Origanum majorana L. As Flavoring Agent: Impact on Quality Indices, Stability, and Volatile and Phenolic Profiles of Extra Virgin Olive Oil (EVOO)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Material—Preparation of Flavored Olive Oil
2.2. Determination of Olive Oil Quality Indices
2.3. Color Measurement
2.4. Determination of Oxidative Stability
2.5. Determination of Antifungal Activity
2.5.1. Inoculum Preparation
2.5.2. Challenge Test
2.6. Identification and Semi-Quantification of Volatile Compounds (VCs) by Solid Phase-Microextraction Gas Chromatography-Mass Spectrometry (SPME-GC-MS)
2.7. Extraction of Phenolic Compounds
2.8. Spectrophotometric Assays
2.9. Identification of Phenolic Compounds by High-Resolution Liquid Chromatography-Mass Spectrometry
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extra Virgin Olive Oil Quality Indices and Lovibond Color Parameters
3.2. Evaluation of Antifungal Activity
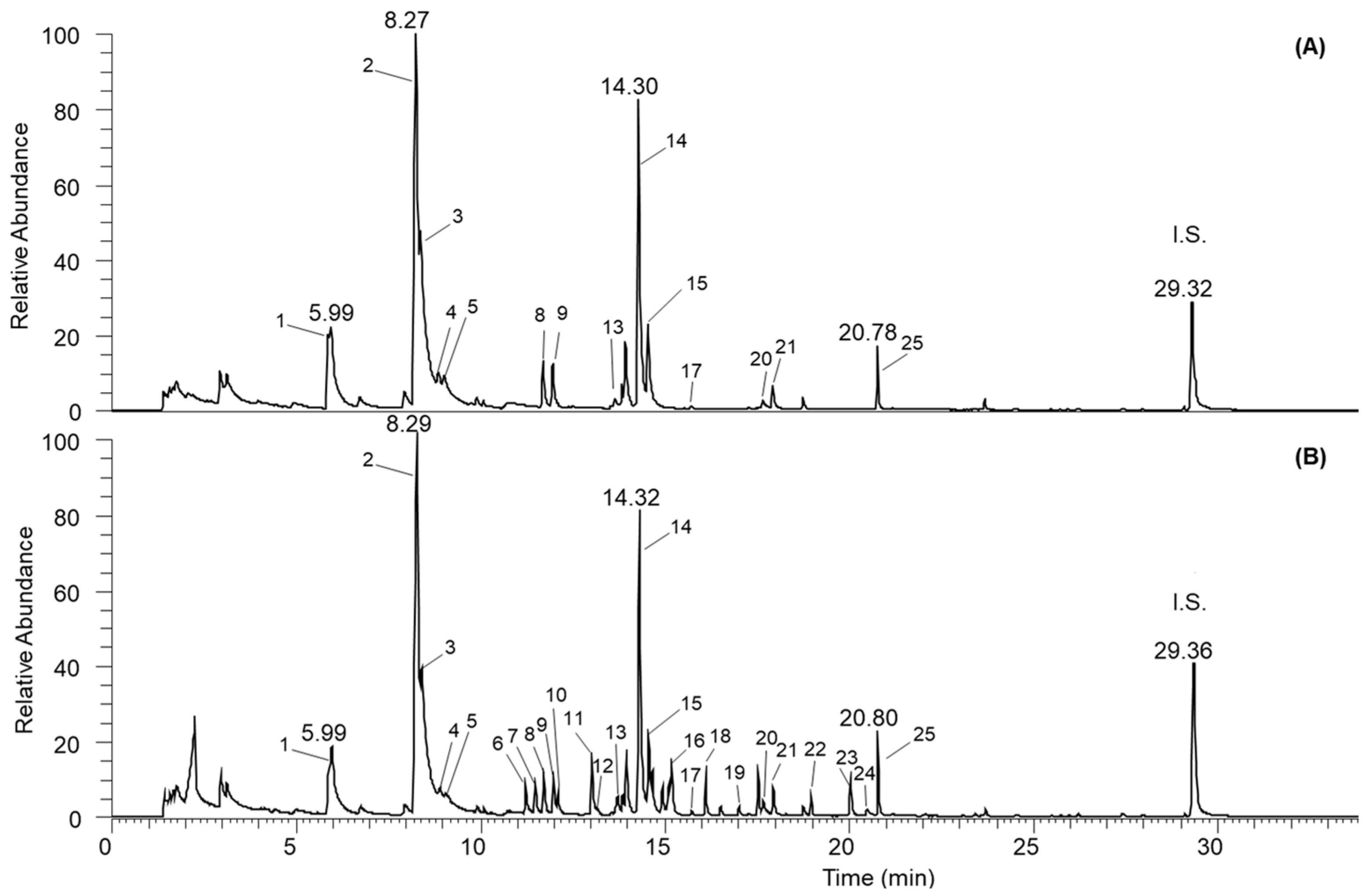
3.3. Analysis of Volatile Compounds
3.4. Photometric Analysis
3.5. Identification of Phenolic Compounds by LC-QToF-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perona, J.; Botham, K. Olive Oil as a Functional Food: Nutritional and Health Benefits. In Handbook of Olive Oil: Analysis and Properties; Springer: Boston, MA, USA, 2013; pp. 677–714. ISBN 978-1-4614-7776-1. [Google Scholar]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive Oil Consumption and Human Health: A Narrative Review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Krause, M.; Schmucker, C.; Hoffmann, G.; Rücker, G.; Meerpohl, J.J. Impact of Different Types of Olive Oil on Cardiovascular Risk Factors: A Systematic Review and Network Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health (Text with EEA Relevance). Off. J. Eur. Union 2021, 55, L136. [Google Scholar]
- Lamas, S.; Rodrigues, N.; Peres, A.M.; Pereira, J.A. Flavoured and Fortified Olive Oils—Pros and Cons. Trends Food Sci. Technol. 2022, 124, 108–127. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Fregapane, G.; Salvador, M.D.; Simal-Gándara, J. Characterisation of Extra Virgin Olive Oils from Galician Autochthonous Varieties and Their Co-Crushings with Arbequina and Picual Cv. Food Chem. 2015, 176, 493–503. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.; Peca, G. Presence of Microorganisms in Flavoured Extra Virgin Olive Oil. Ann. Microbiol. 2004, 54, 161–168. [Google Scholar]
- Napoli, E.; Siracusa, L.; Ruberto, G. New Tricks for Old Guys: Recent Developments in the Chemistry, Biochemistry, Applications and Exploitation of Selected Species from the Lamiaceae Family. Chem. Biodivers. 2020, 17, e1900677. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Soares, V.P.; Fagundes, M.B.; Guerra, D.R.; Leães, Y.S.V.; Speroni, C.S.; Robalo, S.S.; Emanuelli, T.; Cichoski, A.J.; Wagner, R.; Barin, J.S.; et al. Ultrasound Assisted Maceration for Improving the Aromatization of Extra-Virgin Olive Oil with Rosemary and Basil. Food Res. Int. 2020, 135, 109305. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Grati-Kamoun, N.; Attia, H. Physico-Chemical Change and Heat Stability of Extra Virgin Olive Oils Flavoured by Selected Tunisian Aromatic Plants. Food Chem. Toxicol. 2009, 47, 2613–2619. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Silva, P.; Câmara, J.S. Global Volatile Profile of Virgin Olive Oils Flavoured by Aromatic/Medicinal Plants. Food Chem. 2017, 227, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Casal, S.; Malheiro, R.; Lamas, H.; Bento, A.; Pereira, J.A. Aromatized Olive Oils: Influence of Flavouring in Quality, Composition, Stability, Antioxidants, and Antiradical Potential. LWT Food Sci. Technol. 