Comprehensive Assessment of Anti-Inflammatory, Antiproliferative and Neuroprotective Properties of Cauliflower after Dehydration by Different Drying Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Drying Procedure
2.2. Determination of Amino Acid Composition
2.3. Determination of Glucosinolate Content and Hydroxycinnamic Acid and Its Derivatives
2.4. Determination of Isothiocyanate Content
2.5. Extraction Procedure for Determining Anti-Inflammatory, Antiproliferative, and Neuroprotective Properties
2.6. Determination of In Vivo Anti-Inflammatory Potential
2.7. Determination of In Vitro Antiproliferative Potential
2.8. Determination of In Vitro Neuroprotective Potential
2.9. Data Analysis
3. Results and Discussion
3.1. Amino Acids of Dried Cauliflower
3.2. Hydroxycinnamic Acid and Its Derivatives in Dried Cauliflower
3.3. Glucosinolate (GSL) Content in Dried Cauliflower
3.4. Isothiocyanate (ITC) Content in Dried Cauliflower
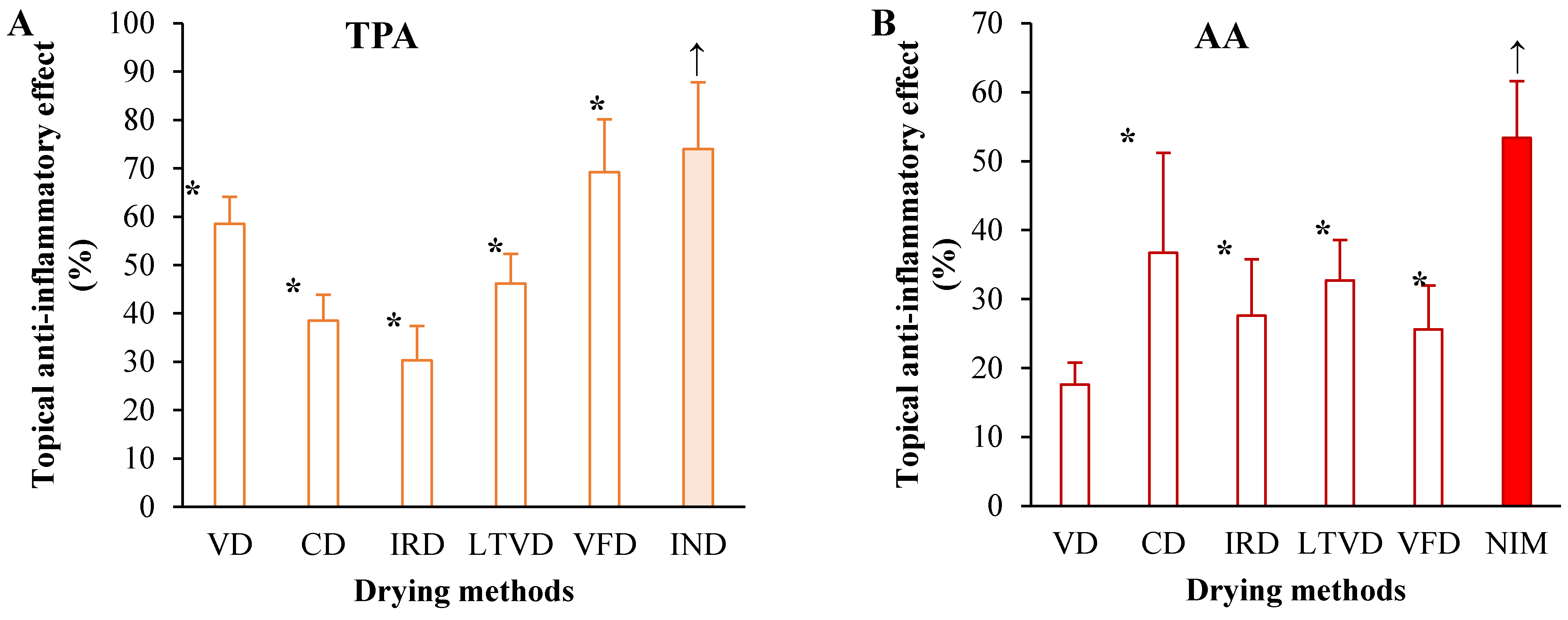
3.5. Anti-Inflammatory Potential in Dried Cauliflower
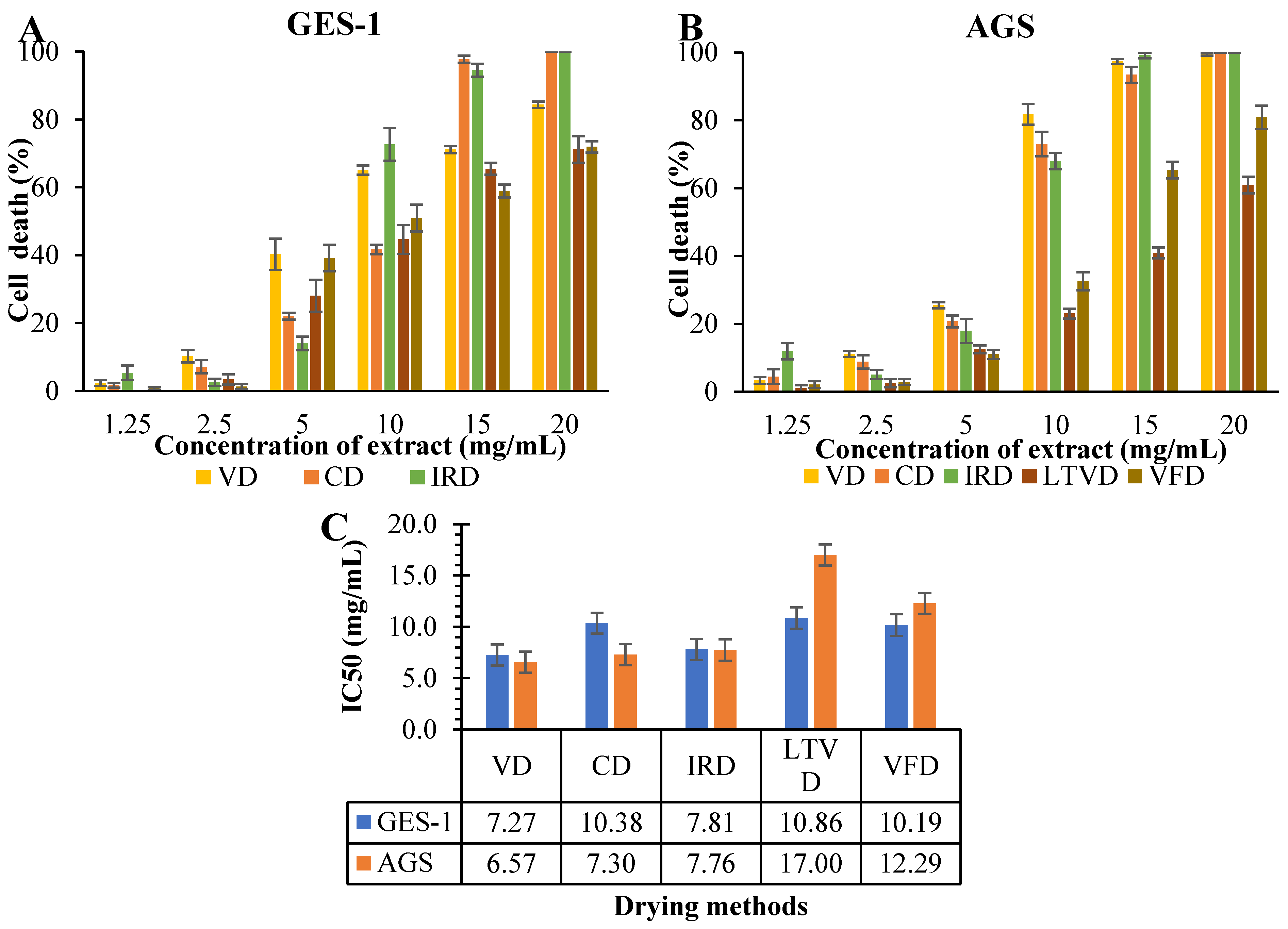
3.6. Antiproliferative Potential in Dried Cauliflower
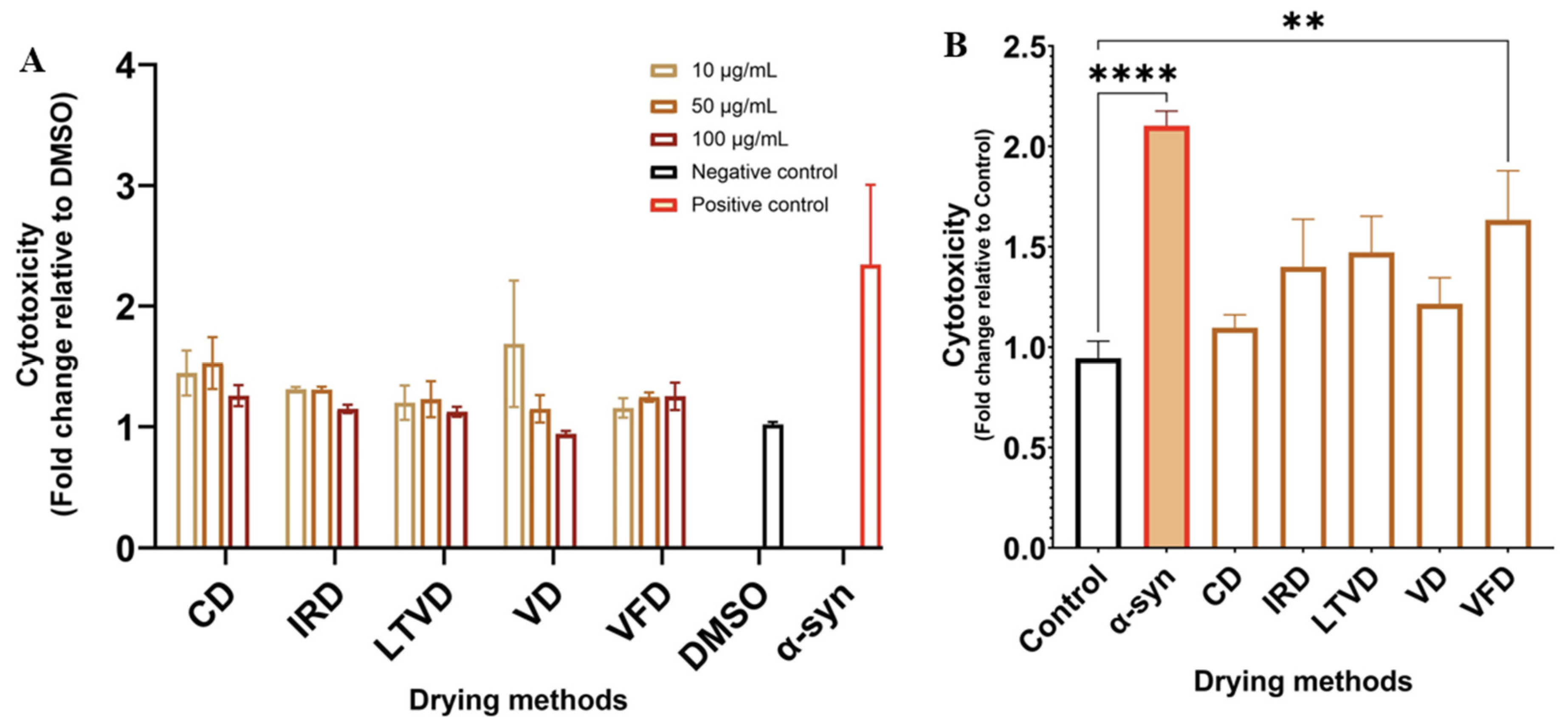
3.7. Neuroprotective Potential in Dried Cauliflower
3.8. Relationship between Bioactive Compounds and Health-Related Properties of Cauliflower
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Lee-Martínez, S.; Larrauri-Rodríguez, M.; Zaldívar-Lelo de Larrea, G.; Pérez-Serrano, R.M.; Camacho-Calderón, N. Isothiocyanate-rich extracts from cauliflower (Brassica oleracea Var. Botrytis) and radish (Raphanus sativus) inhibited metabolic activity and induced ROS in selected human HCT116 and HT-29 colorectal cancer cells. Int. J. Environ. Res. Public Health 2022, 19, 14919. [Google Scholar] [CrossRef]
- Holman, J.; Hurd, M.; Moses, P.L.; Mawe, G.M.; Zhang, T.; Ishaq, S.L.; Li, Y. Interplay of broccoli/broccoli sprout bioactives with gut microbiota in reducing inflammation in inflammatory bowel diseases. J. Nutr. Biochem. 2023, 113, 109238. [Google Scholar] [CrossRef]
- Razis, A.F.A.; Bagatta, M.; De Nicola, G.R.; Iori, R.; Ioannides, C. Up-regulation of cytochrome P450 and phase II enzyme systems in rat precision-cut rat lung slices by the intact glucosinolates, glucoraphanin and glucoerucin. Lung Cancer 2011, 71, 298–305. [Google Scholar] [CrossRef]
- Hwang, E.-S. Effect of cooking method on antioxidant compound contents in cauliflower. Prev. Nutr. Food Sci. 2019, 24, 210–216. [Google Scholar] [CrossRef]
- Sosinska, E.; Obiedzinski, M.W. Effect of processing on the content of glucobrassicin and its degradation products in broccoli and cauliflower. Food Control 2011, 22, 1348–1356. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Kwak, J.-H. Chemical composition and antioxidant activity in different tissues of brassica vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef]
