Chemometric Discrimination of the Geographical Origin of Rheum tanguticum by Stable Isotope Analysis
Abstract
1. Introduction
2. Materials and Methods
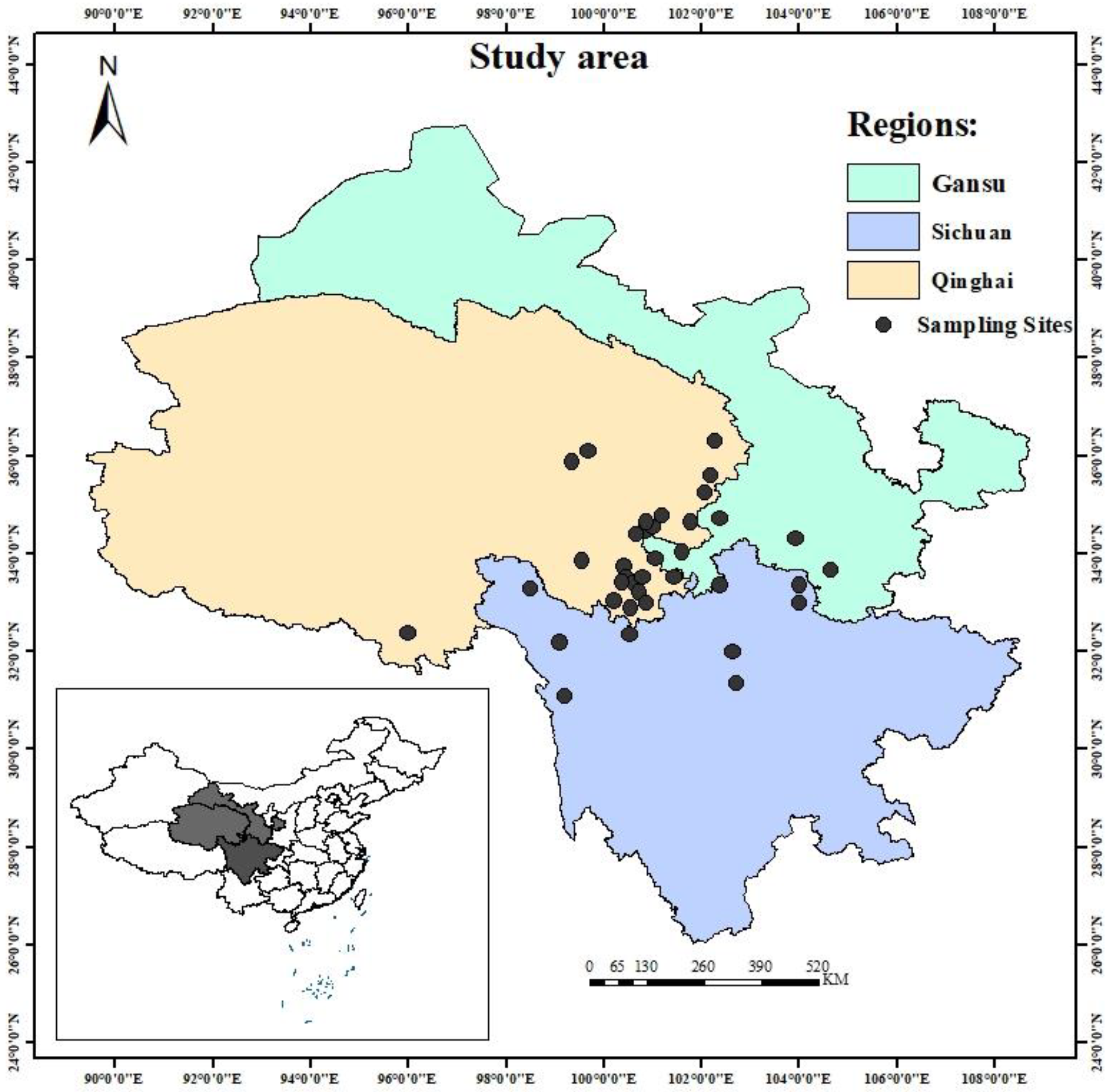
2.1. Sample Collection and Preparation
2.2. Stable Isotope Analysis
2.3. Statistical Analysis
3. Results and Discussion
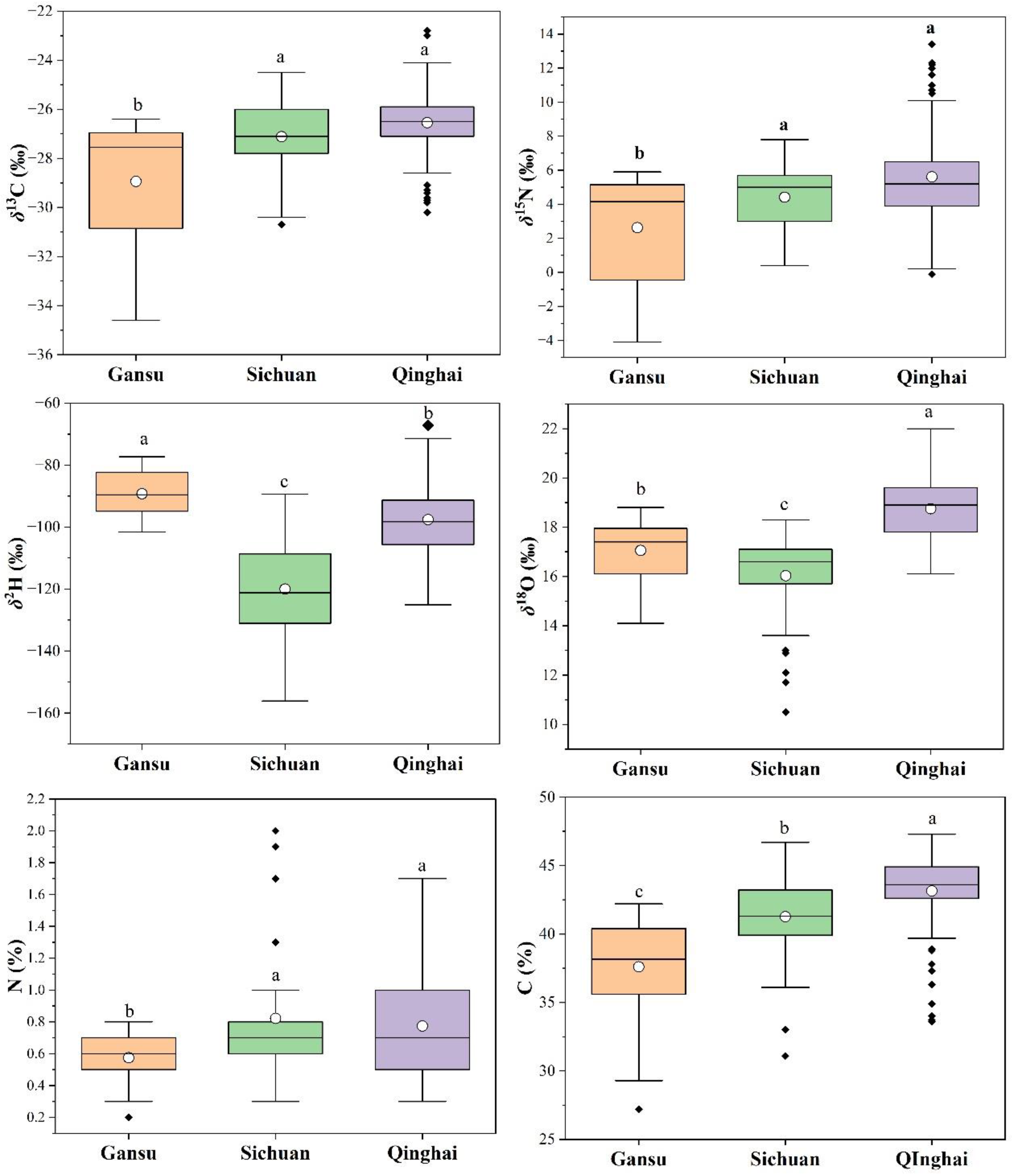
3.1. Stable Isotope Variations in Rhubarb Samples from Different Regions
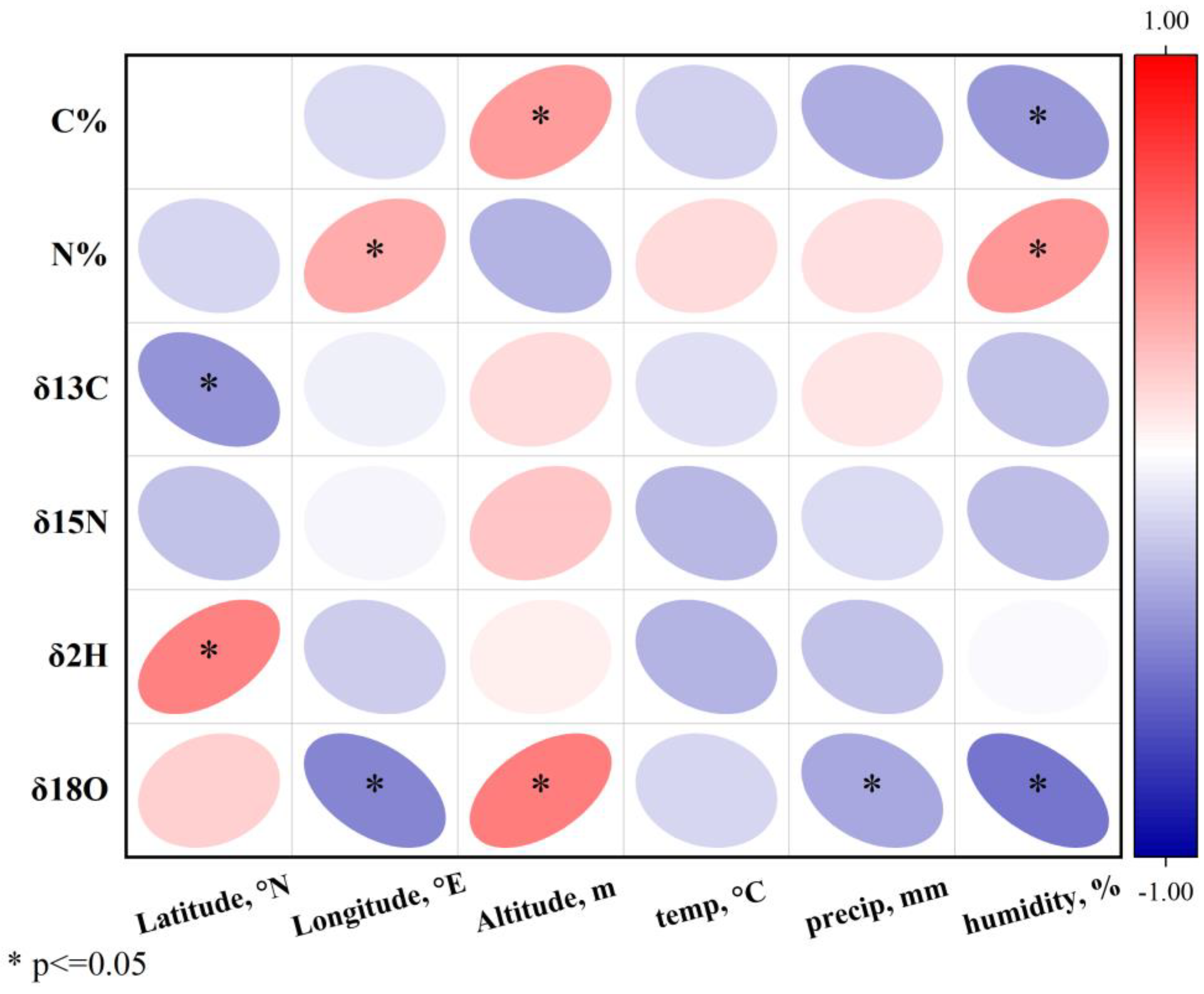
3.2. Correlation Analysis of Stable Isotopes with Environmental Factors
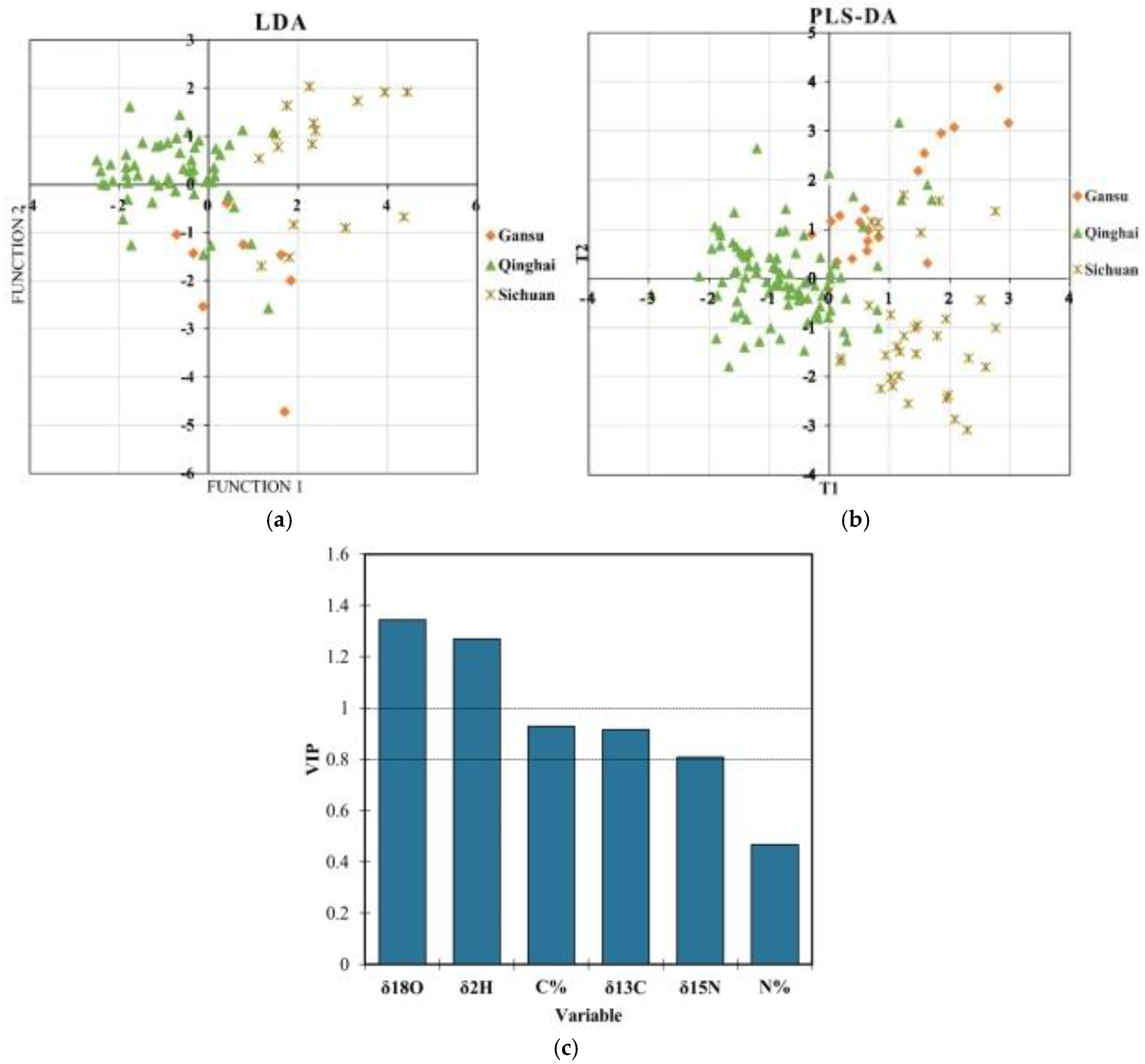
3.3. Geographical Origin Verification Based on Chemometric Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wu, H.; Lin, G. Progresses in applications of stable isotope technology to determining geographical origins of traditional Chinese medicines. J. Isot. 2015, 28, 225–232. [Google Scholar]
- Wang, X.; Yang, R.; Feng, S.; Hou, X.; Zhang, Y.; Li, Y.; Ren, Y. Genetic variation in Rheum palmatum and Rheum tanguticum (Polygonaceae), two medicinally and endemic species in China using ISSR markers. PLoS ONE 2012, 7, e51667. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Chen, Y.; Guo, F.; Cui, Y.; Wei, M.; Cheng, Z. Influence of different drying methods coupled with different process modes on physicochemical qualities and anthraquinones contents of Rheum palmatum L. LWT 2022, 170, 114021. [Google Scholar] [CrossRef]
- Li, J. Studies on Elements and Medicinal Constituents Dynamic Change Characteristics of Rheum tanguticum Maxim. ex Balf. from Qinghai-Plateau. Doctoral Thesis, University of Chinese Academy of Sciences, Beijing, China, 2010. [Google Scholar]