2015, 60, 22–28. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on Virgin Olive Oil Quality. Past, Present and Future—An Overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Rasheed, S.; Nigam, P.S.; Janneh, O.; Sarker, S.D. Composition, Antioxidant and Chemotherapeutic Properties of the Essential Oils from Two Origanum Species Growing in Pakistan. Rev. Bras. Farmacogn. 2011, 21, 943–952. [Google Scholar] [CrossRef]
- El-Ashmawy, I.M.; Saleh, A.; Salama, O.M. Effects of Marjoram Volatile Oil and Grape Seed Extract on Ethanol Toxicity in Male Rats. Basic Clin. Pharmacol. Toxicol. 2007, 101, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; Jaime, L.; López de las Hazas, M.C.; Reglero, G.; Santoyo, S. Supercritical Fluid Extraction as an Alternative Process to Obtain Essential Oils with Anti-Inflammatory Properties from Marjoram and Sweet Basil. Ind. Crops Prod. 2015, 67, 121–129. [Google Scholar] [CrossRef]
- Mossa, A.-T.; Refaie, A.; Ramadan, A.; Bouajila, J. Amelioration of Prallethrin-Induced Oxidative Stress and Hepatotoxicity in Rat by the Administration of Origanum Majorana Essential Oil. BioMed Res. Int. 2013, 2013, 859085. [Google Scholar] [CrossRef]
- Mossa, A.-T.; Nawwar, G. Free Radical Scavenging and Antiacetylcholinesterase Activities of Origanum majorana L. Essential Oil. Hum. Exp. Toxicol. 2011, 30, 1501–1513. [Google Scholar] [CrossRef]
- Veillet, S.; Tomao, V.; Chemat, F. Ultrasound Assisted Maceration: An Original Procedure for Direct Aromatisation of Olive Oil with Basil. Food Chem. 2010, 123, 905–911. [Google Scholar] [CrossRef]
- Benmoussa, H.; Farhat, A.; Elfalleh, W.; Di Maio, I.; Servili, M.; Romdhane, M. A Rapid Application to Flavor the Olive Oil with Dried Rosmarinus officinalis L. Leaves: Microwave-Assisted Maceration. J. Food Process. Preserv. 2017, 41, e12885. [Google Scholar] [CrossRef]
- Assami, K.; Chemat, S.; Meklati, B.Y.; Chemat, F. Ultrasound-Assisted Aromatisation with Condiments as an Enabling Technique for Olive Oil Flavouring and Shelf Life Enhancement. Food Anal. Methods 2016, 9, 982–990. [Google Scholar] [CrossRef]
- Achat, S.; Tomao, V.; Madani, K.; Chibane, M.; Elmaataoui, M.; Dangles, O.; Chemat, F. Direct Enrichment of Olive Oil in Oleuropein by Ultrasound-Assisted Maceration at Laboratory and Pilot Plant Scale. Ultrason. Sonochem. 2012, 19, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.; Marques, M.P.; Mourato, M.; Martins, L.L.; Ferreira-Dias, S. Ultrasound Assisted Coextraction of Cornicabra Olives and Thyme to Obtain Flavored Olive Oils. Molecules 2023, 28, 6898. [Google Scholar] [CrossRef] [PubMed]
- Paduano, A.; Caporaso, N.; Santini, A.; Sacchi, R. Microwave and Ultrasound-Assisted Extraction of Capsaicinoids from Chili Peppers (Capsicum annuum L.) in Flavored Olive Oil. J. Food Res. 2014, 3, p51. [Google Scholar] [CrossRef]
- Bittencourt Fagundes, M.; Ballus, C.A.; Perceval Soares, V.; de Freitas Ferreira, D.; Sena Vaz Leães, Y.; Sasso Robalo, S.; Guidetti Vendruscolo, R.; Bastianello Campagnol, P.C.; Smanioto Barin, J.; Cichoski, A.J.; et al. Characterization of Olive Oil Flavored with Brazilian Pink Pepper (Schinus terebinthifolius Raddi) in Different Maceration Processes. Food Res. Int. 2020, 137, 109593. [Google Scholar] [CrossRef]
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/ (accessed on 20 June 2024).
