Physicochemical Characterization and Thermodynamic Analysis of Avocado Oil Enhanced with Haematococcus pluvialis Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil Extraction and Physicochemical Characterization
2.3. Fatty acid Composition
2.4. Extract of H. pluvialis
2.5. Free Astaxanthin of H. pluvialis Extract by HPLC
2.6. Antiradical Activity
2.7. Stability Cold-Pressed Avocado Oil with H. pluvialis Extract
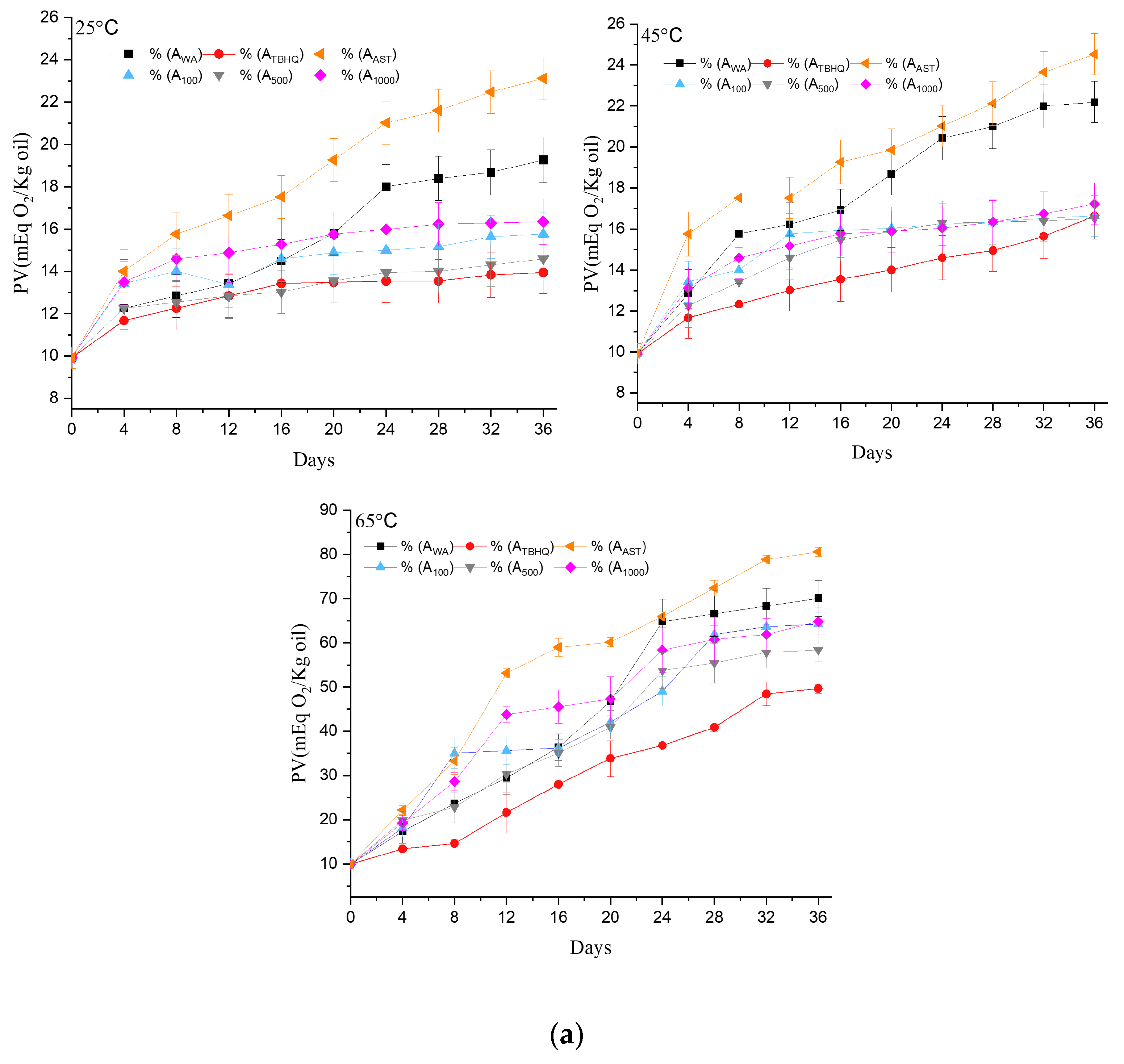
2.8. Peroxide Value
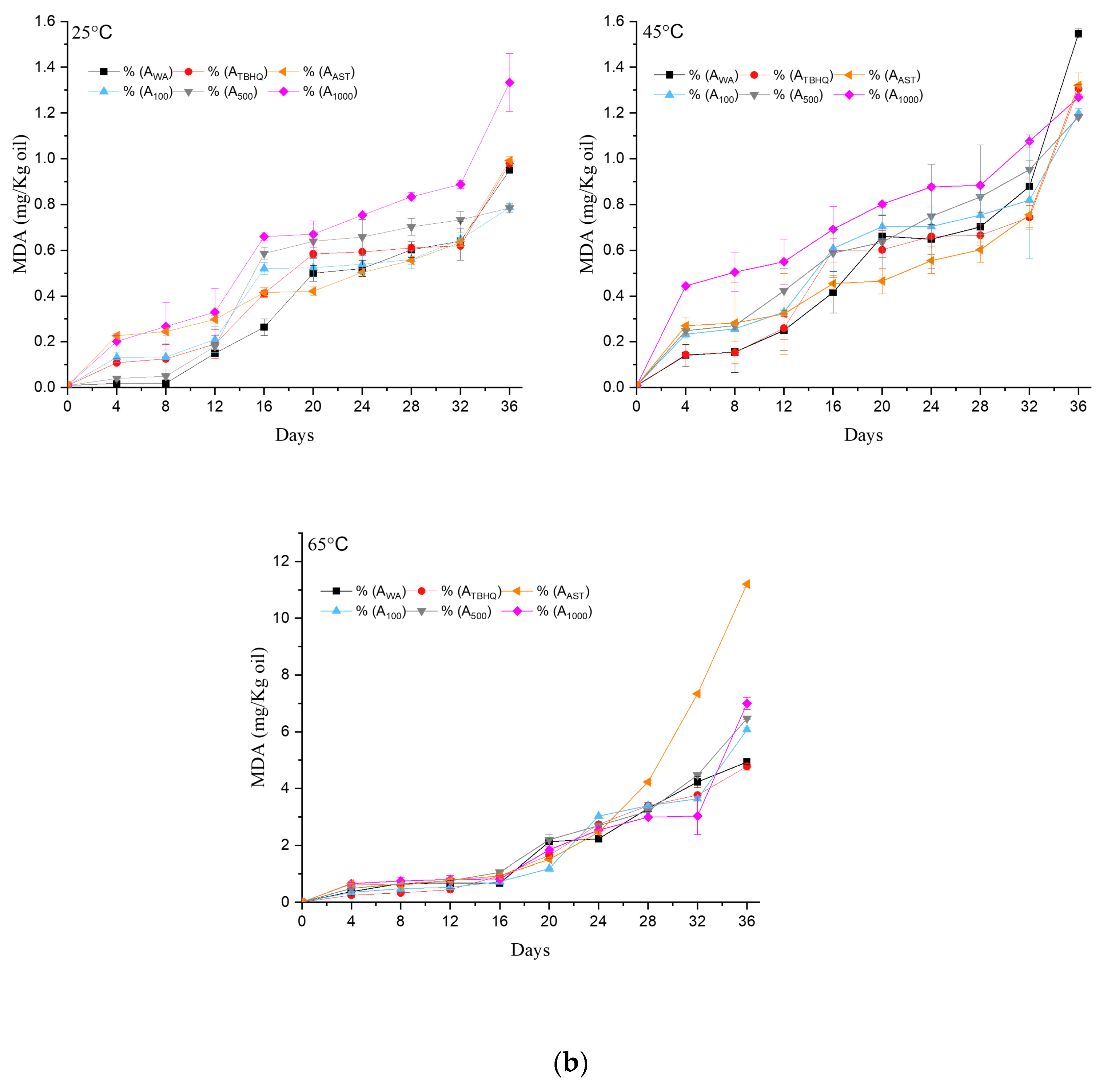
2.9. Thiobarbituric Acid Value (TBARS Method)
2.10. Rate Peroxide and Malondialdehyde
2.11. Thermodynamic Study
2.12. Statistical Analysis
3. Results and Discussion
3.1. Avocado Oil
3.1.1. Physicochemical Characterization
3.1.2. Fatty Acid Composition in Avocado Oil
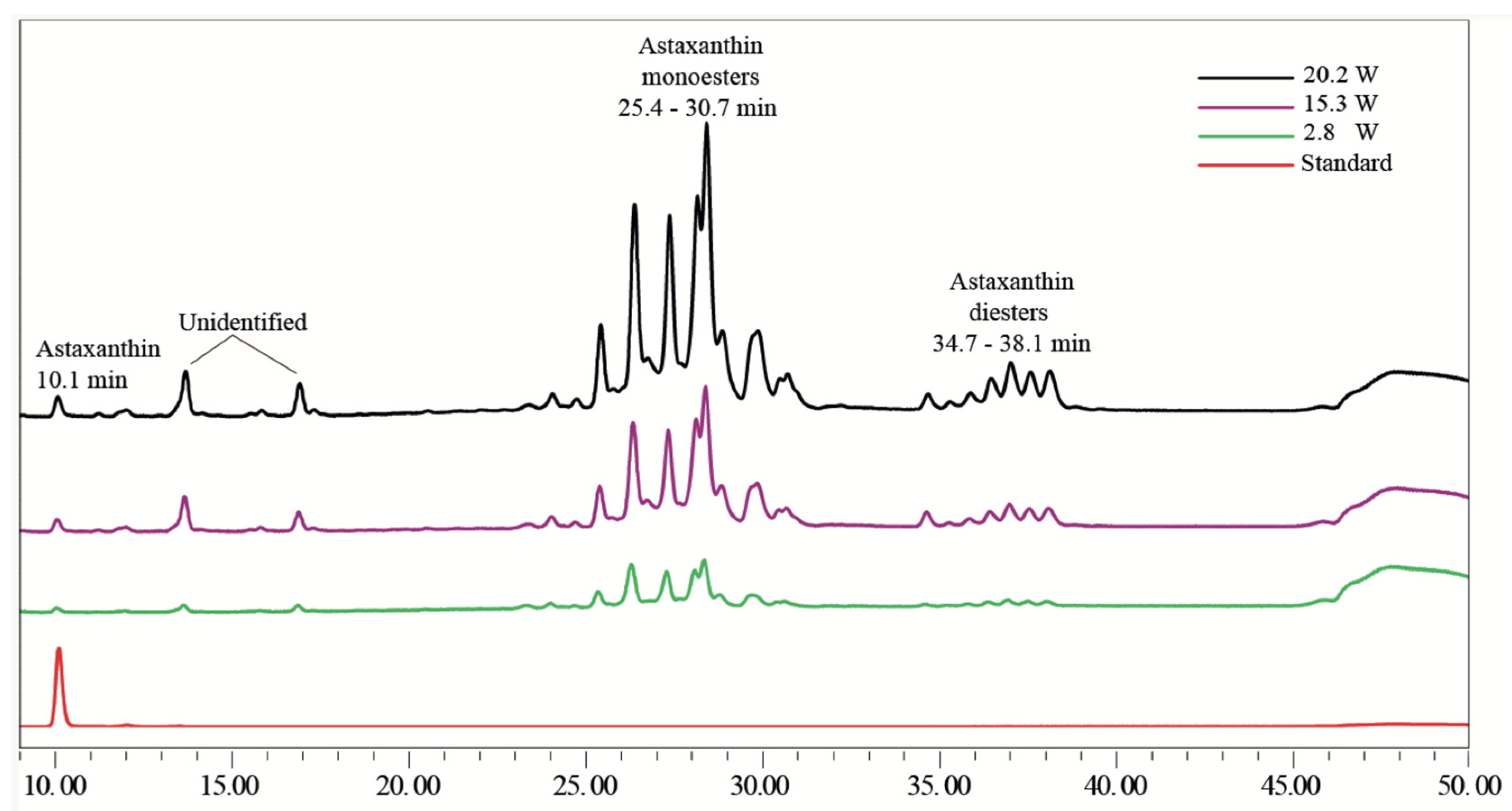
3.1.3. H. pluvialis Extract Characterization and HPLC
3.1.4. Avocado Oil with Haematococcus pluvialis Extract
3.1.5. Kinetics and Thermodynamics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flores, M.; Saravia, C.; Vergara, C.E.; Avila, F.; Valdés, H.; Ortiz-Viedna, J. Avocado Oil: Characteristics, Properties, and Applications. Molecules 2019, 24, 2172. [Google Scholar] [CrossRef] [PubMed]