- Miraj, S. Therapeutic effects of Rheum palmatum L. (Dahuang): A systematic review. Der Pharma Chem. 2016, 8, 50–54. [Google Scholar]
- Wang, X.M.; Ren, Y. Rheum tanguticum, an endangered medicinal plant endemic to China. J. Med. Plants Res. 2009, 3, 1195–1203. [Google Scholar]
- Lu, C.; Wang, H.; Lv, W.; Xu, P.; Zhu, J.; Xie, J.; Liu, B.; Lou, Z. Antibacterial properties of anthraquinones extracted from rhubarb against Aeromonas hydrophila. Fish. Sci. 2011, 77, 375–384. [Google Scholar] [CrossRef]
- Choi, R.J.; Ngoc, T.M.; Bae, K.; Cho, H.J.; Kim, D.D.; Chun, J.; Khan, S.; Kim, Y.S. Anti-inflammatory properties of anthraquinones and their relationship with the regulation of P-glycoprotein function and expression. Eur. J. Pharm. Sci. 2013, 48, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Sun, M.; Salome-Abarca, L.F.; Wang, M.; Choi, Y.H. Investigation of species and environmental effects on rhubarb roots metabolome using (1)H NMR combined with high performance thin layer chromatography. Metabolomics 2018, 14, 137. [Google Scholar] [CrossRef]
- Komatsu, K.; Nagayama, Y.; Tanaka, K.; Ling, Y.; Basnet, P.; Meselhy, M.R. Development of a high performance liquid chromatographic method for systematic quantitative analysis of chemical constituents in rhubarb. Chem. Pharm. Bull. 2006, 54, 941–947. [Google Scholar] [CrossRef]
- Sun, M.; Li, L.; Wang, M.; van Wijk, E.; He, M.; van Wijk, R.; Koval, S.; Hankemeier, T.; van der Greef, J.; Wei, S. Effects of growth altitude on chemical constituents and delayed luminescence properties in medicinal rhubarb. J. Photochem. Photobiol. B Biol. 2016, 162, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Li, L.; Hu, H.; Li, Y.; Liu, C.; Wei, S. Influence of the Environmental Factors on the Accumulation of the Bioactive Ingredients in Chinese Rhubarb Products. PLoS ONE 2016, 11, e0154649. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Wang, M.; Zhang, H.J.; Liu, Y.N.; Ren, X.L.; Deng, Y.R.; Qi, A.D. Comprehensive analysis of Polygoni Multiflori Radix of different geographical origins using ultra-high-performance liquid chromatography fingerprints and multivariate chemometric methods. J. Food Drug Anal. 2018, 26, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Ma, S.; Liu, Z. Comparison and application of fluorescence EEMs and DRIFTS combined with chemometrics for tracing the geographical origin of Radix Astragali. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Drivelos, S.A.; Georgiou, C.A. Multi-element and multi-isotope-ratio analysis to determine the geographical origin of foods in the European Union. TrAC Trends Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Yu, D.-X.; Guo, S.; Yang, J.; Yan, H.; Zhang, Z.-Y.; Duan, J.-A. Application and prospect of stable isotope technology in tracing geographical origin of Chinese herbal medicines. China J. Chin. Mater. Medica 2022, 47, 862–871. [Google Scholar]
- Zhang, T.; Li, S.; Wang, Y.; Hu, Q.; Wang, C.; Yang, H.; Xu, N. Research progress in the application of stable isotope and mineral element analysis in tracing the geographical origin of Chinese medicinal materials. Food Sci. Technol. 2022, 42, e08222. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Wang, B.; Xiong, F.; Xiao, Y.; Zhou, G. Multivariate Statistical Analysis of Multiple Elements in Anisodus Tanguticus (Maxim.) Pascher from Different Regions to Determine Geographical Origins. Res. Sq. 