- Vaishnav, J.; Srivastava, A.K.; Mishra, B.B.; Suprasanna, P.; Variyar, P.S. Glucosinolates breakdown and enhanced nitrile formation in gamma irradiated minimally processed cauliflower (Brassica oleracia). Radiat. Phys. Chem. 2023, 205, 110672. [Google Scholar] [CrossRef]
- Cabello-Hurtado, F.; Gicquel, M.; Esnault, M.A. Evaluation of the antioxidant potential of cauliflower (Brassica Oleracea) from a glucosinolate content perspective. Food Chem. 2012, 132, 1003–1009. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Płatosz, N.; Bartoszek, A. Phytochemical composition and biological activities of differently pigmented cabbage (Brassica oleracea Var. Capitata) and cauliflower (Brassica oleracea Var. Botrytis) varieties. J. Sci. Food Agric. 2019, 99, 5499–5507. [Google Scholar] [CrossRef]
- Nartea, A.; Fanesi, B.; Pacetti, D.; Lenti, L.; Fiorini, D.; Lucci, P.; Frega, N.G.; Falcone, P.M. Cauliflower by-products as functional ingredient in bakery foods: Fortification of pizza with glucosinolates, carotenoids and phytosterols. Curr. Res. Food Sci. 2023, 6, 100437. [Google Scholar] [CrossRef]
- Hengchao, E.; Peng, S.; Zhao, Z.; Yao, X.; Zhang, Y.; Li, X.; Yang, X.; Fan, T.; Zhao, X.; Zhou, C. Molecular networking and equivalently quantitative ion strategy for discovery and quantification of glucosinolates in cauliflower and broccoli by liquid chromatography tandem mass spectrometry. LWT-Food Sci. Technol. 2023, 187, 115318. [Google Scholar] [CrossRef]
- Tabart, J.; Pincemail, J.; Kevers, C.; Defraigne, J.-O.; Dommes, J. Processing effects on antioxidant, glucosinolate, and sulforaphane contents in broccoli and red cabbage. Eur. Food Res. Technol. 2018, 244, 2085–2094. [Google Scholar] [CrossRef]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef] [PubMed]
- Kitainda, V.; Jez, J.M. Structural studies of aliphatic glucosinolate chain-elongation enzymes. Antioxidants 2021, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; An, R.; Zhou, H.; Zhang, Y.; Ling, J.; Hu, H.; Li, P. The glucosinolate profiles of Brassicaceae vegetables responded differently to quick-freezing and drying methods. Food Chem. 2022, 383, 132624. [Google Scholar] [CrossRef]
- Abdelshafeek, K.; El-Shamy, A.M. Review on glucosinolates: Unveiling their potential applications as drug discovery leads in extraction, isolation, biosynthesis, biological activity, and corrosion protection. Food Biosci. 2023, 56, 103071. [Google Scholar] [CrossRef]
- Girgin, N.; El, S.N. Effects of cooking on in vitro sinigrin bioaccessibility, total phenols, antioxidant and antimutagenic activity of cauliflower (Brassica oleraceae L. var. Botrytis). J. Food Compost. Anal. 2015, 37, 119–127. [Google Scholar] [CrossRef]
