F.R.; Stanley, R.; Powell, S.M.; Burke, C.M. Application of electrolysed oxidising water as a sanitiser to extend the shelf-life of seafood products: A review. J. Food Sci. Technol. 2017, 54, 1321–1332. [Google Scholar] [CrossRef]
- Lin, W.-F.; Huang, T.S.; Cornell, J.A.; Lin, C.M.; Wei, C.I. Bactericidal activity of aqueous chlorine and chlorine dioxide solutions in a fish model system. J. Food Sci. 1996, 61, 1030–1034. [Google Scholar] [CrossRef]
- USDA-FSIS. Safe and Suitable Ingredients Used in the Production of Meat and Poultry, and Egg Products; USDA-FSIS: Washington, DC, USA, 2016.
- Ozer, N.P.; Demirci, A. Electrolyzed oxidizing water treatment for decontamination of raw salmon inoculated with Escherichia coli O157: H7 and Listeria monocytogenes Scott A and response surface modeling. J. Food Eng. 2006, 72, 234–241. [Google Scholar] [CrossRef]
- Pao, S.; Kelsey, D.; Khalid, M.; Ettinger, M. Using aqueous chlorine dioxide to prevent contamination of tomatoes with Salmonella enterica and Erwinia carotovora during fruit washing. J. Food Prot. 2007, 70, 629–634. [Google Scholar] [CrossRef]
- Rodgers, S.L.; Cash, J.N.; Siddiq, M.; Ryser, E.T. A comparison of different chemical sanitizers for inactivating Escherichia coli O157: H7 and Listeria monocytogenes in solution and on apples, lettuce, strawberries, and cantaloupe. J. Food Prot. 2004, 67, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Keskinen, L.A.; Burke, A.; Annous, B.A. Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157: H7 from lettuce leaves. Int. J. Food Microbiol. 2009, 132, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Kim, B. Effect of a simple chlorine dioxide method for controlling five foodborne pathogens, yeasts and molds on blueberries. Food Microbiol. 2007, 24, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Linton, R.; Nielsen, S.; Nelson, P. Reduction of Listeria monocytogenes on green peppers (Capsicum annuum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7 C. J. Food Prot. 2001, 64, 1730–1738. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; O’Keefe, S.F.; Wei, C.i. Effect of chlorine dioxide treatment on lipid oxidation and fatty acid composition in salmon and red grouper fillets. J. Am. Oil Chem. Soc. 1997, 74, 539–542. [Google Scholar] [CrossRef]
- Kim, J.; Du, W.X.; Otwell, W.S.; Marshall, M.R.; Wei, C.-I. Nutrients in salmon and red grouper fillets as affected by chlorine dioxide (ClO2) treatment. J. Food Sci. 1998, 63, 629–633. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Choi, J.-K.; Shin, I.-S. Bactericidal effects of hypochlorous acid water against Vibrio parahaemolyticus contaminated on raw fish and shellfish. Korean J. Food Sci. Technol. 2015, 47, 719–724. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, L.; Lai, S.; Yang, H. NMR-based metabolomic investigation of antimicrobial mechanism of electrolysed water combined with moderate heat treatment against Listeria monocytogenes on salmon. Food Control 2021, 125, 107974. [Google Scholar] [CrossRef]
- Huang, Y.-R.; Hsieh, H.-S.; Lin, S.-Y.; Lin, S.-J.; Hung, Y.-C.; Hwang, D.-F. Application of electrolyzed oxidizing water on the reduction of bacterial contamination for seafood. Food Control 2006, 17, 987–993. [Google Scholar] [CrossRef]
- Phuvasate, S.; Su, Y.-C. Effects of electrolyzed oxidizing water and ice treatments on reducing histamine-producing bacteria on fish skin and food contact surface. Food Control 2010, 21, 286–291. [Google Scholar] [CrossRef]