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, Y.; Sun, Y.; Duan, Y. Elemental characteristics of Sanqi (Panax notoginseng) in Yunnan province of China: Multielement determination by ICP-AES and ICP-MS and statistical analysis. Microchem. J. 2019, 146, 931–939. [Google Scholar] [CrossRef]
- Bai, X.; Chen, H.; Long, W.; Lan, W.; Wang, S.; Lei, G.; Guan, Y.; Yang, J.; Fu, H. Accurate Traceability of Stable C, H, O, N Isotope Ratios and Multi-Element Analysis Combined with Chemometrics for Chrysanthemi Flos ‘Hangbaiju’ from Different Origins. Chemosensors 2022, 10, 529. [Google Scholar] [CrossRef]
- Fu, H.; Wei, L.; Chen, H.; Yang, X.; Kang, L.; Hao, Q.; Zhou, L.; Zhan, Z.; Liu, Z.; Yang, J.; et al. Combining stable C, N, O, H, Sr isotope and multi-element with chemometrics for identifying the geographical origins and farming patterns of Huangjing herb. J. Food Compos. Anal. 2021, 102, 103972. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, L.; Wang, Y.; Zhang, S.; Liu, P.; Dong, T.T.X.; Wu, Q.; Duan, J.-A. Comparative metagenomics analysis of the rhizosphere microbiota influence on Radix Angelica sinensis in different growth soil environments in China. Food Sci. Technol. 2021, 41, 775–784. [Google Scholar] [CrossRef]
- Pustjens, A.M.; Muilwijk, M.; Weesepoel, Y.; Van Ruth, S.M. Advances in Authenticity Testing of Geographical Origin of Food Products. In Advances in Food Authenticity Testing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 339–367. [Google Scholar]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Jiménez-Morillo, N.T.; Palma, V.; Garcia, R.; Pereira, J.A.; Dias, C.B.; Cabrita, M.J. Multivariate geostatistical analysis of stable isotopes in Portuguese varietal extra virgin olive oils. Microchem. J. 2020, 157, 105044. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17, 1031–1040. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Krauß, S.; Vieweg, A.; Vetter, W. Stable isotope signatures (delta(2) H-, delta(13) C-, delta(15) N-values) of walnuts (Juglans regia L.) from different regions in Germany. J. Sci. Food Agric. 2020, 100, 1625–1634. [Google Scholar] [CrossRef]
- Mihailova, A.; Abbado, D.; Kelly, S.D.; Pedentchouk, N. The impact of environmental factors on molecular and stable isotope compositions of n-alkanes in Mediterranean extra virgin olive oils. Food Chem. 2015, 173, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Wang, Y.; Zhang, J.; Xiao, S.; Li, H. Characteristics of Stable Isotopes in Precipitation and Their Moisture Sources in the Guanling Region, Guizhou Province. J. Chem. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Jimenez-Morillo, N.T.; Palma, V.; Garcia, R.; Dias, C.B.; Cabrita, M.J. Combination of Stable Isotope Analysis and Chemometrics to Discriminate Geoclimatically and Temporally the Virgin Olive Oils from Three Mediterranean Countries. Foods 2020, 9, 1855. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yao, T.; MacClune, K.; White, J.W.C.; Schilla, A.; Vaughn, B.; Vachon, R.; Ichiyanagi, K. Stable isotopic variations in west China: A consideration of moisture sources. J. Geophys. Res. Atmos. 2007, 112, 1–12. [Google Scholar] [CrossRef]
- Yang, F.; Ran, J.; Liu, H.; Song, J.; Xie, C. Geographic variation of functional components and the climatic response characteristics of Rheum tanguticum Maxim.ex Balf. Acta Ecol. Sin. 2021, 41, 3645–3655. [Google Scholar] [CrossRef]
