Research on Engineering the Saccharomyces uvarum for Constructing a High Efficiency to Degrade Malic Acid and Low Yield of Diacetyl Biosynthesis Pathway
Abstract
1. Introduction
2. Material and Methods
2.1. Strains and Media
2.2. Construction of Plasmids and Mutant Strains
2.3. Fermentation Conditions
2.4. Chemical Analysis
2.5. Statistical Analysis
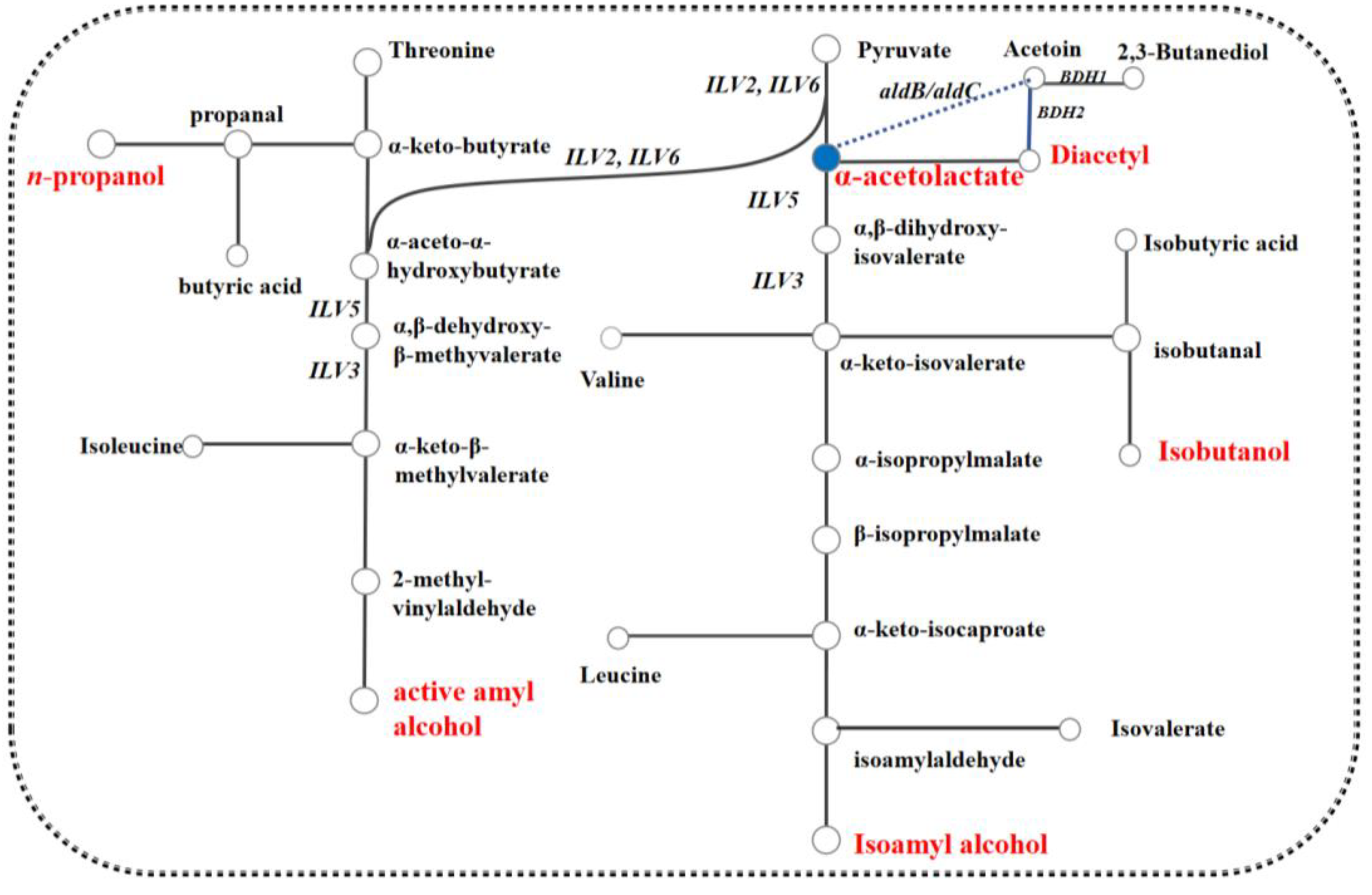
3. Results and Discussion
3.1. The Effect of MLF on Diacetyl Production during Mutant S. uvarum with Low Yield of Diacetyl Fermentation
3.2. The Effect on Diacetyl of Genes Related L-Malic Acid Matebolism Heterologous Expression in S. uvarum
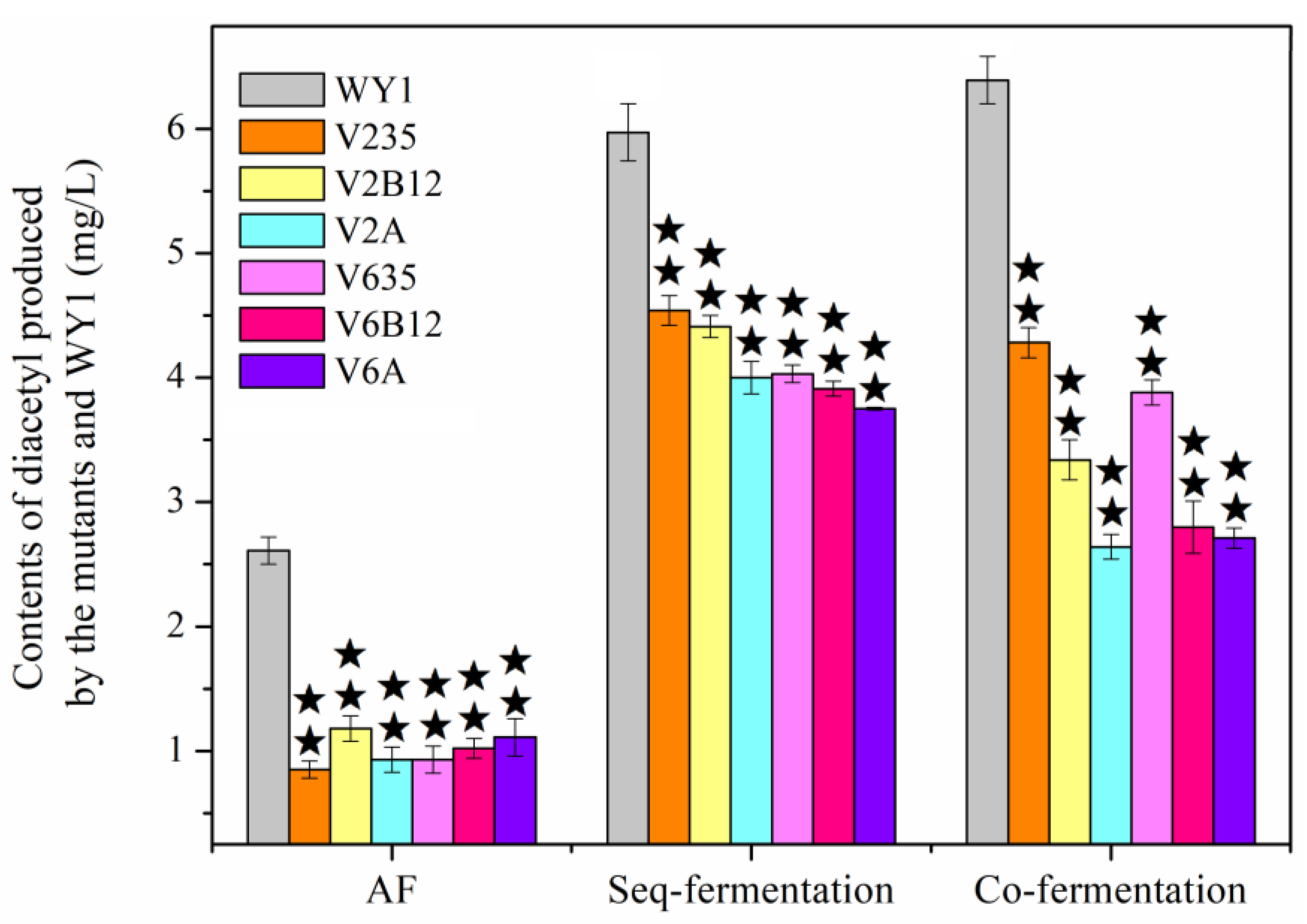
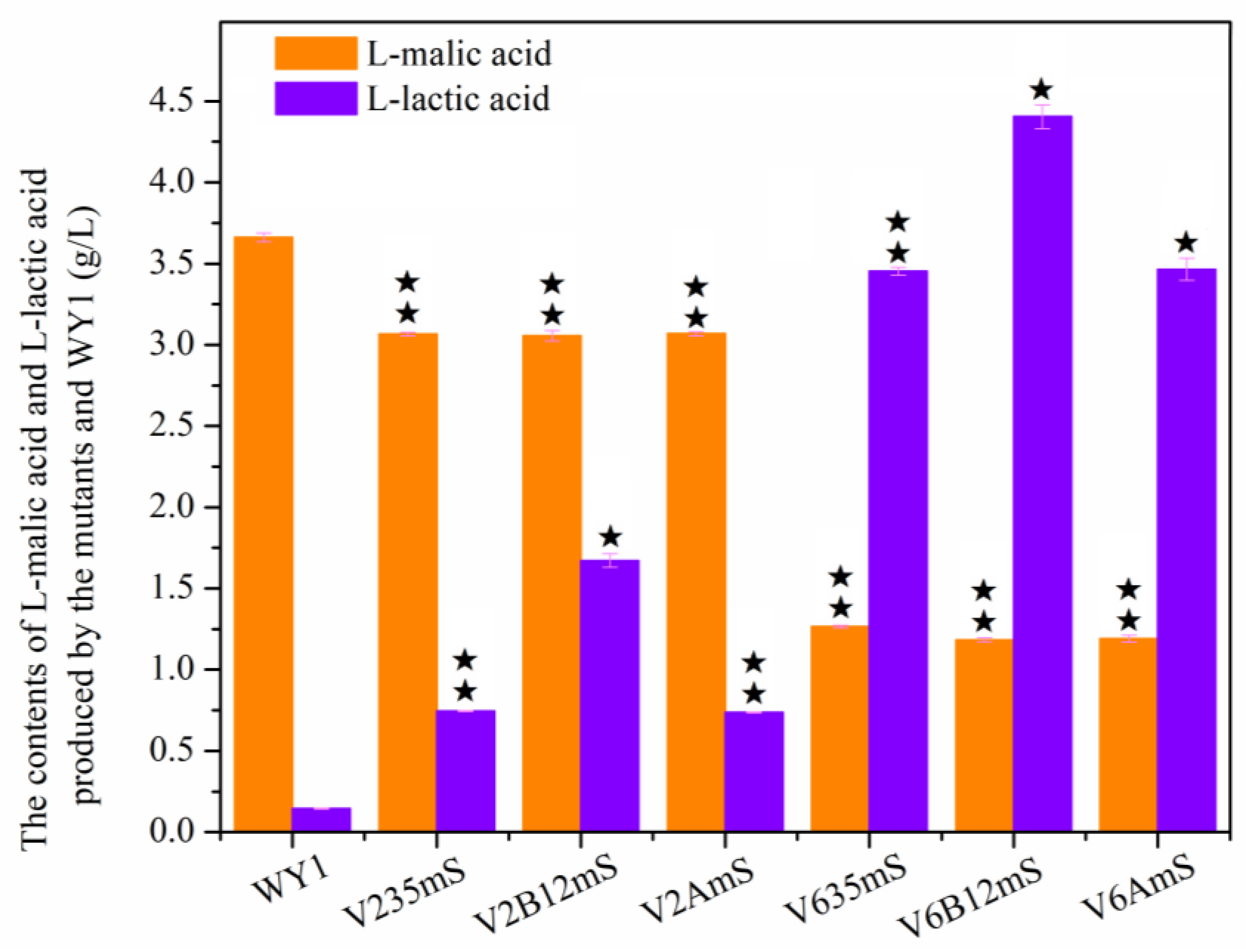
3.3. Effects of Association Mutation of Genes in S. uvarum on Diacetyl and L-Malic Acid Yields Production
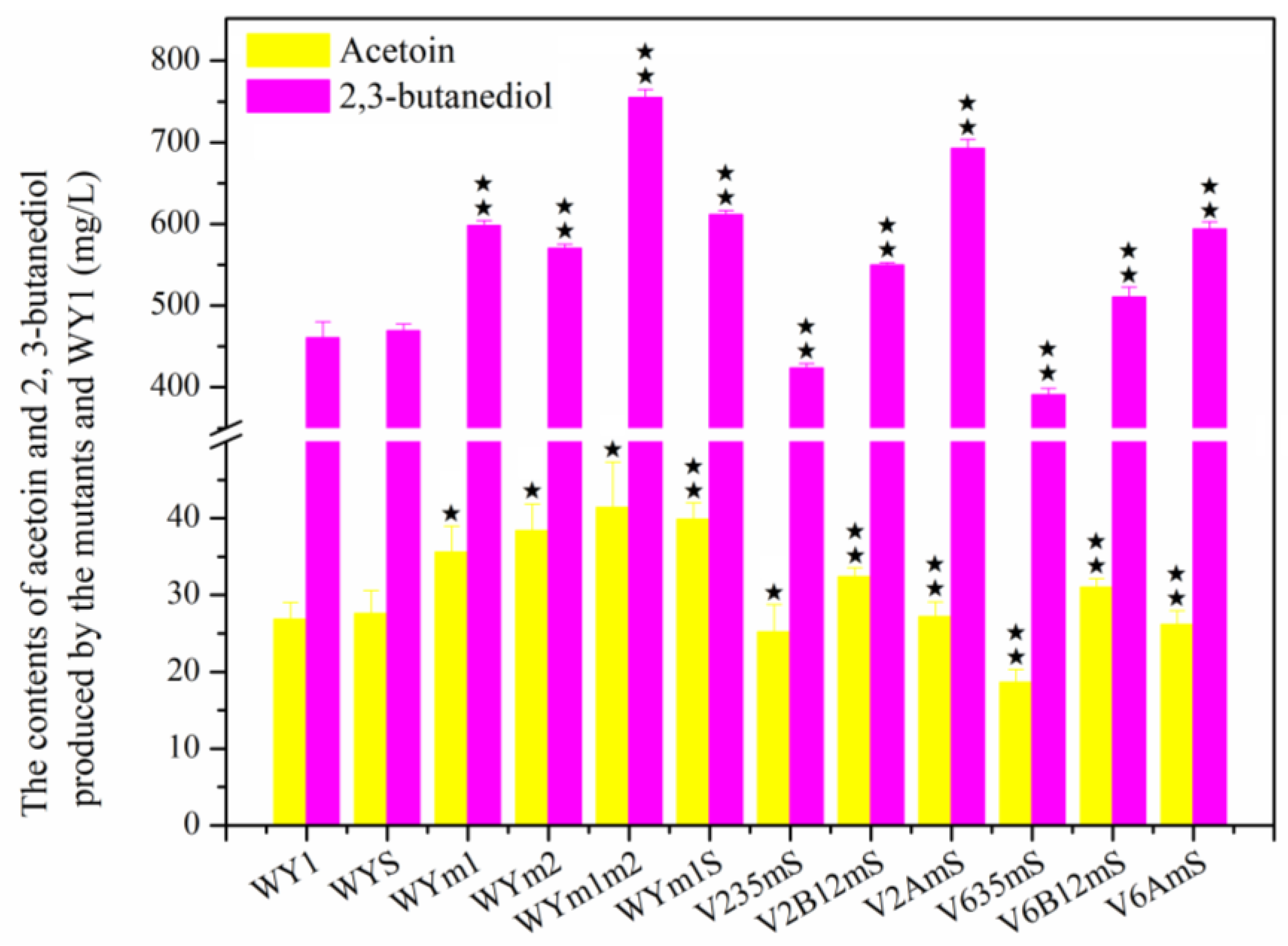
3.4. Effects of Association Mutation of Genes in S. uvarum on Higher Alcohols and Esters Production
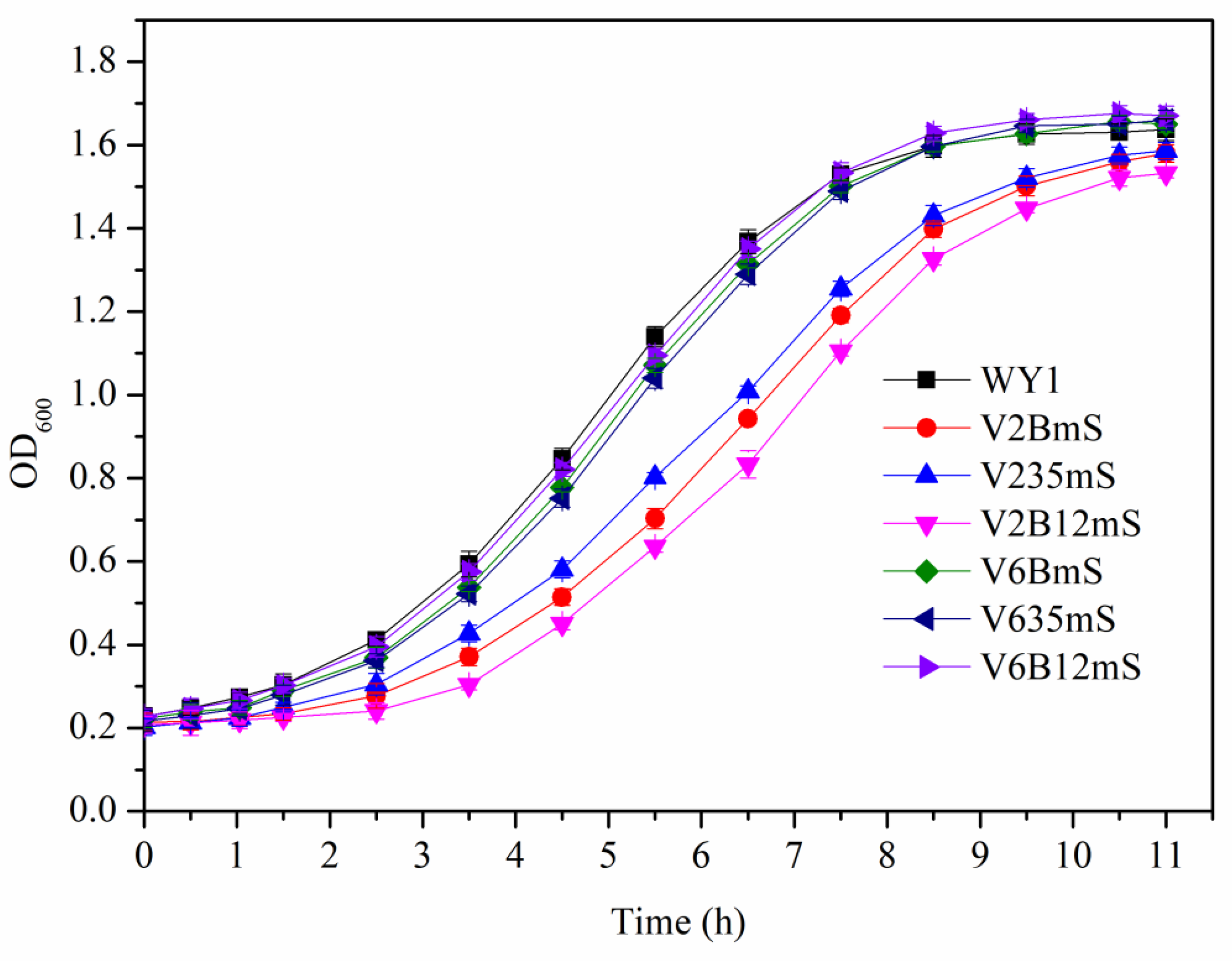
3.5. Effects of Association Mutation of Genes in S. uvarum on Growth and Fermentation Performance
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakker, J.; Clarke, R.J. Wine Flavour Chemistry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q.; et al. Aroma characteristics of volatile compounds brought by variations in microbes in winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef] [PubMed]
- Pelonniermagimel, E.; Cameleyre, M.; Riquier, L.; Barbe, J.C. Sensory specificities involving acetaldehyde and diacetyl in wines produced without added sulfur dioxide. J. Agric. Food Chem. 2023, 71, 9062–9069. [Google Scholar] [CrossRef] [PubMed]
- Jeroen, H. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 165–178. [Google Scholar] [CrossRef]
- Yao, Q.; Heui-Dong, P. Overexpressed acetohydroxyacid reductoisomerase (ILV5) gene in saccharomyces cerevisiae reduces diacetyl contents in korean campbell early and muscat bailey a grape wines. Han’Guk Ungyong Saengmyong Hwahakhoe Chi = J. Korean Soc. Appl. Biol. Chem. 2012, 55, 799–801. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic acid bacteria in wine: Technological advances and evaluation of their functional role. Front. Microbiol. 2021, 11, 612118. [Google Scholar] [CrossRef]