- Al-Holy, M.A.; Rasco, B.A. The bactericidal activity of acidic electrolyzed oxidizing water against Escherichia coli O157: H7, Salmonella Typhimurium, and Listeria monocytogenes on raw fish, chicken and beef surfaces. Food Control 2015, 54, 317–321. [Google Scholar] [CrossRef]
- Hu, Y.; Du, S.; Wu, D.; Luo, H. Study on the antimicrobial activity of strongly acidic electrolysed oxidising water for large yellow croaker. Qual. Assur. Saf. Crop. Foods 2015, 7, 133–139. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Lee, Y.-J.; Park, I.-Y.; Kim, J.-Y.; Oh, S.-J.; Song, K.-B. Effect of chlorine dioxide treatment on microbial growth and qualities of fish paste during storage. Appl. Biol. Chem. 2007, 50, 42–47. [Google Scholar]
- Huang, Y.R.; Shiau, C.Y.; Hung, Y.C.; Hwang, D.F. Change of hygienic quality and freshness in tuna treated with electrolyzed water and carbon monoxide gas during refrigerated and frozen storage. J. Food Sci. 2006, 71, M127–M133. [Google Scholar] [CrossRef]
- McCarthy, S.; Burkhardt, W., III. Efficacy of electrolyzed oxidizing water against Listeria monocytogenes and Morganella morganii on conveyor belt and raw fish surfaces. Food Control 2012, 24, 214–219. [Google Scholar] [CrossRef]
- Kim, J.; Huang, T.S.; Marshall, M.; Wei, C.I. Chlorine dioxide treatment of seafoods to reduce bacterial loads. J. Food Sci. 1999, 64, 1089–1093. [Google Scholar] [CrossRef]
- Khazandi, M.; Deo, P.; Ferro, S.; Venter, H.; Pi, H.; Crabb, S.; Amorico, T.; Ogunniyi, A.D.; Trott, D.J. Efficacy evaluation of a new water sanitizer for increasing the shelf life of Southern Australian King George Whiting and Tasmanian Atlantic Salmon fillets. Food Microbiol. 2017, 68, 51–60. [Google Scholar] [CrossRef]
- Zhu, S.; Ramaswamy, H.; Simpson, B. Effect of high-pressure versus conventional thawing on color, drip loss and texture of Atlantic salmon frozen by different methods. LWT-Food Sci. Technol. 2004, 37, 291–299. [Google Scholar] [CrossRef]
- Fuentes-Amaya, L.F.; Munyard, S.; Fernandez-Piquer, J.; Howieson, J. Sensory, Microbiological and Chemical Changes in Vacuum-Packaged Blue Spotted Emperor (Lethrinus sp), Saddletail Snapper (Lutjanus malabaricus), Crimson Snapper (Lutjanus erythropterus), Barramundi (Lates calcarifer) and Atlantic Salmon (Salmo salar) Fillets Stored at 4 °C. Food Sci. Nutr. 2016, 4, 479–489. [Google Scholar]
- Bourne, M.C. Texture Profile Analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.d.L.S.; de Miranda Gomide, L.A.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Howieson, J.; Chaklader, M.R. The ameliorative effects of low-temperature pasteurization on physicochemical and microbiological quality of raw Akoya pearl oyster (Pinctada fucata). Food Control 2021, 129, 108241. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Lu, W.; Shen, H.; Luo, Y. Quality predictive models of grass carp (Ctenopharyngodon idellus) at different temperatures during storage. Food Control 2011, 22, 1197–1202. [Google Scholar] [CrossRef]
- Özoğul, F.; Özoğul, Y. Comparision of methods used for determination of total volatile basic nitrogen (TVB-N) in rainbow trout (Oncorhynchus mykiss). Turk. J. Zool. 2000, 24, 113–120. [Google Scholar]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Agricultural Chemists (AOAC): Gaithersburg, MD, USA, 1995. [Google Scholar]
- AS 5013.5:2016; Microbiology of the Food Chain–Horizontal Method for the Enumeration of Microorganisms–Colony Count at 30° C by the Pour Plate Technique. Standards Australia (SA): Sydney, Australia, 2016.