- Navarro, S.L.; Li, F.; Lampe, J.W. Mechanisms of action of isothiocyanates in cancer chemoprevention: An update. Food Funct. 2011, 2, 579–587. [Google Scholar] [CrossRef]
- Olayanju, J.B.; Bozic, D.; Naidoo, U.; Sadik, O.A. A comparative review of key isothiocyanates and their health benefits. Nutrients 2024, 16, 757. [Google Scholar] [CrossRef]
- Wang, J.; Barba, F.J.; Sørensen, J.C.; Frandsen, H.B.; Sørensen, S.; Olsen, K.; Orlien, V. The role of water in the impact of high pressure on the myrosinase activity and glucosinolate content in seedlings from Brussels sprouts. Innov. Food Sci. Emerg. Technol. 2019, 58, 102208. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, W.; Zheng, Y.; Xu, R.; Ma, H. Comparison of four drying methods in terms of the drying efficiency and physicochemical properties of chicken meat. Food Phys. 2024, 1, 100010. [Google Scholar] [CrossRef]
- Tetteh, O.N.A.; Ulrichs, C.; Huyskens-Keil, S.; Mewis, I.; Amaglo, N.K.; Oduro, I.N.; Adarkwah, C.; Obeng-Ofori, D.; Förster, N. Effects of harvest techniques and drying methods on the stability of glucosinolates in Moringa oleifera leaves during post-harvest. Sci. Hortic. 2019, 246, 998–1004. [Google Scholar] [CrossRef]
- Korus, A.; Słupski, J.; Gebczynski, P.; Banas, A. Effect of preliminary processing and method of preservation on the content of glucosinolates in kale (Brassica oleracea L. var. acephala) leaves. LWT-Food Sci. Technol. 2014, 59, 1003–1008. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Monteiro, F.; Passos, C.P.; Silva, A.M.S.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Blanching impact on pigments, glucosinolates, and phenolics of dehydrated broccoli by-products. Food Res. Int. 2020, 132, 109055. [Google Scholar] [CrossRef]
- Pasten, A.; Vega-Galvez, A.; Uribe, E.; Carvajal, M.; Mejías, N.; Araya, M.; Goñi, M.G. A Comparison of the effects of Low-Temperature Vacuum Drying and other methods on cauliflower’s nutritional–functional properties. Processes 2024, 12, 1629. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Uribe, E.; Pasten, A.; Camus, J.; Rojas, M.; Garcia, V.; Araya, M.; Valenzuela-Barra, G.; Zambrano, A.; Goñi, M.G. Low-Temperature Vacuum Drying on broccoli: Enhanced anti-inflammatory and anti-proliferative properties regarding other drying methods. Foods 2023, 12, 3311. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; García, S.; Rengel, J.; Pizarro, S.; Álvarez, G. Determination of free and protein amino acid content in microalgae by HPLC-DAD with pre-column derivatization and pressure hydrolysis. Mar. Chem. 2021, 234, 103999. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Gomez-Perez, L.S.; Zepeda, F.; Vidal, R.L.; Grunenwald, F.; Mejías, N.; Pasten, A.; Araya, M.; Ah-Hen, K.S. Assessment of bio-compounds content, antioxidant activity, and neuroprotective effect of red cabbage (Brassica oleracea var. Capitata rubra) processed by convective drying at different temperatures. Antioxidants 2023, 12, 1789. [Google Scholar] [CrossRef]