- Krogerus, K.; Gibson, B.R. Influence of valine and other amino acids on total diacetyl and 2,3-pentanedione levels during fermentation of brewer’s wort. Appl. Microbiol. Biotechnol. 2013, 97, 6919–6930. [Google Scholar] [CrossRef]
- Bruner, J.; Marcus, A.; Fox, G. Changes in diacetyl and amino acid concentration during the fermentation of dry-hopped beer: A look at twelve Saccharomyces species and strains. J. Am. Soc. Brew. Chem. 2022, 81, 242–254. [Google Scholar] [CrossRef]
- Liboà, A.; Genzardi, D.; Núñez-Carmona, E.; Carabetta, S.; Sanzo, R.D.; Russo, M.; Sberveglieri, V. Different diacetyl perception detected through mox sensors in real-time analysis of beer samples. Chemosensors 2023, 11, 147. [Google Scholar] [CrossRef]
- Shi, T.; Li, P.; Chen, S.; Chen, Y.; Guo, X.; Xiao, D. Reduced production of diacetyl by overexpressing BDH2 gene and ILV5 gene in yeast of the lager brewers with one ILV2 allelic gene deleted. J. Ind. Microbiol. Biotechnol. 2017, 44, 397–405. [Google Scholar] [CrossRef]
- Shi, T.; Guo, X.; Li, P.; Zhou, Z.; Xiao, D. Diacetyl content reduction in industrial brewer’s yeast through ILV2 disruption and BDH1 expression. Eur. Food Res. Technol. 2016, 242, 919–926. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, S.J.; Kim, J.W.; Park, Y.C.; Seo, J.H. Molecular cloning and expression of enterobacter aerogenes α-acetolactate decarboxylase in pyruvate decarboxylase-deficient saccharomyces cerevisiae for efficient 2,3-butanediol production. Process Biochem. 2016, 51, 170–176. [Google Scholar] [CrossRef]
- Cejnar, R.; Hlozková, K.; Kotrba, P.; Dostálek, P. Surface-engineered saccharomyces cerevisiae displaying [alpha]-acetolactate decarboxylase from acetobacter aceti ssp xylinum. Biotechnol. Lett. 2016, 38, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Tronchoni, J.; Querol, A.; Belloch, C. Production of aroma compounds by cryotolerant saccharomyces species and hybrids at low and moderate fermentation temperatures. J. Appl. Microbiol. 2013, 114, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.; Gonçalves, C.; Teixeira, S.; Libkind, D.; Bontrager, M.; Masneuf-pomarède, I.; Sampaio, J.P. A gondwanan imprint on global diversity and domestication of wine and cider yeast Saccharomyces uvarum. Nat. Commun. 2014, 5, 4044. [Google Scholar] [CrossRef] [PubMed]