- He, H.-J.; Wu, D.; Sun, D.-W. Rapid and non-destructive determination of drip loss and pH distribution in farmed Atlantic salmon (Salmo salar) fillets using visible and near-infrared (Vis–NIR) hyperspectral imaging. Food Chem. 2014, 156, 394–401. [Google Scholar] [CrossRef]
- Kristoffersen, S.; Vang, B.; Larsen, R.; Olsen, R.L. Pre-rigor filleting and drip loss from fillets of farmed Atlantic cod (Gadus morhua L.). Aquac. Res. 2007, 38, 1721–1731. [Google Scholar] [CrossRef]
- Larsen, R.; Stormo, S.K.; Dragnes, B.T.; Elvevoll, E.O. Losses of taurine, creatine, glycine and alanine from cod (Gadus morhua L.) fillet during processing. J. Food Compos. Anal. 2007, 20, 396–402. [Google Scholar] [CrossRef]
- Biswas, G.; Islam, M.S.; Rahman, S.M.; Al Mamun, S.A. Effect of electrolyzed water on physicochemical and sensory qualities of beef. Theory Pract. Meat Process. 2024, 9, 180–187. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S. New frontiers in understanding drip loss in pork: Recent insights on the role of postmortem muscle biochemistry. J. Anim. Breed. Genet. 2007, 124, 19–26. [Google Scholar] [CrossRef]
- Chan, S.S.; Roth, B.; Jessen, F.; Jakobsen, A.N.; Lerfall, J. Water holding properties of Atlantic salmon. Compr. Rev. Food Sci. Food Saf. 2022, 21, 477–498. [Google Scholar] [CrossRef]
- Duun, A.; Rustad, T. Quality changes during superchilled storage of cod (Gadus morhua) fillets. Food Chem. 2007, 105, 1067–1075. [Google Scholar] [CrossRef]
- Einen, O.; Guerin, T.; Fjæra, S.O.; Skjervold, P.O. Freezing of pre-rigor fillets of Atlantic salmon. Aquaculture 2002, 212, 129–140. [Google Scholar] [CrossRef]
- Bonilla, A.C.; Sveinsdottir, K.; Martinsdottir, E. Development of Quality Index Method (QIM) scheme for fresh cod (Gadus morhua) fillets and application in shelf life study. Food Control 2007, 18, 352–358. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; de Lima, J.T.A.X.; de Paula, F.E.R. Development of Quality Index Method (QIM) scheme for spiny lobster (Panulirus argus, Latreille, 1804) stored in ice. Food Control 2015, 47, 237–245. [Google Scholar] [CrossRef]
- Vaz-Pires, P.; Seixas, P. Development of new quality index method (QIM) schemes for cuttlefish (Sepia officinalis) and broadtail shortfin squid (Illex coindetii). Food Control 2006, 17, 942–949. [Google Scholar] [CrossRef]
- Alexi, N.; Hvam, J.; Lund, B.W.; Nsubuga, L.; de Oliveira Hansen, R.M.; Thamsborg, K.; Lofink, F.; Byrne, D.V.; Leisner, J.J. Potential of novel cadaverine biosensor technology to predict shelf life of chilled yellowfin tuna (Thunnus albacares). Food Control 2021, 119, 107458. [Google Scholar] [CrossRef]
- Calanche, J.; Tomas, A.; Martinez, S.; Jover, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. Relation of quality and sensory perception with changes in free amino acids of thawed seabream (Sparus aurata). Food Res. Int. 2019, 119, 126–134. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Chung, W.H.; Howieson, J.; Fotedar, R. A mixture of full-fat and defatted Hermetia illucens larvae and poultry by-products as sustainable protein sources improved fillet quality traits in farmed Barramundi, Lates calcarifer. Foods 2023, 12, 362. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Chung, W.H.; Howieson, J.; Fotedar, R. A combination of Hermetia illucens reared on fish waste and poultry by-product meal improves sensory and physicochemical quality of farmed Barramundi filets. Front. Nutr. 2022, 8, 788064. [Google Scholar] [CrossRef]
- Shin, J.H.; Chang, S.; Kang, D.H. Application of antimicrobial ice for reduction of foodborne pathogens (Escherichia coli O157:H7, Salmonella Typhimurium, Listeria monocytogenes) on the surface of fish. J. Appl. Microbiol. 2004, 97, 916–922. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Tang, X.; Tang, S.; You, H.; Shi, H.; Gu, R. Combined effect of electrolyzed oxidizing water and chitosan on the microbiological, physicochemical, and sensory attributes of American shad (Alosa sapidissima) during refrigerated storage. Food Control 2014, 46, 397–402. [Google Scholar] [CrossRef]
- Lan, W.; Zhou, Q.; Zhao, X.; Xie, J. Stable chlorine dioxide combined with slightly acidic electrolyzed water as preservation strategies in large yellow croaker (Pseudosciaena crocea) during cold storage: Effects on microbiological, physicochemical, and sensorial qualities. J. Food Saf. 2024, 44, e13113. [Google Scholar] [CrossRef]
- Amanatidou, A.; Schlüter, O.; Lemkau, K.; Gorris, L.; Smid, E.; Knorr, D. Effect of combined application of high pressure treatment and modified atmospheres on the shelf life of fresh Atlantic salmon. Innov. Food Sci. Emerg. Technol. 2000, 1, 87–98. [Google Scholar] [CrossRef]