- Mejías, N.; Vega-Galvez, A.; Gomez-Perez, L.S.; Pasten, A.; Uribe, E.; Cortés, A.; Valenzuela-Barra, G.; Camus, J.; Delporte, C.; Bernal, G. Health-Promoting properties of processed red cabbage (Brassica oleracea var. capitata f. rubra): Effects of drying methods on bio-compound retention. Foods 2024, 13, 830. [Google Scholar] [CrossRef]
- Valenzuela-Barra, G.; Castro, C.; Figueroa, C.; Barriga, A.; Silva, X.; de las Heras, B.; Hortelano, S.; Delporte, C. Anti-inflammatory activity and phenolic profile of propolis from two locations in Región Metropolitana de Santiago, Chile. J. Ethnopharmacol. 2015, 168, 37–44. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Uribe, E.; Pasten, A.; Camus, J.; Gomez-Perez, L.S.; Mejias, N.; Vidal, R.L.; Grunenwald, F.; Aguilera, L.E.; Valenzuela-Barra, G. Comprehensive evaluation of the bioactive composition and neuroprotective and antimicrobial properties of vacuum-dried broccoli (Brassica oleracea var. italica) powder and its antioxidants. Molecules 2023, 28, 766. [Google Scholar] [CrossRef] [PubMed]
- Słupski, J.; Bernas, E.; Kmiecik, W.; Lisiewska, Z. Evaluation of the amino acid content and the quality of protein in florets of white cauliflower: Raw, cooked, and prepared for consumption after freezing. Int. J. Food Sci. Technol. 2009, 44, 629–634. [Google Scholar] [CrossRef]
- Drabinska, N.; Jez, M.; Nogueira, M. Variation in the Accumulation of Phytochemicals and Their Bioactive Properties among the Aerial Parts of Cauliflower. Antioxidants 2021, 10, 1597. [Google Scholar] [CrossRef]
- Yin, M.; Matsuoka, R.; Yanagisawa, T.; Xi, Y.; Zhang, L.; Wang, X. Effect of different drying methods on free amino acid and flavor nucleotides of scallop (patinopecten yessoensis) adductor muscle. Food Chem. 2022, 396, 133620. [Google Scholar] [CrossRef] [PubMed]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in curcuminoids and chemical components of turmeric (Curcuma longa L.) under freeze-drying and low-temperature drying methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Valorization of cauliflower (Brassica oleracea L. var. botrytis) by-products as a source of antioxidant phenolics. J. Agric. Food Chem. 2003, 51, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Gratacós-Cubarsí, M.; Ribas-Agustí, A.; García-Regueiro, J.A.; Castellari, M. Simultaneous evaluation of intact glucosinolates and phenolic compounds by UPLC-DAD-MS/MS in Brassica oleracea L. var. botrytis. Food Chem. 2010, 121, 257–263. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Niciforovic, N.; Abramovic, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Managa, M.G.; Sultanbawa, Y.; Sivakumar, D. Effects of different drying methods on untargeted phenolic metabolites, and antioxidant activity in chinese cabbage (Brassica rapa L. subsp. chinensis) and nightshade (Solanum retroflexum Dun.). Molecules 2020, 25, 1326. [Google Scholar] [CrossRef]