- Stribny, J.; Gamero, A.; Pérez-Torrado, R.; Querol, A. Saccharomyces kudriavzevii and Saccharomyces uvarum differ from Saccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. Int. J. Food Microbiol. 2015, 205, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, X.; Shi, T.; Hu, Z.; Chen, Y.; Du, L.; Xiao, D. Reducing diacetyl production of wine by overexpressing BDH1 and BDH2 in Saccharomyces uvarum. J. Ind. Microbiol. Biotechnol. 2017, 44, 1541–1550. [Google Scholar] [CrossRef]
- Li, P.; Li, T.; Zhang, C.Y.; Xiao, D.G. Effect of ILV2 deletion and ILV3 or/and ILV5 overexpression in saccharomyces uvarum on diacetyl and higher alcohols metabolism during wine fermentation. Eur. Food Res. Technol. 2020, 246, 563–572. [Google Scholar] [CrossRef]
- Li, P.; Gao, Y.; Wang, C.; Zhang, C.Y.; Guo, X.; Xiao, D. Effect of ILV6 deletion and expression of aldB from Lactobacillus plantarum in Saccharomyces uvarum on diacetyl production and wine flavor. J. Agric. Food Chem. 2018, 66, 8556–8565. [Google Scholar] [CrossRef]
- Bauer, R.; Dicks, L.M.T. Control of malolactic fermentation in wine: A review. S. Afr. Soc. Enol. Vitic. 2017, 25, 74–88. [Google Scholar] [CrossRef]
- Costello, P.J.; Kolouchova, R.; Mccarthy, J.M.; Nandorfy, D.E.; Likos, D.; Schmidt, S.A. Transient acetaldehyde production by SO2-producing Saccharomyces cerevisiae promotes the survival of Oenococcus oeni during co-fermentation. OENO One 2023, 57, 399–415. [Google Scholar] [CrossRef]
- Prusova, B.; Licek, J.; Kumsta, M.; Baron, M.; Sochor, J. Effect of new methods for inhibiting malolactic fermentation on the analytical and sensory parameters of wines. Fermentation 2024, 10, 122. [Google Scholar] [CrossRef]