- AOCS. Official Methods and Recommended Practices—Color of Fats and Oil, Lovibond, 6th ed.; Method Cc 13e–92; American Oil Chemists’ Society: Champaign, IL, USA, 2017. [Google Scholar]
- AOCS. Official Methods and Recommended Practices—Oil Stability Index, 6th ed.; Method Cd 12b–92; American Oil Chemists’ Society: Champaign, IL, USA, 2017. [Google Scholar]
- Loureiro, V. Spoilage Yeasts in Foods and Beverages: Characterisation and Ecology for Improved Diagnosis and Control. Food Res. Int. 2000, 33, 247–256. [Google Scholar] [CrossRef]
- Komitopoulou, E. 16—Microbiological Challenge Testing of Foods. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 507–523. ISBN 978-1-84569-701-3. [Google Scholar]
- Revelou, P.-K.; Pappa, C.; Kakouri, E.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Discrimination of Botanical Origin of Olive Oil from Selected Greek Cultivars by SPME-GC-MS and ATR-FTIR Spectroscopy Combined with Chemometrics. J. Sci. Food Agric. 2021, 101, 2994–3002. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp.: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- International Olive Council (IOC). COI/T.20/Doc. No. 29, Official Method of Analysis. Determination of Biophenols in Olive Oil by HPLC; International Olive Council (IOC): Madrid, Spain, 2009. [Google Scholar]
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal Distillates: A New Era of Grape Marc Distillates with Enriched Antioxidant Profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.G.; Glamočlija, J.; Ćirić, A.; Soković, M.; Heropoulos, G.; Proestos, C. Antiradical–Antimicrobial Activity and Phenolic Profile of Pomegranate (Punica granatum L.) Juices from Different Cultivars: A Comparative Study. RSC Adv. 2015, 5, 2602–2614. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Zoumpoulakis, P.; Proestos, C. Comparison of the Antioxidant and Antiradical Activity of Pomegranate (Punica granatum L.) by Ultrasound-Assisted and Classical Extraction. Anal. Lett. 2016, 49, 969–978. [Google Scholar] [CrossRef]
- Pyrovolou, K.; Tataridis, P.; Revelou, P.-K.; Strati, I.F.; Konteles, S.J.; Tarantilis, P.A.; Houhoula, D.; Batrinou, A. Fermentation of a Strong Dark Ale Hybrid Beer Enriched with Carob (Ceratonia siliqua L.) Syrup with Enhanced Polyphenol Profile. Appl. Sci. 2024, 14, 1199. [Google Scholar] [CrossRef]
- European Economic Communities. Characteristics of Olive and Olive Pomace Oils and Their Analytical Methods. Regulation EEC/2568/91 and Latter Modifications. Off. J. Eur. Commun. 1991, 248, 1–82. [Google Scholar]
- Sicaire, A.-G.; Vian, M.A.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Ultrasound Induced Green Solvent Extraction of Oil from Oleaginous Seeds. Ultrason. Sonochem. 2016, 31, 319–329. [Google Scholar] [CrossRef]
- Antoun, N.; Tsimidou, M. Gourmet Olive Oils: Stability and Consumer Acceptability Studies. Food Res. Int. 1997, 30, 131–136. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Gambacorta, G.; Notte, E.L. Changes in Quality Indices, Phenolic Content and Antioxidant Activity of Flavored Olive Oils during Storage. J. Am. Oil Chem. Soc. 2009, 86, 1083–1092. [Google Scholar] [CrossRef]