- Moroney, N.C.; Wan, A.H.; Soler-Vila, A.; FitzGerald, R.D.; Johnson, M.P.; Kerry, J.P. Inclusion of Palmaria palmata (red seaweed) in Atlantic salmon diets: Effects on the quality, shelf-life parameters and sensory properties of fresh and cooked salmon fillets. J. Sci. Food Agric. 2015, 95, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Kittilsen, S.; Schjolden, J.; Beitnes-Johansen, I.; Shaw, J.; Pottinger, T.G.; Sørensen, C.; Braastad, B.O.; Bakken, M.; Øverli, Ø. Melanin-based skin spots reflect stress responsiveness in salmonid fish. Horm. Behav. 2009, 56, 292–298. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Li, K.; Wan, Q.; Wu, G.; Lin, Y.; Huang, T.; Wen, G. The protective role and mechanism of melanin for Aspergillus niger and Aspergillus flavus against chlorine-based disinfectants. Water Res. 2022, 223, 119039. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G. Depigmentation of Melanin-containing Tissues Using Hypochlorous Acid to Enhance Hematoxylin-eosin and Immunohistochemical Staining. Appl. Immunohistochem. Mol. Morphol. 2024, 32, 53–59. [Google Scholar] [CrossRef]
- Mancini, R.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Yu, D.; Regenstein, J.M.; Xia, W. Bio-based edible coatings for the preservation of fishery products: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2481–2493. [Google Scholar] [CrossRef]
- Li, X.P.; Zhou, M.Y.; Liu, J.F.; Xu, Y.X.; Mi, H.B.; Yi, S.M.; Li, J.R.; Lin, H. Shelf-life extension of chilled olive flounder (Paralichthys olivaceus) using chitosan coatings containing clove oil. J. Food Process. Preserv. 2017, 41, e13204. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Jin, W.; Li, L. Effects of tartary buckwheat polysaccharide combined with nisin edible coating on the storage quality of tilapia (Oreochromis niloticus) fillets. J. Sci. Food Agric. 2018, 98, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Steine, G.R.O.; Alfnes, F.; RØRÅ, M.B. The Effect of Color on Consumer WTP for Farmed Salmon. Mar. Resour. Econ. 2005, 20, 211–219. [Google Scholar] [CrossRef]
- Esaiassen, M.; Jensen, T.K.; Edvinsen, G.K.; Otnæs, C.H.A.; Ageeva, T.N.; Mæhre, H.K. Nutritional value and storage stability in commercially produced organically and conventionally farmed Atlantic salmon (Salmo salar L.) in Norway. Appl. Food Res. 2022, 2, 100033. [Google Scholar] [CrossRef]
- Lee, J.; Jahurul, M.; Pua, V.; Shapawi, R.; Chan, P. Effects of chitosan and ascorbic acid coating on the chilled tilapia fish (Oreochromis niloticus) fillet. J. Phys. Conf. Ser. 2019, 1358, 012009. [Google Scholar] [CrossRef]
- Zarandona, I.; López-Caballero, M.E.; Montero, M.P.; Guerrero, P.; de la Caba, K.; Gómez-Guillén, M.C. Horse mackerel (Trachurus trachurus) fillets biopreservation by using gallic acid and chitosan coatings. Food Control 2021, 120, 107511. [Google Scholar] [CrossRef]
- Wenk, J.; Aeschbacher, M.; Salhi, E.; Canonica, S.; Von Gunten, U.; Sander, M. Chemical oxidation of dissolved organic matter by chlorine dioxide, chlorine, and ozone: Effects on its optical and antioxidant properties. Environ. Sci. Technol. 2013, 47, 11147–11156. [Google Scholar] [CrossRef]
- Gordon, G.; Rosenblatt, A.A. Chlorine dioxide: The current state of the art. Ozone Sci. Eng. 2005, 27, 203–207. [Google Scholar] [CrossRef]
- Loke, X.-J.; Chang, C.-K.; Hou, C.-Y.; Cheng, K.-C.; Hsieh, C.-W. Plasma-treated polyethylene coated with polysaccharide and protein containing cinnamaldehyde for active packaging films and applications on tilapia (Orechromis niloticus) fillet preservation. Food Control 2021, 125, 108016. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.W.; Cerqueira, M.A.; Ruiz, H.A.; Martins, J.T.; Casariego, A.; Teixeira, J.A.; Vicente, A.A. Effect of chitosan-based coatings on the shelf life of salmon (Salmo salar). J. Agric. Food Chem. 2010, 58, 11456–11462. [Google Scholar] [CrossRef] [PubMed]
- Rastiani, F.; Jebali, A.; Hekmatimoghaddam, S.; Khalili Sadrabad, E.; Akrami Mohajeri, F.; Dehghani-Tafti, A. Monitoring the freshness of rainbow trout using intelligent PH-sensitive indicator during storage. J. Nutr. Food Secur. 2019, 4, 225–235. [Google Scholar] [CrossRef]
- Fletcher, G.; Summers, G.; Corrigan, V.; Cumarasamy, S.; Dufour, J. Spoilage of king salmon (Oncorhynchus tshawytscha) fillets stored under different atmospheres. J. Food Sci. 2002, 67, 2362–2374. [Google Scholar] [CrossRef]
- Lampel, K.A.; Al-Khaldi, S.; Cahill, S.M. Bad Bug Book: Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins, 2nd ed.; Food and drug administration (FDA), U.S. Department of Health and Human Services: Washington, DC, USA, 2012.