- Li, R.; Shang, H.; Wu, H.; Wang, M.; Duan, M.; Yang, J. Thermal inactivation kinetics and effects of drying methods on the phenolic profile and antioxidant activities of chicory (Cichorium intybus L.) leaves. Sci. Rep. 2018, 8, 9529. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Cheong, L.-Z.; Huang, F.; Zheng, C.; Wan, C.; Zheng, M. Effects of microwave irradiation on the distribution of sinapic acid and its derivatives in rapeseed and the antioxidant evaluation. LWT-Food Sci. Technol. 2019, 108, 310–318. [Google Scholar] [CrossRef]
- Cong, Y.; Zheng, M.; Huang, F.; Liu, C.; Zheng, C. Sinapic acid derivatives in microwave-pretreated rapeseeds and minor components in oils. J. Food Compost. Anal. 2020, 87, 103394. [Google Scholar] [CrossRef]
- Brandão, R.J.; Borel, L.D.M.S.; Marques, L.G.; Prado, M.M. Heat and mass transfer, energy and product quality aspects in drying processes using infrared radiation. In Drying and Energy Technologies; Advanced Structured Materials; Delgado, J., Barbosa de Lima, A., Eds.; Springer: Cham, Switzerland, 2016; Volume 63, pp. 111–130. [Google Scholar]
- Wang, M.; Fan, L.; Li, J. A novel infrared roasting to improve the flavour profile of virgin rapeseed oils. Int. J. Food Sci. Technol. 2023, 58, 6081–6091. [Google Scholar] [CrossRef]
- Sivakumar, G.; Aliboni, A.; Bacchetta, L. HPLC screening of anti-cancer sulforaphane from important European Brassica species. Food. Chem. 2007, 104, 1761–1764. [Google Scholar] [CrossRef]
- Al-Bakheit, A.; Abu-Qatouseh, L. Sulforaphane from broccoli attenuates inflammatory hepcidin by reducing IL-6 secretion in human HepG2 cells. J. Funct. Foods 2020, 75, 104210. [Google Scholar] [CrossRef]
- Passos, G.F.; Medeiros, R.; Marcon, R.; Nascimento, A.F.Z.; Calixto, J.B.; Pianowski, L.F. The role of PKC/ERK1/2 signaling in the anti-inflammatory effect of tetracyclic triterpene euphol on TPA-induced skin inflammation in mice. Eur. J. Pharmacol. 2013, 698, 413–420. [Google Scholar] [CrossRef]
- Aghazadeh-Habashi, A.; Asghar, W.; Jamali, F. Drug-disease interaction: Effect of inflammation and nonsteroidal anti-inflammatory drugs on cytochrome P450 metabolites of arachidonic acid. J. Pharm. Sci. 2018, 107, 756–763. [Google Scholar] [CrossRef]
- Aliabadi, A.; Khanniri, E.; Mahboubi-Rabbani, M.; Bayanati, M. Dual COX-2/15-LOX inhibitors: A new avenue in the prevention of cancer. Eur. J. Med. Chem. 2023, 261, 115866. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Xie, J.-H.; Chen, Y.-J.; Fu, X.-Q.; Wang, R.-J.; Deng, Y.-Y.; Wang, S.; Yu, H.-X.; Liang, C.; Yu, Z.-L. Amelioration of TPA-induced skin inflammation by the leaf extract of Vernonia amygdalina involves ERK/STAT3 (Ser727) signaling inhibition. Phytomedicine 2022, 102, 154194. [Google Scholar] [CrossRef]