- Gambacorta, G.; Faccia, M.; Pati, S.; Lamacchia, C.; Baiano, A.; La Notte, E. Changes in the Chemical and Sensorial Profile of Extra Virgin Olive Oils Flavored with Herbs and Spices during Storage. J. Food Lipids 2007, 14, 202–215. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Hajaij, M.E.; Cioni, P.L.; Hammami, M. Oxidative Evolution of Virgin and Flavored Olive Oils Under Thermo-Oxidation Processes. J. Am. Oil Chem. Soc. 2011, 88, 1339–1350. [Google Scholar] [CrossRef]
- Criado, M.-N.; Romero, M.-P.; Casanovas, M.; Motilva, M.-J. Pigment Profile and Colour of Monovarietal Virgin Olive Oils from Arbequina Cultivar Obtained during Two Consecutive Crop Seasons. Food Chem. 2008, 110, 873–880. [Google Scholar] [CrossRef]
- Rojo, M.C.; Arroyo López, F.N.; Lerena, M.C.; Mercado, L.; Torres, A.; Combina, M. Evaluation of Different Chemical Preservatives to Control Zygosaccharomyces rouxii Growth in High Sugar Culture Media. Food Control 2015, 50, 349–355. [Google Scholar] [CrossRef]
- Pastorkova, E.; Zakova, T.; Landa, P.; Novakova, J.; Vadlejch, J.; Kokoska, L. Growth Inhibitory Effect of Grape Phenolics against Wine Spoilage Yeasts and Acetic Acid Bacteria. Int. J. Food Microbiol. 2013, 161, 209–213. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile Compounds in Virgin Olive Oil: Occurrence and Their Relationship with the Quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Kosma, I.; Vatavali, K.; Kontakos, S.; Kontominas, M.; Kiritsakis, A.; Badeka, A. Geographical Differentiation of Greek Extra Virgin Olive Oil from Late-Harvested Koroneiki Cultivar Fruits. J. Am. Oil Chem. Soc. 2017, 94, 1373–1384. [Google Scholar] [CrossRef]
- Kandylis, P.; Vekiari, A.S.; Kanellaki, M.; Grati Kamoun, N.; Msallem, M.; Kourkoutas, Y. Comparative Study of Extra Virgin Olive Oil Flavor Profile of Koroneiki Variety (Olea Europaea Var. Microcarpa Alba) Cultivated in Greece and Tunisia during One Period of Harvesting. LWT Food Sci. Technol. 2011, 44, 1333–1341. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Sacchi, R. Flavor Chemistry of Virgin Olive Oil: An Overview. Appl. Sci. 2021, 11, 1639. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Alonso, M.V. Relationship between Volatile Compounds and Sensory Attributes of Olive Oils by the Sensory Wheel. J. Am. Oil Chem. Soc. 1996, 73, 1253–1264. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio, R. Effect of Extraction Conditions on Sensory Quality of Virgin Olive Oil. J. Am. Oil Chem. Soc. 1999, 76, 295–300. [Google Scholar] [CrossRef]
- Baccouri, O.; Bendini, A.; Cerretani, L.; Guerfel, M.; Baccouri, B.; Lercker, G.; Zarrouk, M.; Daoud Ben Miled, D. Comparative Study on Volatile Compounds from Tunisian and Sicilian Monovarietal Virgin Olive Oils. Food Chem. 2008, 111, 322–328. [Google Scholar] [CrossRef]
- Kakouri, E.; Daferera, D.; Kanakis, C.; Revelou, P.-K.; Kaparakou, E.H.; Dervisoglou, S.; Perdikis, D.; Tarantilis, P.A. Origanum Majorana Essential Oil—A Review of Its Chemical Profile and Pesticide Activity. Life 2022, 12, 1982. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; Durante, V.; Pesce, V.; Maiellaro, I.; Lovece, A.; Mercurio, A.; Laghezza, A.; Corbo, F.; et al. Comparison Between Different Flavored Olive Oil Production Techniques: Healthy Value and Process Efficiency. Plant Foods Hum. Nutr. 2016, 71, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.-E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional Use, Phytochemistry, Toxicology, and Pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef] [PubMed]