- Forero-Doria, O.; García, M.F.; Vergara, C.E.; Guzman, L. Thermal Analysis and Antioxidant Activity of Oil Extracted from Pulp of Ripe Avocados. J. Therm. Anal. Calorim. 2017, 130, 959–966. [Google Scholar] [CrossRef]
- Resende, L.M.B.; de Souza, V.R.; Ferreira, G.M.D.; Nunes, C.A. Changes in Quality and Phytochemical Contents of Avocado Oil under Different Temperatures. J. Food Sci. Technol. 2019, 56, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic Phenolic Antioxidants: Metabolism, Hazards and Mechanism of Action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, Occurrence, Toxicity and Environmental Health Risks of Synthetic Phenolic Antioxidants: A Review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Carbajal-Sánchez, J.A.; Ramírez-Durán, N.; Gamboa-Angulo, M.; Moreno-Pérez, P.A. Estado de La Información del Consumo en México de Antioxidantes Sintéticos en Alimentos Ultra-Procesados, Basados en los Productos de la Canasta Básica. Estud. Sociales. Rev. Aliment. Contemp. Desarro. Reg. 2021, 31, 58. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and Modification of the Carcinogenic Response by Bha, Bht, and Other Antioxidants. Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Roy, S.; Deshmukh, R.K.; Tripathi, S.; Gaikwad, K.K.; Das, S.S.; Sharma, D. Recent Advances in the Carotenoids Added to Food Packaging Films: A Review. Foods 2023, 12, 4011. [Google Scholar] [CrossRef]
- Todorović, B.; Jaša Grujić, V.; Krajnc, A.U.; Kranvogl, R.; Ambrožic-Dolin, J. Identification and Content of Astaxanthin and Its Esters from Microalgae Haematococcus pluvialis by HPLC-DAD and LC-QTOF-MS after Extraction with Various Solvents. Plants 2021, 10, 2413. [Google Scholar] [CrossRef]
- Camacho Kurmen, J.E.; González, G.; Klotz, B. Producción de Astaxantina En Haematococcus pluvialis Bajo Diferentes Condiciones de Estrés. Nova 2013, 11, 93–104. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Cerecedo-Cruz, L.; Azuara-Nieto, E.; Hernández-Álvarez, A.J.; González-González, C.R.; Melgar-Lalanne, G. Evaluation of the Oxidative Stability of Chipotle Chili (Capsicum annuum L.) Oleoresins in Avocado Oil. Grasas Y Aceites 2018, 69, e240. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Fereidoon, S.; Ying, Z. Lipid Oxidation and Improving the Oxidative Stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Calvo, N.S.; Reynoso, C.M.; Resnik, S.; Cortés-Jacinto, E.; Collins, P. Thermal Stability of Astaxanthin in Oils for Its Use in Fish Food Technology. Anim. Feed Sci. Technol. 2020, 270, 114668. [Google Scholar] [CrossRef]
- Singh, P.K.; Chopra, R.; Garg, M. Comparative Study on the Use of Rosemary Bioactive for Enhancing the Oxidative Stability of Blended Perilla Seed Oil: A Multivariant Kinetic Approach. Food Chem. Adv. 2023, 3, 100447. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Xu, Z.; Liu, R.; Zhang, H.; Wang, X.; Huang, J.; Jin, Q. Comparison of the Characteristics and Oxidation Kinetic Parameters of Flaxseed (Linum usitatissimum L.) Oil Products with Different Refining Degree. J. Food Process. Preserv. 2020, 44, e14753. [Google Scholar] [CrossRef]
- Cheung, S.C.M.; Szeto, Y.T.; Benzie, I.F.F. Antioxidant Protection of Edible Oils. Plant Foods Hum. Nutr. 2007, 62, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.D.; White, P.J. Adaptation of the AOCS Official Method for Measuring Hydroperoxides from Small-Scale Oil Samples. J. Am. Oil Chem. Soc. 2001, 78, 1267–1269. [Google Scholar] [CrossRef]
- ISO 660:2009; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2009.