- Xiong, F.; Nie, X.; Zhao, X.; Yang, L.; Zhou, G. Effect of plant density and harvest stage on yield and quality of Rheum tanguticum. Int. J. Agric. Biol. 2018, 20, 2408–2416. [Google Scholar]
- Xiong, F.; Nie, X.; Zhao, X.; Yang, L.; Zhou, G. Effects of different nitrogen fertilizer levels on growth and active compounds of rhubarb from Qinghai plateau. J. Sci. Food Agric. 2019, 99, 2874–2882. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Jones, H.G.; Archer, N. Plant Physiological Ecology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- O’Leary, M.H.; Madhavan, S.; Paneth, P. Physical and chemical basis of carbon isotope fractionation in plants. Plant Cell Environ. 2006, 15, 1099–1104. [Google Scholar] [CrossRef]
- Handley, L.L.; Scrimgeour, C.M. Terrestrial Plant Ecology and 15N Natural Abundance: The Present Limits to Interpretation for Uncultivated Systems with Original Data from a Scottish Old Field. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: Cambridge, MA, USA, 1997; Volume 27, pp. 133–212. [Google Scholar]
- Rozanski, K.; Araguás-Araguás, L.; Gonfiantini, R. Isotopic Patterns in Modern Global Precipitation. In Climate Change in Continental Isotopic Records; Geophysical Monograph Series; American Geophysical Union: Washington, DC, USA, 2013; pp. 1–36. [Google Scholar]
- Tarapoulouzi, M.; Skiada, V.; Agriopoulou, S.; Psomiadis, D.; Rebufa, C.; Roussos, S.; Theocharis, C.R.; Katsaris, P.; Varzakas, T. Chemometric Discrimination of the Geographical Origin of Three Greek Cultivars of Olive Oils by Stable Isotope Ratio Analysis. Foods 2021, 10, 336. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Q.; Guo, M.; Wang, J.; Kou, R.; Ye, L. Comparative study of the Rheum tanguticum’s chemical contents based on spatial distribution characteristics. PLoS ONE 2022, 17, e0278113. [Google Scholar] [CrossRef] [PubMed]