- Boz, H. P-Coumaric Acid in Cereals: Presence, Antioxidant and Antimicrobial Effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Boskou, D. Antioxidant Effect of Natural Phenols on Olive Oil. J. Am. Oil Chem. Soc. 1991, 68, 669–671. [Google Scholar] [CrossRef]
- Cheung, S.C.M.; Szeto, Y.T.; Benzie, I.F.F. Antioxidant Protection of Edible Oils. Plant Foods Hum. Nutr. 2007, 62, 39–42. [Google Scholar] [CrossRef]
- Tsuzuki, W.; Nagata, R.; Yunoki, R.; Nakajima, M.; Nagata, T. Cis/Trans-Isomerisation of Triolein, Trilinolein and Trilinolenin Induced by Heat Treatment. Food Chem. 2008, 108, 75–80. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Thakorlal, J.; Zhou, J. Effects of Added Phenolics on the Storage Stability of Avocado and Coconut Oils. Int. J. Food Sci. Technol. 2011, 46, 1575–1585. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. P-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Wang, J.; Wang, F.; Mao, J. P-Coumaric Acid: Advances in Pharmacological Research Based on Oxidative Stress. Curr. Top. Med. Chem. 2024, 24, 416–436. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.-Y.; Lee, J.H. Effects of Flavonoids on Physical and Oxidative Stability of Soybean Oil O/W Emulsions. Food Sci. Biotechnol. 2015, 24, 851–858. [Google Scholar] [CrossRef]
- Lien, T.F.; Yeh, H.S.; Su, W.T. Effect of Adding Extracted Hesperetin, Naringenin and Pectin on Egg Cholesterol, Serum Traits and Antioxidant Activity in Laying Hens. Arch. Anim. Nutr. 2008, 62, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.H.; Severo, A.C. Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology of Hesperetin. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–26. ISBN 978-3-030-94753-8. [Google Scholar]
- Fathi, M.; Varshosaz, J.; Mohebbi, M.; Shahidi, F. Hesperetin-Loaded Solid Lipid Nanoparticles and Nanostructure Lipid Carriers for Food Fortification: Preparation, Characterization, and Modeling. Food Bioprocess Technol. 2013, 6, 1464–1475. [Google Scholar] [CrossRef]
- Kesen, S.; Kelebek, H.; Sen, K.; Ulas, M.; Selli, S. GC–MS–Olfactometric Characterization of the Key Aroma Compounds in Turkish Olive Oils by Application of the Aroma Extract Dilution Analysis. Food Res. Int. 2013, 54, 1987–1994. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Rodríguez-Pérez, C.; Gerasopoulos, D.; Segura-Carretero, A. Olive Oil Enrichment in Phenolic Compounds during Malaxation in the Presence of Olive Leaves or Olive Mill Wastewater Extracts. Eur. J. Lipid Sci. Technol. 2017, 119, 1600425. [Google Scholar] [CrossRef]
- Siliani, S.; Mattei, A.; Innocenti, L.B.; Zanoni, B. Bitter Taste and Phenolic Compounds in Extra Virgin Olive Oil: An Empirical Relationship. J. Food Qual. 2006, 29, 431–441. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; De Luca, G.; Maiuolo, J.; Oliverio, M.; Sindona, G.; Procopio, A. Biomimetic Synthesis and Antioxidant Evaluation of 3,4-DHPEA-EDA [2-(3,4-Hydroxyphenyl) Ethyl (3S,4E)-4-Formyl-3-(2-Oxoethyl)Hex-4-Enoate]. Food Chem. 2014, 162, 89–93. [Google Scholar] [CrossRef]