- AOCS. Sampling and Analysis of Commercial Fats and Oils, 5th ed.; Official Methods and Recommended Practices of the American Oil Chemists Society; AOCS: Urbana, IL, USA, 1998; pp. 7–25. [Google Scholar]
- Rozema, B.; Mitchell, B.; Winters, D.; Kohn, A.; Sullivan, D.; Meinholz, E. Proposed Modifications to AOAC 996.06, Optimizing the Determination of Trans Fatty Acids: Presentation of Data. J. AOAC Int. 2008, 91, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Ruen-Ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of Extraction Methods for Recovery of Astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2011, 46, 64–70. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Fu, L.; Zhu, H.; Zhang, B. Effect of Extraction and Drying Methods on Antioxidant Activity of Astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 197–203. [Google Scholar] [CrossRef]
- Orosa, M.; Franqueira, D.; Cid, A.; Abalde, J. Analysis and Enhancement of Astaxanthin Accumulation in Haematococcus pluvialis. Bioresour. Technol. 2005, 96, 373–378. [Google Scholar] [CrossRef]
- Minioti, K.S.; Georgiou, C.A. Comparison of Different Tests Used in Mapping the Greek Virgin Olive Oil Production for the Determination of Its Total Antioxidant Capacity. Grasas Aceites 2010, 61, 45–51. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS Assays to Assess the Antioxidant Profile of Untreated Oils. Food Anal. Methods 2015, 8, 1294–1302. [Google Scholar] [CrossRef]
- Guzmán-Díaz, D.A.; Treviño-Garza, M.Z.; Rodríguez-Romero, B.A.; Gallardo-Rivera, C.T.; Amaya-Guerra, C.A.; Báez-González, J.G. Development and Characterization of Gelled Double Emulsions Based on Chia (Salvia hispanica L.) Mucilage Mixed with Different Biopolymers and Loaded with Green Tea Extract (Camellia sinensis). Foods 2019, 8, 677. [Google Scholar] [CrossRef]
- Poyato, C.; Navarro-Blasco, I.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Oxidative Stability of O/W and W/O/W Emulsions: Effect of Lipid Composition and Antioxidant Polarity. Food Res. Int. 2013, 51, 132–140. [Google Scholar] [CrossRef]
- Piedrahita, A.M.; Peñaloza, J.; Cogollo, Á.; Rojano, B.A. Kinetic Study of the Oxidative Degradation of Choibá Oil (Dipteryx oleifera Benth.) with Addition of Rosemary Extract (Rosmarinus officinalis L.). Food Nutr. Sci. 2015, 6, 466–479. [Google Scholar] [CrossRef]
- Gülmez, Ö.; Şahin, S. Evaluation of Oxidative Stability in Hazelnut Oil Treated with Several Antioxidants: Kinetics and Thermodynamics Studies. LWT 2019, 111, 478–483. [Google Scholar] [CrossRef]
- NMX-F-811-SCFI-2021; Aceites y Grasas—Aceite de Aguacate—Especificaciones (Cancela a La NMX-F-052-SCFI-2008). Secretaría de Economía Direccion General de Normas: Mexico City, Mexico, 2021.