- Laursen, K.H.; Schjoerring, J.K.; Kelly, S.D.; Husted, S. Authentication of organically grown plants—Advantages and limitations of atomic spectroscopy for multi-element and stable isotope analysis. TrAC Trends Anal. Chem. 2014, 59, 73–82. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wadood, S.A.; Xia, Y.; Liu, Y.; Guo, H.; Guo, B.L.; Gan, R.Y. Wheat authentication: An overview on different techniques and chemometric methods. Crit. Rev. Food Sci. Nutr. 2021, 63, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Maione, C.; Barbosa, R.M. Recent applications of multivariate data analysis methods in the authentication of rice and the most analyzed parameters: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xiong, F.; Wang, L.; Wang, B.; Chen, K.; Chen, C.; Zhou, G. Study on the quality characteristics and geographical origin authentication of wild Rheum tanguticum in three authentic regions. J. Food Compos. Anal. 2023, 123, 105463. [Google Scholar] [CrossRef]
| LDA | Regions | Predicted | Total | ||
|---|---|---|---|---|---|
| Gansu | Sichuan | Qinghai | |||
| Training | Gansu | 15 | 0 | 1 | 16 |
| Sichuan | 3 | 25 | 0 | 28 | |
| Qinghai | 9 | 2 | 90 | 101 | |
| %, correct | 93.8 | 89.3 | 89.1 | 89.7 | |
| Validation | Gansu | 3 | 0 | 1 | 4 |
| Sichuan | 2 | 12 | 3 | 17 | |
| Qinghai | 2 | 0 | 22 | 24 | |
| %, correct | 75.0 | 70.6 | 91.7 | 82.2 | |
| k-NN | |||||
| Training | Gansu | 13 | 0 | 4 | 17 |
| Sichuan | 1 | 30 | 4 | 35 | |