- Ahmad, G.M.; Serie, M.M.A.; Ghoneem, T.; Ghareeb, D.A.; Yacout, G.A.; Abdel-Latif, M.S. Apoptotic-antiproliferative activity of Salix mucronata and Triticum spelta against human breast, lung, and liver cancer cells: A comparative study with other plant extracts containing phenolics and flavonoids. S. Afr. J. Bot. 2024, 171, 788––801. [Google Scholar] [CrossRef]
- Mitra, S.; Emran, T.B.; Chandran, D.; Zidan, B.M.R.M.; Das, R.; Mamada, S.S.; Masyita, A.; Salampe, M.; Nainu, F.; Khandaker, M.U.; et al. Cruciferous vegetables as a treasure of functional foods bioactive compounds: Targeting p53 family in gastrointestinal tract and associated cancers. Front. Nutr. 2022, 9, 951935. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Lenzi, M.; Hrelia, P. Apoptosis induction by sulfur-containing compounds in malignant and nonmalignant human cells. Environ. Mol. Mutagen. 2009, 50, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.B.; Patel, J.K. Preparation, characterization, and in vitro cytotoxicity activity of allyl-isothiocyanate-embedded polymeric nanoparticles for potential breast cancer targeting. Breast Cancer 2023, 30, 1065–1078. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.S.; Singh, M.; Kumar, D.; Kumar, M. Antiinflammatory action of sinapic and its esters in carrageenan induced rat paw oedema model. Indian J. Pharm. Sci. 1993, 55, 184–187. [Google Scholar]
- Kim, G.; Jang, M.; Hwang, I.; Cho, J.; Kim, S. Radish sprout alleviates DSS-induced colitis via regulation of NF-kB signaling pathway and modifying gut microbiota. Biomed. Pharmacother. 2021, 144, 112365. [Google Scholar] [CrossRef]
- Xian, Y.-F.; Hu, Z.; Ip, S.-P.; Chen, J.-N.; Su, Z.-R.; Lai, X.-P.; Lin, Z.-X. Comparison of the anti-inflammatory effects of Sinapis alba and Brassica juncea in mouse models of inflammation. Phytomedicine 2018, 15, 196–204. [Google Scholar] [CrossRef]
- Zang, Q.; Cao, W.; Yang, C.; Hong, L.; Geng, S.; Han, H.; Zhong, C. Isothiocyanates attenuate immune checkpoint blockage therapy in gastric cancer via induction of PD-L1 expression. J. Nutr. Biochem. 2023, 112, 109226. [Google Scholar]
- Choi, Y.H. ROS-mediated activation of AMPK plays a critical role in sulforaphane-induced apoptosis and mitotic arrest in AGS human gastric cancer cells. Gen. Physiol. Biophys. 2018, 37, 129–140. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, W.; Zhou, Z.; Sun, C. Benefits and risks of the hormetic effects of dietary isothiocyanates on cancer prevention. PLoS ONE 2014, 9, e114764. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Larocca, M.; Milella, S.; Fasano, A.; Rossano, R.; Liuzzi, G.M. Neuroprotective potential of isothiocyanates in an in vitro model of neuroinflammation. Inflammopharmacol. 