- Balmaseda, A.; Lorentzen, M.; Dutilh, L.; Bauduin, R.; Guichard, H.; Ollivier, S.; Miot-Sertier, C.; Lucas, P.M. Alcoholic fermentation drives the selection of Oenococcus oeni strains in wine but not in cider. Int. J. Food Microbiol. 2023, 400, 110276. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.G.; Reynolds, A.G.; Rodriguez, A.V.; Semon, M.J.; Mills, J.M. Implication of acetic acid in the induction of slow/stuck grape juice fermentations and inhibition of yeast by Lactobacillus sp. Am. J. Enol. Vitic. 1999, 50, 204–210. [Google Scholar] [CrossRef]
- Miotto, S.P.S.; Fensterseifer, L.C.; de Souza Hassemer, G.; Martins, G.; Ficagna, E.; Steffens, J.; Cansian, R.L. Malolactic fermentation of lactic acid bacteria isolated from southern brazilian red wine. World J. Microbiol. Biotechnol. 2023, 39, 201. [Google Scholar] [CrossRef]
- Zelle, R.M.; Harrison, J.C.; Pronk, J.T.; Maris, A.J.A.V. Anaplerotic role for cytosolic malic enzyme in engineered Saccharomyces cerevisiae strains. Appl. Environ. Microbiol. 2011, 77, 732–738. [Google Scholar] [CrossRef]
- Schümann, C.; Michlmayr, H.; Eder, R.; Del Hierro, A.M.; Kulbe, K.D.; Mathiesen, G.; Nguyen, T. Heterologous expression of Oenococcus oeni malolactic enzyme in Lactobacillus plantarum for improved malolactic fermentation. AMB Express 2012, 2, 19. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Monedero, V.; Zuniga, M. Malic enzyme and malolactic enzyme pathways are functionally linked but independently regulated in Lactobacillus casei BL23. Appl. Environ. Microbiol. 2013, 79, 5509–5518. [Google Scholar] [CrossRef]
- Vilela, A. Biological demalication and deacetification of musts and wines: Can wine yeasts make the wine taste better? Fermentation 2017, 3, 51. [Google Scholar] [CrossRef]
- Husnik, J.I.; Volschenk, H.; Bauer, J.; Colavizza, D.; Luo, Z.; van Vuuren, H.J. Metabolic engineering of malolactic wine yeast. Metab. Eng. 2006, 8, 315–323. [Google Scholar] [CrossRef]
- Annica, S.; Marinda, V.B.; Heber, V.Z.W. Constructing recombinant saccharomyces cerevisiae strains for malic-to-fumaric acid conversion. FEMS Microbiol. Lett. 2023, 370, fnad003. [Google Scholar] [CrossRef]