- Sindona, G.; Caruso, A.; Cozza, A.; Fiorentini, S.; Lorusso, B.; Marini, E.; Nardi, M.; Procopio, A.; Zicari, S. Anti-Inflammatory Effect of 3,4-DHPEA-EDA [2-(3,4-Hydroxyphenyl) Ethyl (3S, 4E)-4-Formyl-3-(2-Oxoethyl)Hex-4-Enoate] on Primary Human Vascular Endothelial Cells. Curr. Med. Chem. 2012, 19, 4006–4013. [Google Scholar] [CrossRef]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Selvaggini, R.; Montedoro, G.F.; Di Saverio, C.; Morozzi, G. Virgin Olive Oil Phenols Inhibit Proliferation of Human Promyelocytic Leukemia Cells (HL60) by Inducing Apoptosis and Differentiation. J. Nutr. 2006, 136, 614–619. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The Biological Activities of Natural Lignans from Olives and Virgin Olive Oils: A Review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Touillaud, M.S.; Thiébaut, A.C.M.; Fournier, A.; Niravong, M.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F. Dietary Lignan Intake and Postmenopausal Breast Cancer Risk by Estrogen and Progesterone Receptor Status. JNCI J. Natl. Cancer Inst. 2007, 99, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.U.; Chen, J.M.; Li, T.; Strasser-Weippl, K.; Goss, P.E. Dietary Flaxseed Alters Tumor Biological Markers in Postmenopausal Breast Cancer. Clin. Cancer Res. 2005, 11, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
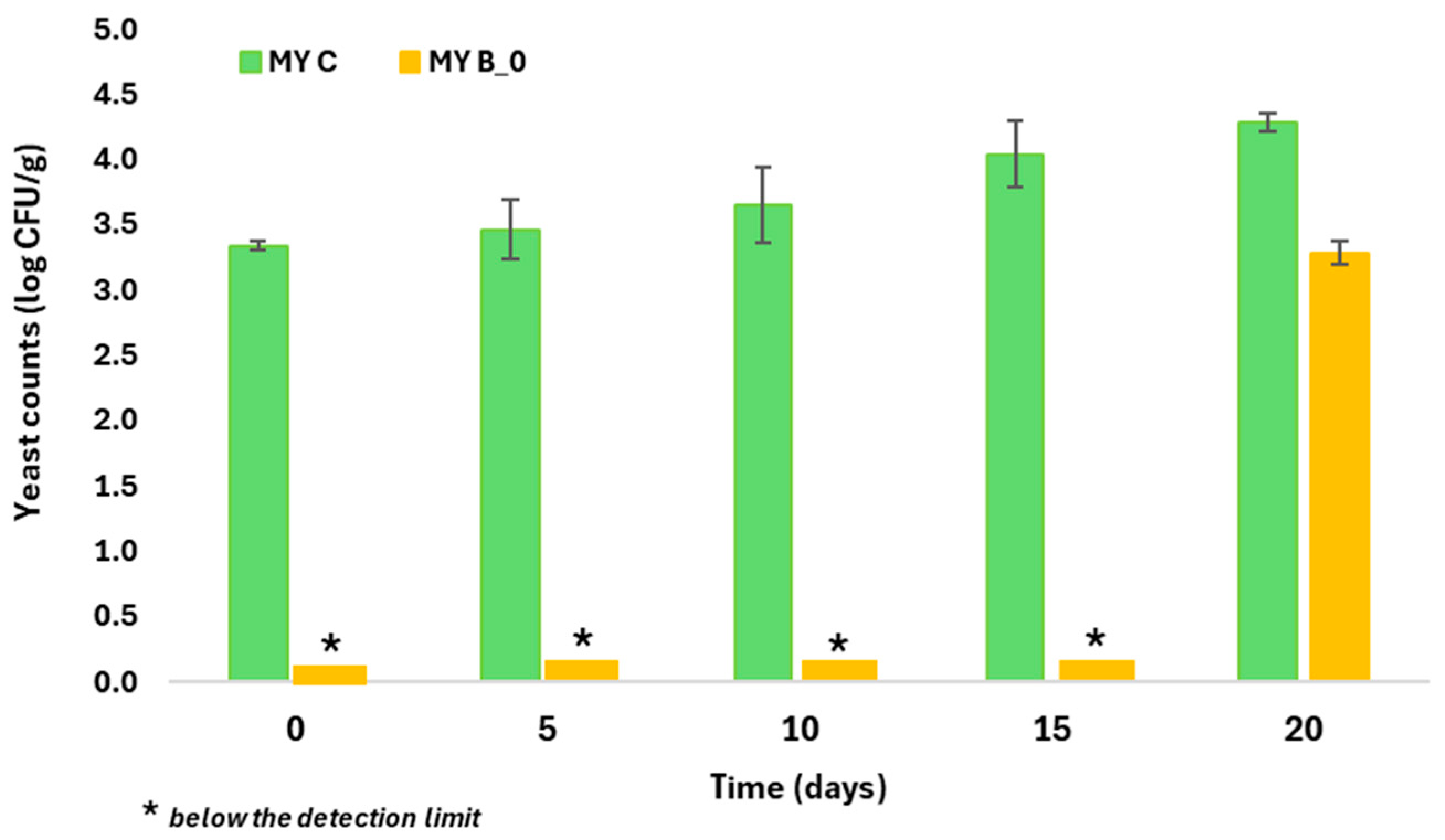
| Quality Indices | EVOO Samples | ||||
|---|---|---|---|---|---|
| C_0 | A_0 | B_0 | A_14 | B_14 | |
| Free Fatty Acids (FFA, % w/w oleic acid) | 0.56 ± 0.00 a | 0.56 ± 0.00 a | 0.55 ± 0.01 a | 0.50 ± 0.07 a | 0.59 ± 0.08 a |
| K232 | 1.26 ± 0.03 a | 1.78 ± 0.01 d | 1.45 ± 0.04 c | 2.25 ± 0.02 e | 1.37 ± 0.02 b |
| K270 | 0.10 ± 0.01 a | 0.09 ± 0.01 a | 0.18 ± 0.01 c | 0.15 ± 0.01 b | 0.22 ± 0.01 d |
| ΔΚ | 0.011 ± 0.001 a | 0.011 ± 0.001 a | 0.011 ± 0.001 a | 0.010 ± 0.001 a | 0.016 ± 0.001 b |
| Peroxide Value (PV, meq O2/kg) | 15.35 ± 1.30 a | 12.50 ± 2.32 a | 11.86 ± 1.79 a | 14.86 ± 2.65 a | 13.88 ± 2.74 a |
| Oxidation Induction Time (OIT, h) | 11.16 ± 0.02 a | 11.67 ± 0.07 b | 12.70 ± 0.23 d | 11.94 ± 0.12 c | 13.34 ± 0.18 e |
| Lovibond color parameters | |||||
| R (Red) | 1.0 | 1.2 | 1.3 | 1.3 | 1.2 |
| Y (Yellow) | 35.5 | 35 | 35.5 | 36 | 38 |
| B (Blue) | 0 | 0 | 0 | 0 | 0 |
| N (Neutral) | 0.3 | 0.3 | 0.8 | 0.6 | 0.5 |
| Chlorophyll (ppm) | 6.84 ± 0.02 a | 6.86 ± 0.00 a | 6.92 ± 0.04 b | 6.86 ± 0.01 a | 7.31 ± 0.06 c |
| b-carotene (ppm) | 44.85 ± 0.01 a | 44.86 ± 0.01 a | 45.17 ± 0.03 c | 45.04 ± 0.02 b | 46.33 ± 0.10 d |
| Peak No | Compound | 1 RI Exp. | 2 RI Lit. | EVOO Samples | |||||
|---|---|---|---|---|---|---|---|---|---|
| C_0 3 | C_14 4 | A_0 5 | A_14 6 | B_0 7 | B_14 8 | ||||
| 1 | (Z)-3-hexenal | <800 | 797 | 6.33 ± 2.01 a | 4.88 ± 0.43 a | 7.91 ± 0.30 a | 4.87 ± 0.10 a | 7.14 ± 1.50 a | 3.89 ± 0.90 a |
| 2 | (E)-2-hexenal | 849 | 846 | 49.51 ± 13.31 a | 64.92 ± 0.98 a | 70.06 ± 5.94 a | 72.79 ± 1.58 a | 75.87 ± 15.49 a | 62.25 ± 6.40 a |
| 3 | 3-hexen-1-ol | 852 | 847 | 42.45 ± 16.28 a | 55.35 ± 4.63 a | 58.36 ± 4.70 a | 52.09 ± 2.40 a | 59.41 ± 15.20 a | 44.25 ± 6.49 a |