- Quitral, V.; Donoso, M.L.; Ortiz, J.; Herrera, M.V.; Araya, H.; Aubourg, S.P. Chemical changes during the chilled storage of Chilean jack mackerel (Trachurus murphyi): Effect of a plant-extract icing system. LWT-Food Sci. Technol. 2009, 42, 1450–1454. [Google Scholar] [CrossRef]
- Ramírez Orejel, J.C.; Cano-Buendía, J.A. Applications of electrolyzed water as a sanitizer in the food and animal-by products industry. Processes 2020, 8, 534. [Google Scholar] [CrossRef]

| Solution | pH |
|---|---|
| 3.5% brine | 7.64 ± 0.09 a |
| 5 ppm ClO2 | 7.18 ± 0.01 c |
| 10 ppm ClO2 | 6.84 ± 0.11 d |
| 50 ppm HOCl | 7.50 ± 0.04 ab |
| 100 ppm HOCl | 7.35 ± 0.01 bc |
| Treatments | Two-Way ANOVA (P) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | ClO2 Ice (5 ppm) | ClO2 (5 ppm) | ClO2 (10 ppm) | HOCl (50 ppm) | HOCl (100 ppm) | T | ST | T × ST | |
| Texture Profile | |||||||||
| Springiness (mm) | 0.419 | 0.139 | 0.673 | ||||||
| Day 1 | 1.00 ± 0.00 aA | 1.00 ± 0.01 aA | 0.99 ± 0.03 aA | 1.00 ± 0.00 aA | 0.99 ± 0.03 aA | 1.00 ± 0.00 aA | |||
| Day 4 | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | |||
| Day 7 | 0.99 ± 0.02 aA | 0.99 ± 0.02 aA | 1.00 ± 0.01 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | 1.00 ± 0.00 aA | |||
| 1 B (r) | −8.24 × 10−4 (−0.21) | −5.56 × 10−4 (−0.10) | 1.94 × 10−3 (0.31) | - | 1.62 × 10−3 (0.31) | - | |||
| Cohesiveness (ratio) | 0.066 | 0.008 * | 0.897 | ||||||
| Day 1 | 0.23 ± 0.01 aB | 0.26 ± 0.04 aA | 0.25 ± 0.03 aA | 0.26 ± 0.05 aA | 0.24 ± 0.11 aA | 0.20 ± 0.03 aA | |||
| Day 4 | 0.26 ± 0.05 aAB | 0.29 ± 0.03 aA | 0.26 ± 0.04 aA | 0.32 ± 0.08 aA | 0.27 ± 0.05 aA | 0.25 ± 0.05 aA | |||
| Day 7 | 0.27 ± 0.02 aA | 0.28 ± 0.06 aA | 0.26 ± 0.04 aA | 0.27 ± 0.04 aA | 0.27 ± 0.03 aA | 0.25 ± 0.03 aA | |||
| B (r) | 6.58 × 10−3 (0.42) | 3.06 × 10−3 (0.18) | 2.50 × 10−3 (0.16) | 4.27 × 10−5 (0.00) | 4.02 × 10−3 (0.16) | 9.00 × 10−3 (0.49) | |||
| Gumminess (g) | 0.310 | 0.022 * | 0.486 | ||||||
| Day 1 | 679.61 ± 211.84 aB | 1050.72 ± 473.39 aA | 898.04 ± 271.88 aA | 788.1625 ± 368.76 aA | 817.66 ± 513.72 aA | 609.58 ± 182.19 aA | |||
| Day 4 | 901.96 ± 256.29 aB | 1001.01 ± 329.93 aA | 826.83 ± 398.21 aA | 1079.79 ± 518.26 aA | 987.88 ± 293.71 aA | 789.01 ± 242.40 aA | |||
| Day 7 | 1307.61 ± 274.14 aA | 1034.23 ±413.41 aA | 950.76 ± 269.90 aA | 1067.77 ± 295.07 aA | 985.69 ± 291.78 aA | 927.82 ± 246.25 aA | |||