- Li, P.; Song, W.; Wang, Y.; Li, X.; Wu, S.; Li, B.; Zhang, C. Effects of heterologous expression of genes related L–malic acid metabolism in Saccharomyces uvarum on flavor substances production in Wine. Foods 2024, 13, 2038. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.C.; Liu, Z.; Lagarde, M.M.M.; Zuo, J. Conditional gene expression in the mouse inner ear using cre-loxP. J. Assoc. Res. Otolaryngol. 2012, 13, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, M.; Volschenk, H.; Young, R.A.; Van Vuuren, H.J.J. Transcriptional regulation of the schizosaccharomyces pombe malic enzyme gene, mae2. J. Biol. Chem. 1999, 274, 9969–9975. [Google Scholar] [CrossRef] [PubMed]
- Goupil-Feuillerat, N.; Corthier, G.; Godon, J.J.; Ehrlich, S.D.; Renault, P. Transcriptional and translational regulation of alpha-acetolactate decarboxylase of lactococcus lactis subsp. lactis. J. Bacteriol. 2000, 182, 5399–5408. [Google Scholar] [CrossRef]
- Bony, M.; Bidart, F.; Camarasa, C.; Ansanay, V.; Dulau, L.; Barre, P.; Dequin, S. Metabolic analysis of S. cerevisiae strains engineered for malolactic fermentation. FEBS Lett. 1997, 410, 452–456. [Google Scholar] [CrossRef]




| Strains or Plasmids | Relevant Characteristic | Reference or Source |
|---|---|---|
| Strain | ||
| E. coli DH5α | supE44ΔlacU169 (ϕ80lacZΔM15) hsdR17 recAl endAl gyrA96 thi-1 relA | This lab |
| WY1 | Wild-type industrial Saccharomyces uvarum | This lab (the parenal strain) |
| Lactococcus lactis | Wild-type industrial Lactococcus lactis | This lab |
| Lactobacillus plantarum | Wild-type industrial Lactobacillus plantarum | This lab |
| Schizosaccharomyces pombe | Wild-type industrial Schizosaccharomyces pombe | This lab |
| WYS | PGK1p-mleS (Lactococcus lactis)-PGK1t-loxP-KanMX-loxP | Previous study [32] |
| WYm1 | PGK1p-mae1 (Schizosaccharomyces pombe)-PGK1t-loxP-KanMX-loxP | Previous study [32] |
| WYm1S | PGK1p-mae1 (Schizosaccharomyces pombe)-PGK1t-PGK1p-mleSN (Lactococcus lactis)-PGK1t-loxP- KanMX-loxP | Previous study [32] |
| V22 | ILV2 (n-2)/Δilv2 | Previous study [18] |
| V61 | ILV6 (n-1)/Δilv6 | Previous study [19] |
| V6A | ILV6 (n-2)/Δilv6, PGK1p-aldB-PGK1-loxP-KanMX-loxP | Previous study [19] |
| V235 | ILV2 (n-3)/Δilv2, PGK1p-ILV3-PGK1t-PGK1p-ILV5-PGK1t-loxP- KanMX-loxP | Previous study [18] |
| V2B12 | ILV2 (n-3)/Δilv2, PGK1p-BDH1-PGK1t-PGK1p-BDH2-PGK1t-loxP- KanMX-loxP | This study |