- FAO/WHO. Codex Stan 210-1999: Codex Standard for Named Vegetable Oils; FAO/WHO: Rome, Italy, 1999.
- Kilic Buyukkurt, O. Characterization of Aroma Compounds of Cold-Pressed Avocado Oil Using Solid-Phase Microextraction Techniques with Gas Chromatography–Mass Spectrometry. Food Anal. Group 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-Pressed Oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef]
- Santana, I.; Castelo-Branco, V.N.; Guimarães, B.M.; de Oliveira Silva, L.; Peixoto, V.O.D.S.; Cabral, L.M.C.; Freitas, S.P.; Torres, A.G. Hass Avocado (Persea americana Mill.) Oil Enriched in Phenolic Compounds and Tocopherols by Expeller-Pressing the Unpeeled Microwave Dried Fruit. Food Chem. 2019, 286, 354–361. [Google Scholar] [CrossRef]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasarán, I. Stability of Avocado Oil during Heating: Comparative Study to Olive Oil. Food Chem. 2012, 132, 439–446. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Yan, B.; Yao, X. Supercritical Fluid Extraction of Astaxanthin from Haematococcus pluvialis and Its Antioxidant Potential in Sunflower Oil. Innov. Food Sci. Emerg. Technol. 2012, 13, 120–127. [Google Scholar] [CrossRef]
- Desai, R.K.; Streefland, M.; Wijffels, R.H.; Eppink, M.H.M. Novel Astaxanthin Extraction from Haematococcus pluvialis Using Cell Permeabilising Ionic Liquids. Green Chem. 2016, 18, 1261–1267. [Google Scholar] [CrossRef]
- Nemani, N.; Dehnavi, S.M.; Pazuki, G. Extraction and Separation of Astaxanthin with the Help of Pre-Treatment of Haematococcus pluvialis Microalgae Biomass Using Aqueous Two-Phase Systems Based on Deep Eutectic Solvents. Sci. Rep. 2024, 14, 5420. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Espinosa, C.; Paredes, A.; Palma, J.; Jaime, C.; Vílchez, C.; Cerezal, P. Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants. Molecules 2019, 24, 4073. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, Y.Y.; Ma, H.L.; Wang, Z.B. Ultrasonic Effects on the Degradation Kinetics, Preliminary Characterization and Antioxidant Activities of Polysaccharides from Phellinus Linteus Mycelia. Ultrason. Sonochem. 2016, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Annegowda, H.V.; Anwar, L.N.; Mordi, M.N.; Ramanathan, S.; Mansor, S.M. Influence of Sonication on the Phenolic Content and Antioxidant Activity of Terminalia catappa L. Leaves. Pharmacogn. Res. 2010, 2, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Castillo, A.; Pereira, S.; Otero, A.; Garcia-Jares, C.; Lores, M. Multicomponent Bioactive Extract from Red Stage Haematococcus pluvialis Wet Paste: Avoiding the Drying Step and Toxic Solvents. J. Appl. Phycol. 2022, 34, 1537–1553. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant Potential of Microalgae in Relation to Their Phenolic and Carotenoid Content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Lipid Peroxidation Triggers Neurodegeneration: A Redox Proteomics View into the Alzheimer Disease Brain. Free Radic. Biol. Med. 2013, 62, 157–169. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estevez, M. Avocado (Persea americana Mill.) Phenolics, in Vitro Antioxidant and Antimicrobial Activities, and Inhibition of Lipid and Protein Oxidation in Porcine Patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef]