| 4 | (E)-2-hexen-1-ol | 864 | 851 | 5.96 ± 1.82 a | 6.84 ± 1.03 a | 7.91 ± 0.62 a | 6.80 ± 0.56 a | 7.35 ± 1.62 a | 6.03 ± 1.14 a |
| 5 | 1-hexanol | 868 | 863 | 10.44 ± 4.88 a | 13.47 ± 0.83 a | 14.33 ± 1.34 a | 11.78 ± 1.85 a | 12.54 ± 2.71 a | 10.22 ± 0.84 a |
| 6 | β-thujene | 923 | 920 | − | − | 2.72 ± 0.42 ab | 4.22 ± 0.31 b | 2.46 ± 0.87 ac | 3.34 ± 0.14 ab |
| 7 | a-pinene | 929 | 932 | − | − | 1.67 ± 0.24 a | 2.56 ± 0.27 ab | 3.84 ± 1.14 c | 3.38 ± 0.06 a |
| 8 | 3-Ethyl-1,5-octadiene isomer 1 | 936 | 930 | 3.83 ± 1.22 a | 4.61 ± 0.59 a | 5.23 ± 0.53 a | 4.57 ± 0.24 a | 5.04 ± 1.59 a | 3.93 ± 0.30 a |
| 9 | 3-Ethyl-1,5-octadiene isomer 2 | 943 | 930 | 4.12 ± 1.33 a | 4.90 ± 0.63 a | 4.80 ± 0.40 a | 4.03 ± 0.10 a | 4.35 ± 1.15 a | 3.36 ± 0.27 a |
| 10 | Camphene | 946 | 946 | − | − | 1.54 ± 0.03 a | 1.83 ± 0.11 ab | 3.47 ± 1.17 b | 2.51 ± 0.15 ab |
| 11 | Sabinene | 970 | 969 | − | − | 2.87 ± 0.11 a | 6.23 ± 0.18 b | 3.19 ± 0.51 a | 5.67 ± 0.50 bc |
| 12 | β-pinene | 974 | 974 | − | − | 0.24 ± 0.03 a | 0.32 ± 0.05 a | 0.29 ± 0.40 a | 0.37 ± 0.11 a |
| 13 | 6-methyl-5-hepten-2-one | 984 | 989 | 0.22 ± 0.01 a | 0.25 ± 0.02 a | 0.17 ± 0.03 a | 0.30 ± 0.09 a | 0.25 ± 0.11 a | 0.33 ± 0.02 a |
| 14 | (Z)-3-hexenyl acetate | 1004 | 1004 | 30.38 ± 8.32 a | 34.37 ± 2.52 a | 39.65 ± 5.12 a | 35.12 ± 2.01 a | 35.62 ± 10.67 a | 32.65 ± 3.07 a |
| 15 | Hexyl acetate | 1012 | 1007 | 10.28 ± 3.09 a | 12.82 ± 1.05 a | 10.32 ± 1.55 a | 10.15 ± 3.04 a | 9.07 ± 0.11 a | 7.22 ± 0.72 a |
| 16 | Eucalyptol | 1028 | 1026 | − | − | 3.76 ± 0.76 a | 3.89 ± 0.79 a | 3.42 ± 2.85 a | 5.08 ± 0.14 a |
| 17 | (E)-β-ocimene | 1047 | 1044 | 0.26 ± 0.05 a | 0.42 ± 0.04 a | 0.40 ± 0.05 a | 0.36 ± 0.00 a | 0.45 ± 0.11 a | 0.36 ± 0.02 a |
| 18 | γ-terpinene | 1085 | 1086 | − | − | 2.86 ± 0.32 ab | 4.52 ± 0.03 c | 2.73 ± 0.78 a | 3.67 ± 0.28 abc |
| 19 | Terpinolene | 1085 | 1086 | − | − | 0.69 ± 0.00 a | 1.00 ± 0.01 a | 0.68 ± 0.21 a | 0.79 ± 0.00 a |
| 20 | Nonanal | 1105 | 1100 | 1.35 ± 0.03 a | 1.70 ± 0.06 a | 1.98 ± 0.13 a | 1.76 ± 0.01 a | 2.03 ± 1.12 a | 2.15 ± 0.06 a |
| 21 | 2-ethenyl-1.1-dimethyl-3-methylenecyclohexane | 1114 | − | 2.43 ± 0.40 a | 2.92 ± 0.19 a | 3.26 ± 0.05 a | 2.60 ± 0.02 a | 3.19 ± 1.30 a | 2.81 ± 0.12 a |
| 22 | (+)-camphor | 1146 | 1141 | − | − | 0.71 ± 0.01 ab | 0.77 ± 0.01 a | 1.98 ± 0.60 c | 1.58 ± 0.02 abc |
| 23 | (-)-terpinen-4-ol | 1181 | 1174 | − | − | 1.52 ± 0.03 a | 2.76 ± 0.03 b | 1.71 ± 0.60 a | 3.69 ± 0.17 c |
| 24 | α-terpineol | 1196 | 1186 | − | − | 0.37 ± 0.07 a | 0.58 ± 0.03 a | 0.50 ± 0.30 a | 1.14 ± 0.06 b |
| 25 | Methylcyclodecane | 1205 | 1202 | 4.42 ± 0.95 a | 5.11 ± 0.25 a | 5.74 ± 0.25 a | 4.19 ± 0.11 a | 6.43 ± 2.05 a | 5.73 ± 0.33 a |
| Olive Oil Samples | Total Phenolic Content (TPC) Expressed as mg Gallic Acid Equivalents (GAE)/kg Oil | Antiradical Activity Expressed as mg Trolox Equivalents (TE)/kg Oil | Antioxidant Activity by Ferric Reducing Antioxidant Power (FRAP) Assay Expressed as mg of FeSO4 x 7H2O/kg Oil |
|---|---|---|---|
| C_0 | 117.75 ± 17.10 a | 204.81 ± 13.54 ab | 1354.74 ± 99.71 ab |
| A_0 | 179.18 ± 11.43 b | 192.42 ± 14.42 a | 1581.28 ± 136.20 b |