| V2A | ILV2 (n-3)/Δilv2, PGK1p-aldB-PGK1-loxP-KanMX-loxP | This study |
| V635 | ILV6 (n-2)/Δilv6, PGK1p-ILV3-PGK1t-PGK1p-ILV5-PGK1t-loxP- KanMX-loxP | This study |
| V6B12 | ILV6 (n-2)/Δilv6, PGK1p-BDH1-PGK1t-PGK1p-BDH2-PGK1t-loxP- KanMX-loxP | This study |
| V2mSK | ILV2 (n-3)/Δilv2, PGK1p-mae1-PGK1t-PGK1p-mleSNZ-PGK1t-loxP-loxP- KanMX-loxP | This study |
| V2mS | ILV2 (n-3)/Δilv2, PGK1p-mae1-PGK1t-PGK1p-mleSNZ-PGK1t | This study |
| V235mS | ILV2 (n-3)/Δilv2, PGK1p-mae1-PGK1t-PGK1p-mleSNZ-PGK1t-loxP, PGK1p-ILV3-PGK1t-PGK1p-ILV5-PGK1t-loxP-KanMX-loxP | This study |
| V2AmS | ILV2 (n-3)/Δilv2, PGK1p-mae1-PGK1t-PGK1p-mleSNZ-PGK1t-loxP, PGK1p-ALDB-PGK1-loxP-KanMX-loxP | This study |
| V2B12mS | ILV2 (n-3)/Δilv2, PGK1p-mae1-PGK1t-PGK1p-mleSNZ-PGK1t-loxP, PGK1p-BDH1-PGK1t-PGK1p-BDH2-PGK1t-loxP-KanMX-loxP | This study |
| V6mSK | ILV6 (n-2)/Δilv6, PGK1p-mae1-PGK1t-PGK1p-mleSNZ-PGK1t-loxP-KanMX-loxP | This study |
| V6mS | ILV6 (n-2)/Δilv6, PGK1p-mae1-PGK1t-PGK1p-2mleSNZ-PGK1t | This study |
| V635mS | ILV6 (n-2)/Δilv6, PGK1p-mae1-PGK1t-PGK1p-mleSNZ-PGK1t-loxP, PGK1p-ILV3-PGK1t-PGK1p-ILV5-PGK1t-loxP-KanMX-loxP | This study |
| V6AmS | ILV6 (n-2)/Δilv6, PGK1p-mae1-PGK1t-PGK1p-mleSNZ-PGK1t-loxP, PGK1p-ALDB-PGK1-loxP-KanMX-loxP | This study |
| Plasmids | ||
| Yep352 pUG6 | Apr, cloning vector E. coli/S. cerevisiae shuttle vector, containing Amp+ and loxP-KanMX-loxP cassette | This lab This lab |
| pPGK1 | E. coli/S. cerevisiae shuttle vector, containing Amp+ and PGK1p and PGK1t | This lab |
| pSH-Zeocin | Zeor, Cre expression vector | This lab |
| Yep-Pm1SK | Ampr, Kanr, PGK1p-mae1-PGK1t-PGK1p-mleS-PGK1t-loxP-KanMX-loxP | Previous study [32] |
| Yep-PAK | Ampr, Kanr, PGK1p-aldB-PGK1t-loxP-KanMX-loxP | Previous study [19] |
| Yep-PB1B2K | Ampr, Kanr, PGK1p-BDH1-PGK1t-PGK1p-BDH2-PGK1t-loxP-KanMX-loxP | Previous study [17] |
| Yep-PV35K | Ampr, Kanr, PGK1p-ILV3-PGK1t-PGK1p-ILV5-PGK1t-loxP-KanMX-loxP | Previous study [18] |
| Primers | Sequence (5′→3′) |
|---|---|
| PGK-U | CGCGGATCCTCTAACTGATCTATCCAAAACTG |
| PGK-D | ACGCGTCGACTAACGAACGCAGAATTTTCGAG |
| MAE-U | GAATTCCAGATCTCCTCGAGATGCTTAGAACCAGACTATCCG |
| MAE-D | TCTATCGCAGATCCCTCGAGCTACAATTGGTTGGTGTGCAC |