- Nair, A.; Ahirwar, A.; Singh, S.; Lodhi, R.; Lodhi, A.; Rai, A.; Jadhav, D.A.; Harish, D.A.; Varjani, S.; Singh, G.; et al. Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties. Mar. Drugs 2023, 21, 176. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, J.; Yang, L.; Gu, C.; Xue, C. Thermal Stability and Oral Absorbability of Astaxanthin Esters from Haematococcus pluvialis in Balb/c Mice. J. Sci. Food Agric. 2019, 99, 3662–3671. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, X.; Gu, J.; Li, X.; Yunrui, C.; Jie, X.; Changlu, X. Influence of Molecular Structure of Astaxanthin Esters on Their Stability and Bioavailability. Food Chem. 2020, 343, 128497. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Sathivel, S. Kinetics of Lipid Oxidation and Degradation of Flaxseed Oil Containing Crawfish (Procambarus clarkii) Astaxanthin. J. Am. Oil Chem. Soc. 2011, 88, 595–601. [Google Scholar] [CrossRef]
- Espinaco, B.Y.; Niizawa, I.; Marino, F.; Zorrilla, S.E.; Sihufe, G.A. Storage Stability of Chia (Salvia hispanica L.) Oil Incorporated with Astaxanthin. J. Food Process. Preserv. 2021, 45, e15184. [Google Scholar] [CrossRef]
- Ambati Ranga, R.; Ravi, S.; Gokare Aswathanarayana, R. Stabilization of Astaxanthin in Edible Oils and Its Use as an Antioxidant. J. Sci. Food Agric. 2007, 87, 957–965. [Google Scholar] [CrossRef]
- Vandemoortele, A.; Heynderickx, P.M.; Leloup, L.; De Meulenaer, B. Kinetic Modeling of Malondialdehyde Reactivity in Oil to Simulate Actual Malondialdehyde Formation upon Lipid Oxidation. Food Res. Int. 2021, 140, 110063. [Google Scholar] [CrossRef]
- Frankel, E.N.; Neff, W.E. Formation of Malonaldehyde from Lipid Oxidation Products. Biochim. Et Biophys. Acta (BBA)/Lipids Lipid Metab. 1983, 754, 264–270. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Formation of Hydroperoxy- and Hydroxyalkenals during Thermal Oxidative Degradation of Sesame Oil Monitored by Proton NMR. Eur. J. Lipid Sci. Technol. 2004, 106, 680–687. [Google Scholar] [CrossRef]
- Yeşilsu, A.F.; Özyurt, G. Oxidative Stability of Microencapsulated Fish Oil with Rosemary, Thyme and Laurel Extracts: A Kinetic Assessment. J. Food Eng. 2019, 240, 171–182. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, Y.P.; Kim, H.S. Effect of Natural Antioxidants on the Lipid Oxidation of Microencapsulated Seed Oil. Food Control 2012, 23, 528–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, C.; Meng, X.; Dong, W.; Guo, H.; Su, C.; Zhang, X. Effect of Storage Condition on Oil Oxidation of Flat-European Hybrid Hazelnut. J. Oleo Sci. 2019, 68, 939–950. [Google Scholar] [CrossRef]
- Conte, L.; Milani, A.; Calligaris, S.; Rovellini, P.; Lucci, P.; Nicoli, M.C. Temperature Dependence of Oxidation Kinetics of Extra Virgin Olive Oil (EVOO) and Shelf-Life Prediction. Foods 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Alonso, S.; Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Oxidation Kinetics in Olive Oil Triacylglycerols under Accelerated Shelf-Life Testing (25–75 °C). Eur. J. Lipid Sci. Technol. 2004, 106, 369–375. [Google Scholar] [CrossRef]