| B (r) | 1.06 × 102 (0.74) | −2.75 (−0.02) | 8.79 (0.07) | 4.01 × 10 (0.23) | 2.58 × 10 (0.18) | 5.30 × 10 (0.52) | |||
| Chewiness (g/mm) | 0.335 | 0.032 * | 0.511 | ||||||
| Day 1 | 771.38 ± 225.73 aB | 1115.04 ± 462.08 aA | 979.45 ± 295.81 aA | 848.50 ± 376.76 aA | 888.76 ± 440.32 aA | 707.67 ± 172.35 aA | |||
| Day 4 | 961.34 ± 240.34 aB | 1068.11 ± 331.35 aA | 894.82 ± 394.96 aA | 1100.87 ± 481.03 aA | 1035.35 ± 260.33 aA | 879.46 ± 250.71 aA | |||
| Day 7 | 1349.62 ± 260.64 aA | 1079.91 ± 368.58 aA | 1020.26 ± 208.57 aA | 1157.65 ± 250.71 aA | 1021.52 ± 290.82 aA | 968.77 ± 225.40 aA | |||
| B (r) | 9.74 × 10 (0.72) | −5.85 (−0.04) | 6.80 (0.05) | 4.65 × 10 (0.29) | 2.01 × 10 (0.16) | 4.35 × 10 (0.46) | |||
| Adhesiveness (g/s) | 0.414 | 0.030 * | 0.464 | ||||||
| Day 1 | −157.19 ± 97.85 aB | −96.58 ± 61.15 aA | −97.71 ± 42.21 aA | −95.02 ± 35.44 aA | −115.21 ± 39.79 aA | −94.51 ± 50.14 aA | |||
| Day 4 | −102.09 ± 55.87 aAB | −120.43 ± 31.14 aA | −97.85 ± 54.41 aA | −113.91 ± 59.25 aA | −94.73 ± 35.06 aA | −67.58 ± 36.29 aA | |||
| Day 7 | −56.10 ± 32.71 aA | −96.23 ± 45.51 aA | −55.61 ± 25.17 aA | −114.02 ± 86.60 aA | −63.71 ± 40.07 aA | −65.88 ± 54.00 aA | |||
| B (r) | 1.68 (0.56) | 0.06 (0.00) | 7.02 (0.39) | −2.93 (−0.11) | 8.72 (0.51) | 4.77 (0.26) | |||
| Hardness (g) | 0.735 | 0.006 * | 0.168 | ||||||
| Day 1 | 2941.80 ± 812.39 aB | 3888.33 ± 1221.31 aA | 3617.83 ± 796.22 aA | 2990.00 ± 831.56 aA | 3208.00 ± 690.23 aA | 3048.60 ± 482.02 aA | |||
| Day 4 | 3380.17 ± 515.34 aB | 3386.00 ± 859.30 aA | 3084.83 ± 1116.81 aA | 3487.83 ± 1039.67 aA | 3543.83 ± 601.23 aA | 3207.83 ± 723.00 aA | |||
| Day 7 | 4880.83 ± 1015.24 aA | 3613.00 ± 877.50 aA | 3632.00 ± 842.76 aA | 3956.00 ± 584.87 aA | 3660.83 ± 774.69 aA | 3633.40 ± 653.56 aA | |||
| B (r) | 3.29 × 102 (0.71) | −4.59 × 10 (0.12) | 2.36 (0.01) | 1.61 × 102 (0.44) | 7.27 × 10 (0.26) | 9.75 × 10 (0.37) | |||
| Colour (Flesh) | |||||||||
| L * | 0.240 | 0.000 * | 0.000 * | ||||||
| Day 1 | 51.97 ± 2.11 aB | 52.64 ± 2.46 aB | 52.48 ± 2.44 aB | 53.00 ± 1.08 aAB | 52.11 ± 2.22 aA | 52.72 ± 2.64 aB | |||
| Day 4 | 52.98 ± 1.31 cB | 55.13 ± 1.54 bcAB | 57.72 ± 3.47 abA | 55.99 ± 1.81 abcA | 53.56 ± 1.60 cA | 59.16 ± 3.14 aA | |||
| Day 7 | 58.17 ± 5.94 aA | 57.53 ± 4.92 aA | 54.60 ± 6.46 aAB | 52.23 ± 5.07 aB | 53.22 ± 2.66 aA | 52.03 ± 6.08 aB | |||