| mae2-U | GAATTCCAGATCTCCTCGAGGCACGTGGACCGTCTTACC |
| mae2-D | TCTATCGCAGATCCCTCGAGAGTTGATGAATAACAATAGGAGAAA |
| mleSNZ-U | GAATTCCAGATCTCCTCGAGATGCGTGCACATGAAATTT |
| mleSNZ-D | TCTATCGCAGATCCCTCGAGTTAGTACTCTGGATACCATTTAAGA |
| K(ApaI)-U | CCGCTAACAATACCTGGGCCCCAGCTGAAGCTTCGTACGC |
| K(ApaI)-D | GCACACGGTGTGGTGGGCCCGCATAGGCCACTAGTGGATCTG |
| mae1-U | GAATTCCAGATCTCCTCGAGTTCATTTTCTCTCTTGGCCAC |
| mae1-D | TCTATCGCAGATCCCTCGAGCTTTTGTCATGAAATCCCTCTTA |
| mlePNZ-U | GAATTCCAGATCTCCTCGAGATGAAAAAACTTAAAGAAACGA |
| mlePNZ-D | TCTATCGCAGATCCCTCGAGTTAATAAAAGAATCGTATAAGAATT |
| PGK(SmaI)-U | GTACCCGGGTCTAACTGATCTATCCAAAACTGA |
| PGK(SmaI)-D | GATCCCCGGGTAACGAACGCAGAATTTTC |
| GAL80A-U | GTGCCTCTATGATGGGTATG |
| GAL80A-D | TACCGAGCTCGAATTCGTAATAAGAACGGGAAACCAACTATC |
| GAL80B-U | TCCACTAGTGGCCTATGCACCTTGATGGATGCTCTGATA |
| GAL80B-D | ATTCCTGGAGAACCACCTAA |
| ReGAL80-U | GATAGTTGGTTTCCCGTTCTTATTACGAATTCGAGCTCGGTA |
| ReGAL80-D | TATCAGAGCATCCATCAAGGTGCATAGGCCACTAGTGGAT |
| V2m1SA-U | CGTTATTACAGTGCGTCTC |
| V2m1SA-D | TACCGAGCTCGAATTCGTAATCCCAGAAGTAACCAAGACAA |
| V2m1SB-U | ATCCACTAGTGGCCTATGCGCCATCGGTGCTCAAGTT |
| V2m1SB-D | GCTACCACCTGCCACCA |
| V2m1S-U | TTGTCTTGGTTACTTCTGGGATTACGAATTCGAGCTCGGTA |
| V2m1S-D | AACTTGAGCACCGATGGCGCATAGGCCACTAGTGGAT |
| K-U | CAGCTGAAGCTTCGTACGC |
| K-D | GCATAGGCCACTAGTGGATCTG |
| ApaI-U | CCTGCTTCAAACCGCTAACAATA |
| ApaI-D | CGAATGCACACGGTGTGGT |
| SmaI-U | TTCGAGCTCGGTACCCG |
| SmaI-D | AGTTAGAGGATCCCCGGG |
| 1YGAL80-U | GATCATCGTAGTGCCCAATT |
| 1YGAL80-D | GTACCGAGCTCGAATTCGT |
| 2YGAL80-U | GGTTTGGTTGATGCGAGTG |
| 2YGAL80-D | CCATTCATCGTGTTGTTTTGG |
| 1YV2m1S-U | TGTAAACAGAACTTTGCCACTA |
| 1YV2m1S-D | GTACCGAGCTCGAATTCGT |
| 2YV2m1S-U | GGTTTGGTTGATGCGAGTG |
| 2YV2m1S-D | TTCATTTATGGCTTCGTCGGA |
| Strain | WY1 | V235mS | V2B12mS | V2AmS | V635mS | V6B12mS | V6AmS |
|---|---|---|---|---|---|---|---|
| Residual sugar (g/L) | 2.57 ± 0.10 | 2.96 ± 0.14 a | 2.96 ± 0.08 a | 3.11 ± 0.05 a | 2.66 ± 0.08 | 2.61 ± 0.15 | 2.57 ± 0.25 |
| Ethanol (%, v/v) | 11.03 ± 0.02 | 10.52 ± 0.05 a | 10.45 ± 0.17 a | 10.70 ± 0.11 a | 11.02 ± 0.15 | 11.05 ± 0.10 | 11.00 ± 0.21 |
| pH | 2.88 ± 0.11 | 2.80 ± 0.10 | 2.83 ± 0.06 | 2.85 ± 0.15 | 3.20 ± 0.20 a | 3.23 ± 0.12 a | 3.25 ± 0.18 a |