2021, 29, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Montaut, S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases. Fitoterapia 2015, 106, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Jaafaru, M.S.; Karim, N.A.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Razis, A.F.A. Protective effect of glucosinolates hydrolytic products in neurodegenerative diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef]
- Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial health effects of glucosinolates-derived isothiocyanates on cardiovascular and neurodegenerative diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]

| Parameters (g/100 g) | Drying Methods | ||||
|---|---|---|---|---|---|
| VD | CD | IRD | LTVD | VFD | |
| Essential amino acids (Eaa) | |||||
| Histidine | ND | ND | 0.23 ± 0.01 b | 0.21 ± 0.04 b | 0.33 ± 0.03 a |
| Threonine | 0.46 ± 0.01 b | 0.10 ± 0.00 c | 1.06 ± 0.07 a | 0.65 ± 0.07 b | 1.07 ± 0.20 a |
| Valine | 0.43 ± 0.02 c | 0.31 ± 0.00 c | 0.95 ± 0.10 a | 0.59 ± 0.17 bc | 0.85 ± 0.21 ab |
| Phenylalanine | 0.66 ± 0.08 c | 0.51 ± 0.07 c | 1.58 ± 0.21 a | 1.13 ± 0.10 b | 1.45 ± 0.27 ab |
| Isoleucine | 1.11 ± 0.03 b | 0.80 ± 0.10 b | 1.64 ± 0.18 a | 1.06 ± 0.03 b | 1.69 ± 0.26 a |
| Leucine | 0.77 ± 0.02 a | 0.62 ± 0.11 a | 0.75 ± 0.21 a | 0.58 ± 0.02 a | 0.80 ± 0.01 a |
| Lysine | 0.46 ± 0.01 d | 0.26 ± 0.02 e | 0.98 ± 0.07 b | 0.72 ± 0.07 c | 1.33 ± 0.40 a |
| Total Eaa | 3.90 ± 0.18 | 2.59 ± 0.30 | 7.21 ± 0.84 | 4.94 ± 0.49 | 7.53 ± 1.39 |
| Nonessential amino acids (NEaa) | |||||
| Aspartic acid | 0.86 ± 0.02 bc | 0.56 ± 0.07 c | 1.48 ± 0.16 a | 0.68 ± 0.22 c | 1.35 ± 0.36 ab |
| Glutamic acid | 1.53 ± 0.00 bc | 1.00 ± 0.13 c | 2.25 ± 0.27 ab | 1.57 ± 0.12 bc | 2.53 ± 0.71 a |
| Serine | 0.52 ± 0.21 b | 0.55 ± 0.06 b | 0.96 ± 0.12 a | 0.69 ± 0.04 ab | 0.97 ± 0.19 a |
| Glycine | 0.93 ± 0.02 a | 1.03 ± 0.15 a | 0.63 ± 0.12 bc | 0.56 ± 0.11 c | 0.86 ± 0.01 ab |
| Arginine | 0.77 ± 0.04 ab | 0.56 ± 0.06 c | 0.84 ± 0.10 a | 0.64 ± 0.05 bc | 0.87 ± 0.05 a |
| Alanine | 0.63 ± 0.02 bc | 0.50 ± 0.06 c | 0.96 ± 0.12 a | 0.67 ± 0.05 bc | 0.81 ± 0.16 ab |
| Tyrosine | 0.32 ± 0.04 a | 0.36 ± 0.06 a | 0.41 ± 0.01 a | 0.24 ± 0.05 a | 0.43 ± 0.07 a |
| Total NEaa | 5.56 ± 0.34 | 4.56 ± 0.59 | 7.54 ± 0.91 | 5.05 ± 0.64 | 7.81 ± 1.55 |
| Total aa | 9.46 ± 0.52 | 7.15 ± 0.89 | 14.75 ± 1.75 | 9.99 ± 1.13 | 15.34 ± 2.94 |
| N° | Identification | [M-H]-Theoretical m/z | [M-H]-Observed m/z | Mass Error (ppm) | Drying Methods | ||||
|---|---|---|---|---|---|---|---|---|---|
| VD | CD | IRD | LTVD | VFD | |||||
| 1 | Chlorogenic acid | 353.0878 | 353.0876 | −0.56 | 2.63 ± 0.37 d | 14.05 ± 0.64 b | 1.33 ± 0.43 d | 48.35 ± 2.67 a | 7.47 ± 0.46 c |
| 2 | Ferulic acid acyl-β-D-glucoside | 355.1035 | 355.1043 | 2.25 | 12.49 ± 0.41 b | 4.67 ± 0.15 c | 0.85 ± 0.05 d | 6.12 ± 0.42 c | 51.79 ± 2.57 a |