| B_0 | 215.98 ± 7.63 cd | 238.74 ± 14.05 b | 1402.36 ± 98.43 ab |
| A_14 | 183.80 ± 17.30 bc | 205.10 ± 17.12 ab | 1420.26 ± 97.31 ab |
| B_14 | 249.84 ± 8.02 d | 199.17 ± 7.02 a | 1236.74 ± 50.54 a |
| Compound | Sample | tR (min) | Formula [M-H]− | Theoretical m/z [M-H]− | Experimental m/z [M-H]− | Mass Error |
|---|---|---|---|---|---|---|
| Hydroxytyrosol | C_0, A_14, B_14 | 1.88 | C8H10O3 | 153.0557 | 153.0553 | 2.73 |
| Tyrosol | C_0, A_14, B_14 | 2.76 | C8H10O2 | 137.0608 | 137.0606 | 1.49 |
| Vanillic acid | C_0, A_14, B_14 | 3.67 | C8H8O4 | 167.0350 | 167.0350 | 0.00 |
| p-coumaric acid | A_14, B_14 | 5.30 | C9H8O3 | 163.0400 | 163.0404 | −2.39 |
| 3,4-DHPEA-EDA | C_0, A_14, B_14 | 7.46 | C17H20O6 | 319.1187 | 319.1188 | −0.27 |
| Oleuropein aglycon isomer 1 | C_0, A_14, B_14 | 7.90 | C19H22O8 | 377.1242 | 377.1229 | 3.43 |
| Oleuropein aglycon isomer 2 | C_0, A_14, B_14 | 8.30 | C19H22O8 | 377.1242 | 377.1232 | 2.63 |
| Oleocanthal | C_0, A_14, B_14 | 9.15 | C17H20O5 | 303.1238 | 303.1238 | 0.00 |
| Oleuropein aglycon isomer 3 | C_0, A_14, B_14 | 9.42 | C19H22O8 | 377.1242 | 377.1235 | 1.83 |
| Luteolin | C_0, A_14, B_14 | 9.86 | C15H10O6 | 285.0405 | 285.0405 | 0.00 |
| Oleuropein aglycon isomer 4 | C_0, A_14, B_14 | 9.96 | C19H22O8 | 377.1242 | 377.1228 | 3.69 |
| 1-Acetoxypinoresinol | C_0, A_14, B_14 | 10.09 | C22H24O8 | 415.1398 | 415.1386 | 2.99 |
| Oleuropein aglycon isomer 5 | C_0, A_14, B_14 | 10.33 | C19H22O8 | 377.1242 | 377.1228 | 3.69 |
| Oleuropein aglycon isomer 6 | C_0, A_14, B_14 | 10.57 | C19H22O8 | 377.1242 | 377.1231 | 2.89 |
| Oleuropein aglycon isomer 7 | C_0, A_14, B_14 | 10.77 | C19H22O8 | 377.1242 | 377.1232 | 2.63 |
| Naringenin | C_0, A_14, B_14 | 10.94 | C15H12O5 | 271.0612 | 271.0611 | 0.37 |
| Apigenin | C_0, A_14, B_14 | 11.48 | C15H10O5 | 269.0456 | 269.0455 | 0.19 |
| Oleuropein aglycon isomer 8 | C_0, A_14, B_14 | 11.68 | C19H22O8 | 377.1242 | 377.1234 | 2.10 |
| Oleuropein aglycon isomer 9 | C_0, A_14, B_14 | 11.92 | C19H22O8 | 377.1242 | 377.1234 | 2.10 |
| Oleuropein aglycon isomer 10 | C_0, A_14, B_14 | 12.29 | C19H22O8 | 377.1242 | 377.1234 | 2.10 |
| Hesperetin | A_14, B_14 | 12.63 | C16H14O6 | 301.0718 | 301.0714 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revelou, P.K.; Konteles, S.J.; Batrinou, A.; Xagoraris, M.; Tarantilis, P.A.; Strati, I.F. Origanum majorana L. As Flavoring Agent: Impact on Quality Indices, Stability, and Volatile and Phenolic Profiles of Extra Virgin Olive Oil (EVOO). Foods 2024, 13, 3164. https://doi.org/10.3390/foods13193164
Revelou PK, Konteles SJ, Batrinou A, Xagoraris M, Tarantilis PA, Strati IF. Origanum majorana L. As Flavoring Agent: Impact on Quality Indices, Stability, and Volatile and Phenolic Profiles of Extra Virgin Olive Oil (EVOO). Foods. 2024; 13(19):3164. https://doi.org/10.3390/foods13193164
Chicago/Turabian StyleRevelou, Panagiota Kyriaki, Spyridon J. Konteles, Anthimia Batrinou, Marinos Xagoraris, Petros A. Tarantilis, and Irini F. Strati. 2024. "Origanum majorana L. As Flavoring Agent: Impact on Quality Indices, Stability, and Volatile and Phenolic Profiles of Extra Virgin Olive Oil (EVOO)" Foods 13, no. 19: 3164. https://doi.org/10.3390/foods13193164