| Weight losses of CO2 (g) | 14.97 ± 0.10 | 14.65 ± 0.14a | 14.52 ± 0.20a | 14.44 ± 0.20 a | 14.92 ± 0.06 | 14.97 ± 0.10 | 14.95 ± 0.18 |
| L-malic acid (g/L) | 3.661 ± 0.026 | 3.067 ± 0.010 | 3.056 ± 0.031 | 3.069 ± 0.012 | 1.266 ± 0.008 a | 1.183 ± 0.011 a | 1.190 ± 0.021 a |
| L-lactic acid (g/L) | 0.144 ± 0.003 | 0.745 ± 0.003 | 1.672 ± 0.041 | 0.737 ± 0.002 | 3.453 ± 0.024 a | 4.405 ± 0.074 a | 3.465 ± 0.068 a |
| Citric acid (g/L) | 0.771 ± 0.004 | 0.770 ± 0.001 | 0.782 ± 0.002 | 0.759 ± 0.012 | 0.750 ± 0.009 a | 0.743 ± 0.015 a | 0.752 ± 0.002 a |
| Tartaric Acid (g/L) | 2.394 ± 0.055 | 2.486 ± 0.094 | 2.414 ± 0.094 | 2.384 ± 0.087 | 2.620 ± 0.213 | 2.376 ± 0.040 | 2.488 ± 0.019 |
| Succinic acid | 1.324 ± 0.014 | 1.248 ± 0.014 | 1.233 ± 0.031 | 1.200 ± 0.018 | 1.016 ± 0.037 a | 0.996 ± 0.014 a | 0.967 ± 0.026 a |
| acetic acid (g/L) | 0.193 ± 0.006 | 0.188 ± 0.008 | 0.191 ± 0.012 | 0.176 ± 0.012 | 0.201 ± 0.006 | 0.193 ± 0.024 | 0.207 ± 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Song, W.; Wu, S.; Wang, Y.; Fan, Y.; Zhang, C. Research on Engineering the Saccharomyces uvarum for Constructing a High Efficiency to Degrade Malic Acid and Low Yield of Diacetyl Biosynthesis Pathway. Foods 2024, 13, 3161. https://doi.org/10.3390/foods13193161
Li P, Song W, Wu S, Wang Y, Fan Y, Zhang C. Research on Engineering the Saccharomyces uvarum for Constructing a High Efficiency to Degrade Malic Acid and Low Yield of Diacetyl Biosynthesis Pathway. Foods. 2024; 13(19):3161. https://doi.org/10.3390/foods13193161
Chicago/Turabian StyleLi, Ping, Wenjun Song, Shankai Wu, Yumeng Wang, Yicong Fan, and Cuiying Zhang. 2024. "Research on Engineering the Saccharomyces uvarum for Constructing a High Efficiency to Degrade Malic Acid and Low Yield of Diacetyl Biosynthesis Pathway" Foods 13, no. 19: 3161. https://doi.org/10.3390/foods13193161
APA StyleLi, P., Song, W., Wu, S., Wang, Y., Fan, Y., & Zhang, C. (2024). Research on Engineering the Saccharomyces uvarum for Constructing a High Efficiency to Degrade Malic Acid and Low Yield of Diacetyl Biosynthesis Pathway. Foods, 13(19), 3161. https://doi.org/10.3390/foods13193161