- Villanueva, E.; Rodríguez, G.; Aguirre, E.; Castro, V. Influence of Antioxidants on Oxidative Stability of the Oil Chia (Salvia hispanica L.) by Rancimat. Sci. Agropecu. 2017, 8, 19–27. [Google Scholar] [CrossRef]
| Total Fatty Acids | Fatty Acid | Relative Percentage |
|---|---|---|
| Polyunsaturated fatty acids | ||
| Linoleic acid | 11.00 ± 0.35 | |
| Monounsaturated fatty acids | 71.80 | |
| Palmitoleic acid | 14.25 ± 0.17 | |
| Oleic acid | 48.10 ± 0.11 | |
| Vaccenic acid | 9.54 ± 0.21 | |
| Saturated fatty acid | ||
| Palmitic acid | 17.20 ± 0.34 |
| Power (W) | Free Astaxanthin (mg g−1) | DPPH (μmol eq. Trolox g−1 DW) | ABTS (μmol eq. Trolox g−1 DW) |
|---|---|---|---|
| 2.80 | 3.53 ± 0.07 a | 3.50 ± 0.64 a | 4.95 ± 0.04 a |
| 15.3 | 4.71 ± 0.03 b | 6.21 ± 0.91 b | 6.21 ± 0.59 b |
| 20.2 | 8.26 ± 2.42 c | 6.81 ± 1.45 b | 7.30 ± 0.26 c |
| Kinetics and Thermodynamics | Oil Samples | |||||
|---|---|---|---|---|---|---|
| AWA | ATBHQ | A100 | A500 | A1000 | AAST | |
| (kJ mol−1) | 37.84 | 50.51 | 49.22 | 51.72 | 46.89 | 33.01 |
| (kJ mol−1 K−1) | −157.68 | −123.83 | −126.80 | −119.13 | −133.56 | −172.04 |
| 25 °C (kJ mol−1) | 84.83 | 87.41 | 87.01 | 87.22 | 86.70 | 84.27 |
| 45 °C (kJ mol−1) | 87.98 | 89.89 | 89.54 | 89.60 | 89.37 | 87.72 |
| 65 °C (kJ mol−1) | 91.13 | 92.36 | 92.08 | 91.99 | 92.04 | 91.16 |
| Ea (Kj mol−1) | 40.47 | 53.14 | 51.85 | 54.35 | 49.53 | 35.64 |
| k (meq O2 kg−1 oil h−1) 25 °C | 10.84 × 10−3 | 3.78 × 10−3 | 4.99 × 10−3 | 4.26 × 10−3 | 5.64 × 10−3 | 14.02 × 10−3 |
| k (meq O2 kg−1 oil h−1) 45 °C | 13.57 × 10−3 | 6.72 × 10−3 | 6.01 × 10−3 | 6.84 × 10−3 | 6.46 × 10−3 | 13.93 × 10−3 |
| k (meq O2 kg−1 oil h−1) 65 °C | 77.59 × 10−3 | 49.58 × 10−3 | 62.37 × 10−3 | 59.42 × 10−3 | 63.03 × 10−3 | 80.02 × 10−3 |
| R2 | 0.81 | 0.88 | 0.78 | 0.85 | 0.76 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Báez-González, J.G.; Gallegos-Garza, M.M.; Gallardo-Rivera, C.T.; Treviño-Garza, M.Z.; Amaya-Guerra, C.A.; Rodríguez-Rodríguez, J.; Obregón-Solís, E.; García-Márquez, E. Physicochemical Characterization and Thermodynamic Analysis of Avocado Oil Enhanced with Haematococcus pluvialis Extract. Foods 2024, 13, 3184. https://doi.org/10.3390/foods13193184
Báez-González JG, Gallegos-Garza MM, Gallardo-Rivera CT, Treviño-Garza MZ, Amaya-Guerra CA, Rodríguez-Rodríguez J, Obregón-Solís E, García-Márquez E. Physicochemical Characterization and Thermodynamic Analysis of Avocado Oil Enhanced with Haematococcus pluvialis Extract. Foods. 2024; 13(19):3184. https://doi.org/10.3390/foods13193184
Chicago/Turabian StyleBáez-González, Juan G., Melissa M. Gallegos-Garza, Claudia T. Gallardo-Rivera, Mayra Z. Treviño-Garza, Carlos A. Amaya-Guerra, José Rodríguez-Rodríguez, Efraín Obregón-Solís, and Eristeo García-Márquez. 2024. "Physicochemical Characterization and Thermodynamic Analysis of Avocado Oil Enhanced with Haematococcus pluvialis Extract" Foods 13, no. 19: 3184. https://doi.org/10.3390/foods13193184