| Qinghai | 1 | 2 | 90 | 93 | |
| %, correct | 76.5 | 85.7 | 96.8 | 91.7 | |
| Validation | Gansu | 3 | 0 | 0 | 3 |
| Sichuan | 0 | 6 | 4 | 10 | |
| Qinghai | 0 | 2 | 30 | 32 | |
| %, correct | 100.0 | 60.0 | 93.8 | 86.7 | |
| PLS-DA | |||||
| Training | Gansu | 6 | 1 | 11 | 18 |
| Sichuan | 2 | 26 | 6 | 34 | |
| Qinghai | 4 | 2 | 87 | 93 | |
| %, correct | 33.3 | 76.5 | 93.6 | 82.1 | |
| Validation | Gansu | 1 | 0 | 1 | 2 |
| Sichuan | 1 | 8 | 2 | 11 | |
| Qinghai | 0 | 0 | 32 | 32 | |
| %, correct | 50.0 | 72.7 | 100.0 | 91.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuralykyzy, B.; Nie, J.; Zhou, G.; Mei, H.; Zhao, S.; Li, C.; M. Rogers, K.; Zhang, Y.; Yuan, Y. Chemometric Discrimination of the Geographical Origin of Rheum tanguticum by Stable Isotope Analysis. Foods 2024, 13, 3176. https://doi.org/10.3390/foods13193176
Nuralykyzy B, Nie J, Zhou G, Mei H, Zhao S, Li C, M. Rogers K, Zhang Y, Yuan Y. Chemometric Discrimination of the Geographical Origin of Rheum tanguticum by Stable Isotope Analysis. Foods. 2024; 13(19):3176. https://doi.org/10.3390/foods13193176
Chicago/Turabian StyleNuralykyzy, Bayan, Jing Nie, Guoying Zhou, Hanyi Mei, Shuo Zhao, Chunlin Li, Karyne M. Rogers, Yongzhi Zhang, and Yuwei Yuan. 2024. "Chemometric Discrimination of the Geographical Origin of Rheum tanguticum by Stable Isotope Analysis" Foods 13, no. 19: 3176. https://doi.org/10.3390/foods13193176
APA StyleNuralykyzy, B., Nie, J., Zhou, G., Mei, H., Zhao, S., Li, C., M. Rogers, K., Zhang, Y., & Yuan, Y. (2024). Chemometric Discrimination of the Geographical Origin of Rheum tanguticum by Stable Isotope Analysis. Foods, 13(19), 3176. https://doi.org/10.3390/foods13193176