| B (r) | 1.03 (0.58) | 0.81 (0.54) | 0.35 (0.18) | −0.13 (−0.09) | 0.19 (0.21) | −0.12 (−0.05) | |||
| a * | 0.017 * | 0.000 * | 0.022 * | ||||||
| Day 1 | 25.20 ± 3.20 aA | 24.16 ± 1.63 aA | 26.13 ± 2.25 aA | 27.45 ± 0.90 aA | 26.76 ± 1.79 aA | 25.91 ± 2.56 aA | |||
| Day 4 | 24.21 ± 1.02 aA | 24.62 ± 1.36 aA | 24.07 ± 0.84 aA | 23.42 ± 1.98 aB | 23.64 ± 1.08 aB | 24.05 ± 1.06 aA | |||
| Day 7 | 23.11 ± 1.92 abA | 22.18 ± 4.08 bA | 24.29 ± 2.41 abA | 25.46 ± 1.70 abC | 23.83 ± 1.39 abB | 25.57 ± 2.09 aA | |||
| B (r) | −0.35 (−0.38) | −0.33 (−0.30) | −0.31 (−0.36) | −0.33 (−0.13) | −0.49 (−0.60) | −0.06 (−0.06) | |||
| b * | 0.001 * | 0.000 * | 0.002 * | ||||||
| Day 1 | 26.95 ± 2.82 abA | 25.64 ± 1.52 bA | 27.53 ± 1.74 abA | 29.00 ± 1.16 aA | 28.67 ± 2.76 aA | 27.47 ± 2.41 abA | |||
| Day 4 | 24.35 ± 1.00 aB | 25.10 ± 0.87 aA | 24.93 ± 1.00 aB | 24.54 ± 1.30 aB | 24.28 ± 0.69 aB | 24.23 ± 1.00 aB | |||
| Day 7 | 24.06 ± 1.28 cB | 24.66 ± 2.50 bcA | 26.98 ± 3.08 abcAB | 27.41 ± 2.72 abAB | 25.73 ± 1.56 abcB | 28.26 ± 2.44 aA | |||
| B (r) | −0.48 (−0.54) | −0.16 (−0.23) | −0.09 (−0.10) | −0.27 (−0.25) | −0.49 (−0.47) | 0.13 (0.12) | |||
| C * | 0.002 * | 0.000 * | 0.005 * | ||||||
| Day 1 | 36.90 ± 4.20 abA | 35.23 ± 2.15 bA | 37.97 ± 2.71 abA | 39.94 ± 1.34 aA | 39.23 ± 3.19 abA | 37.76 ± 3.45 abA | |||
| Day 4 | 34.34 ± 1.38 aAB | 35.27 ± 1.46 aA | 34.66 ± 0.98 aB | 33.93 ± 2.18 aB | 33.89 ± 1.02 aB | 34.15 ± 1.23 aB | |||
| Day 7 | 33.37 ± 2.22 bB | 33.22 ± 4.36 bA | 36.33 ± 3.64 abAB | 37.42 ± 3.12 abA | 35.07 ± 2.03 abB | 38.12 ± 3.13 aA | |||
| B (r) | −0.59 (−0.47) | −0.34 (−0.28) | −0.27 (−0.23) | −0.42 (−0.31) | −0.69 (−0.54) | 0.06 (0.04) | |||
| Colour (Skin) | |||||||||
| L * | 0.002 * | 0.000 * | 0.000 * | ||||||
| Day 1 | 54.87 ± 3.88 aB | 57.56 ± 3.28 aA | 54.53 ± 2.27 aA | 56.80 ± 2.03 aA | 49.51 ± 4.41 bB | 54.36 ± 3.11 aA | |||
| Day 4 | 58.68 ± 1.10 aA | 51.88 ± 5.41 cAB | 54.86 ± 0.84 abcA | 55.48 ± 5.33 abcA | 57.50 ± 0.80 abA | 52.99 ± 3.55 bcA | |||
| Day 7 | 52.88 ± 2.27 aB | 50.54 ± 6.55 abB | 45.55 ± 3.92 bB | 45.32 ± 7.10 bB | 46.01 ± 5.00 abB | 50.29 ± 4.04 abA | |||
| B (r) | −0.33 (−0.23) | −1.17 (−0.49) | −1.50 (−0.73) | −1.91 (−0.66) | −0.58 (−0.24) | −0.68 (−0.42) | |||
| a * | 0.006 * | 0.000 * | 0.000 * | ||||||
| Day 1 | 1.27 ± 0.29 bB | 1.06 ± 0.34 bB | 1.20 ± 0.27 bB | 1.10 ± 0.16 bB | 2.81 ± 1.65 aA | 1.23 ± 0.41 bA | |||