| 3 | (3-Sinapoyl)fructofuranosyl-(6-sinapoyl)glucopyranoside | 753.2247 | 753.2267 | 2.65 | 18.01 ± 0.68 d | 32.88 ± 2.41 b | 18.24 ± 2.14 d | 28.35 ± 0.58 c | 44.17 ± 4.20 a |
| 4 | Sinapoyl D-glucoside | 385.1140 | 385.1154 | 3.64 | 125.91 ± 6.10 b | 125.05 ± 11.68 b | 35.34 ± 1.27 c | 108.62 ± 8.05 b | 344.14 ± 17.81 a |
| 5 | 3′-O-sinapoyl-6-O-feruloylsucrose | 723.2141 | 723.2185 | 6.08 | 9.18 ± 0.65 cb | 11.13 ± 0.48 b | 6.46 ± 0.44 c | 9.00 ± 0.35 bc | 37.06 ± 4.27 a |
| 6 | Glucoerucin | 420.0351 | 420.0371 | 4.76 | 18.47 ± 1.18 c | ND d | 8.31 ± 0.74 b | ND d | 50.24 ± 1.32 a |
| 7 | Glucobrassicin | 447.0537 | 447.0556 | 4.25 | 1034.83 ± 35.03 b | 863.83 ± 39.63 c | 502.57 ± 23.56 d | 880.62 ± 23.89 c | 2950.36 ± 166.4 a |
| 8 | Neoglucobrassicin | 477.0643 | 447.0648 | 1.05 | 703.55 ± 51.07 c | 1412.46 ± 84.09 b | 251.43 ± 20.99 e | 516.58 ± 25.15 d | 2520.91 ± 109.4 a |
| 9 | 4-methoxy-glucobrassicin | 477.0643 | 447.0688 | 9.43 | 243.88 ± 15.63 c | 225.47 ± 24.15 c | 145.32 ± 7.79 d | 340.19 ± 16.01 b | 693.76 ± 18.81 a |
| Parameters | Drying Methods | ||||
|---|---|---|---|---|---|
| VD | CD | IRD | LTVD | VFD | |
| SFN | 8.00 ± 0.10 c | 9.00 ± 0.10 b | 12.04 ± 0.10 a | 7.00 ± 0.10 d | 5.40 ± 0.10 e |
| AITC | 6.00 ± 0.30 c | ND | 22.02 ± 3.00 ab | 26.02 ± 3.00 a | 18.01 ± 1.30 b |
| PITC | ND | ND | ND | ND | ND |
| I3C | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Galvez, A.; Pasten, A.; Uribe, E.; Mejias, N.; Araya, M.; Vidal, R.L.; Valenzuela-Barra, G.; Delporte, C. Comprehensive Assessment of Anti-Inflammatory, Antiproliferative and Neuroprotective Properties of Cauliflower after Dehydration by Different Drying Methods. Foods 2024, 13, 3162. https://doi.org/10.3390/foods13193162
Vega-Galvez A, Pasten A, Uribe E, Mejias N, Araya M, Vidal RL, Valenzuela-Barra G, Delporte C. Comprehensive Assessment of Anti-Inflammatory, Antiproliferative and Neuroprotective Properties of Cauliflower after Dehydration by Different Drying Methods. Foods. 2024; 13(19):3162. https://doi.org/10.3390/foods13193162
Chicago/Turabian StyleVega-Galvez, Antonio, Alexis Pasten, Elsa Uribe, Nicol Mejias, Michael Araya, René L. Vidal, Gabriela Valenzuela-Barra, and Carla Delporte. 2024. "Comprehensive Assessment of Anti-Inflammatory, Antiproliferative and Neuroprotective Properties of Cauliflower after Dehydration by Different Drying Methods" Foods 13, no. 19: 3162. https://doi.org/10.3390/foods13193162
APA StyleVega-Galvez, A., Pasten, A., Uribe, E., Mejias, N., Araya, M., Vidal, R. L., Valenzuela-Barra, G., & Delporte, C. (2024). Comprehensive Assessment of Anti-Inflammatory, Antiproliferative and Neuroprotective Properties of Cauliflower after Dehydration by Different Drying Methods. Foods, 13(19), 3162. https://doi.org/10.3390/foods13193162