| Day 4 | 1.54 ± 0.44 aAB | 1.85 ± 0.46 aA | 1.70 ± 0.51 aB | 1.69 ± 0.33 aB | 1.59 ± 0.27 aA | 1.88 ± 0.54 aA | |||
| Day 7 | 1.95 ± 0.57 abA | 1.47 ± 0.37 bA | 2.91 ± 0.91 abA | 3.14 ± 1.17 aA | 2.39 ± 0.83 abA | 2.20 ± 1.88 abA | |||
| B (r) | 0.11 (0.55) | 0.08 (0.42) | 0.29 (0.75) | 0.34 (0.77) | −0.07 (−0.15) | 0.16 (0.34) | |||
| b * | 0.090 | 0.000 * | 0.000 * | ||||||
| Day 1 | 2.62 ± 0.38 bcB | 2.46 ± 0.87 bcB | 1.58 ± 0.48 cC | 2.67 ± 0.54 bcB | 4.99 ± 2.13 aA | 3.17 ± 0.41 bA | |||
| Day 4 | 3.43 ± 0.75 aA | 3.34 ± 0.84 aAB | 3.42 ± 0.89 aB | 3.12 ± 1.12 aB | 3.07 ± 0.73 aB | 4.06 ± 1.08 aA | |||
| Day 7 | 3.78 ± 0.46 aA | 3.68 ± 0.89 aA | 5.05 ± 1.03 aA | 5.38 ± 1.34 aA | 3.89 ± 1.25 aAB | 4.11 ± 3.25 aA | |||
| B (r) | 0.19 (0.66) | 0.20 (0.57) | 0.58 (0.87) | 0.45 (0.71) | −0.18 (−0.28) | 0.16 (0.20) | |||
| C * | 0.045 * | 0.000 * | 0.000 * | ||||||
| Day 1 | 2.93 ± 0.30 bB | 2.69 ± 0.92 bB | 2.02 ± 0.40 bC | 2.89 ± 0.53 bB | 5.74 ± 2.66 aA | 3.41 ± 0.49 bA | |||
| Day 4 | 3.76 ± 0.85 aA | 3.83 ± 0.92 aA | 3.83 ± 0.99 aB | 3.60 ± 0.98 aB | 3.46 ± 0.75 aB | 4.48 ± 1.19 aA | |||
| Day 7 | 4.27 ± 0.62 aA | 4.02 ± 0.45 aA | 5.84 ± 1.33 aA | 6.32 ± 1.36 aA | 4.57 ± 1.48 aAB | 4.69 ± 3.71 aA | |||
| B (r) | 0.19 (0.66) | 0.20 (0.57) | 0.58 (0.87) | 0.45 (0.71) | −0.18 (−0.28) | 0.16 (0.20) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, W.H.; Chaklader, M.R.; Howieson, J. Efficacy Evaluation of Chlorine Dioxide and Hypochlorous Acid as Sanitisers on Quality and Shelf Life of Atlantic Salmon (Salmo salar) Fillets. Foods 2024, 13, 3156. https://doi.org/10.3390/foods13193156
Chung WH, Chaklader MR, Howieson J. Efficacy Evaluation of Chlorine Dioxide and Hypochlorous Acid as Sanitisers on Quality and Shelf Life of Atlantic Salmon (Salmo salar) Fillets. Foods. 2024; 13(19):3156. https://doi.org/10.3390/foods13193156
Chicago/Turabian StyleChung, Wing H., Md Reaz Chaklader, and Janet Howieson. 2024. "Efficacy Evaluation of Chlorine Dioxide and Hypochlorous Acid as Sanitisers on Quality and Shelf Life of Atlantic Salmon (Salmo salar) Fillets" Foods 13, no. 19: 3156. https://doi.org/10.3390/foods13193156
APA StyleChung, W. H., Chaklader, M. R., & Howieson, J. (2024). Efficacy Evaluation of Chlorine Dioxide and Hypochlorous Acid as Sanitisers on Quality and Shelf Life of Atlantic Salmon (Salmo salar) Fillets. Foods, 13(19), 3156. https://doi.org/10.3390/foods13193156







