Assessment of Fungal and Contamination of Ochratoxin A and Patulin in Foods Susceptible to Contamination in the Yangzhou Market, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Standards
2.2. Sampling Collection and Preparation
2.3. Extraction of OTA from the Samples
2.4. Determination of OTA by Using HPLC
2.5. Extraction of PAT from the Samples
2.6. Determination of PAT by Using HPLC
2.7. Fungal Isolation and Purification
2.8. Identification of Fungi by Morphological Observation
2.9. Identification of Fungi by Using ITS Sequence
2.10. Statistical Analysis
3. Results
3.1. OTA Contamination in the Cereal, Nut, Coffee, and Wine Food Samples
3.2. PAT Contamination in the Fruits and Fruit Derivate Foods
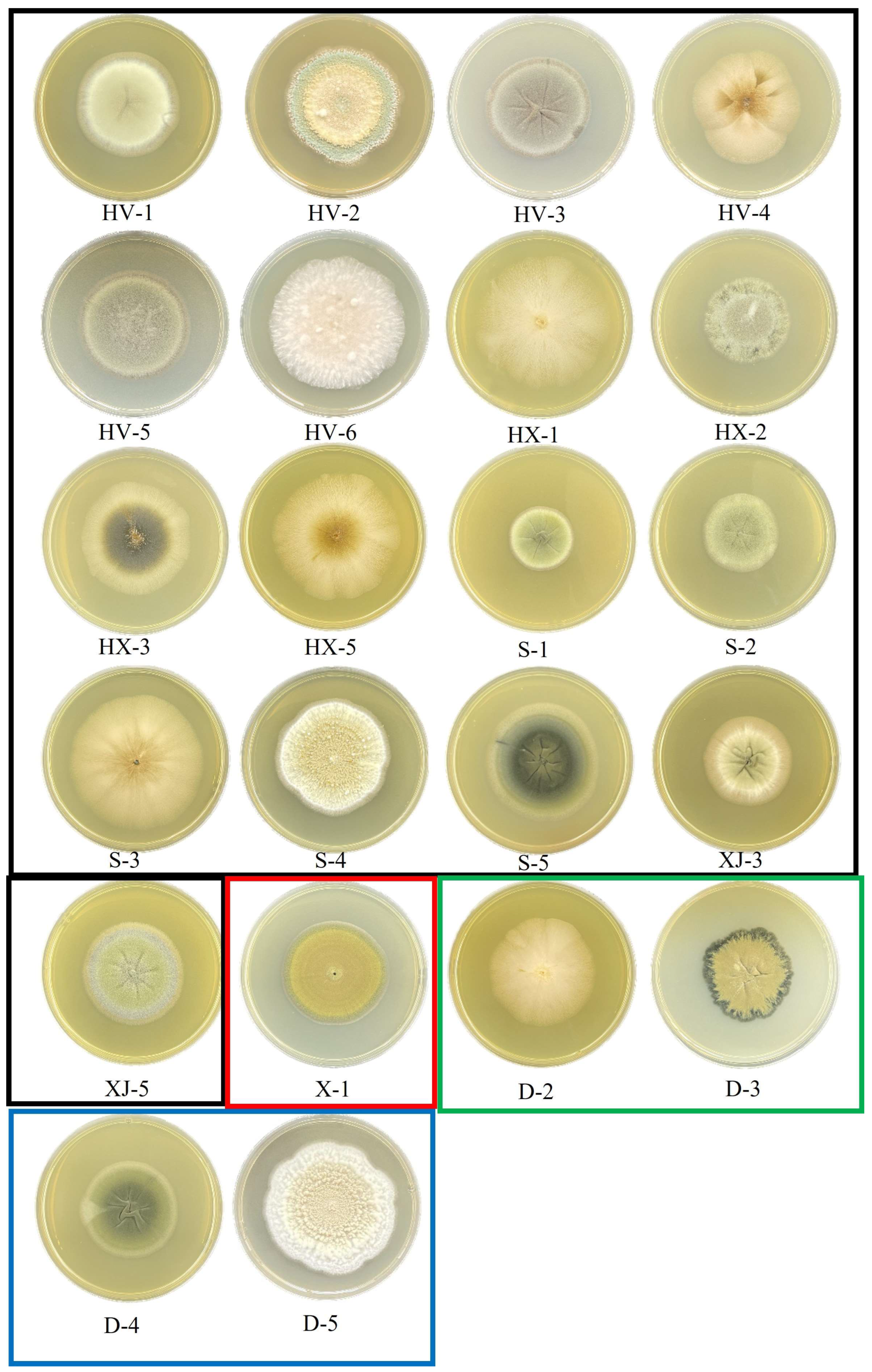
3.3. Fungal in the Cereal, Nut, Coffee and Wine Food Samples
3.4. Fungal Diversity in Apples, Hawthorn, Pears, and Their Derivatives
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amezqueta, S.; Schorr-Galindo, S.; Murillo-Arbizu, M.; Gonzalez-Penas, E.; Lopez de Cerain, A.; Guiraud, J.P. OTA-producing fungi in foodstuffs: A review. Food Control 2012, 26, 259–268. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 1192. [Google Scholar] [CrossRef]
- IARC monographs on the evaluation of carcinogenic risk to humans, Vol. 56, Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. Anal. Chim. Acta 1993, 294, 341.
- European Commission Regulation. Setting Maxi-Mum Levels for Certain Contaminants in Foodstuffs; European Union: Luxembourg, 2006; Volume 364, pp. 5–24, No. 1881/2006 of 19 December 2006.
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in roasted coffee from french supermarkets and transfer in coffee beverages: Comparison of analysis methods. Toxins 2010, 2, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Codex Stand. Codex General Standard for Contaminants and Toxins in Food and Feed; FAO: Rome, Italy, 1995; pp. 31–32. [Google Scholar]
- GB 2761; China National Standard for Food Safety: Limits of Mycotoxins in Food. Standards Press of China: Beijing, China, 2017.
- European Commission 2023. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. OJEU. 119:103–157. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 24 April 2023).
- van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef]
- Amezqueta, S.; Gonzalez-Penas, E.; Murillo-Arbizu, M.; de Cerain, A.L. Ochratoxin A decontamination: A review. Food Control 2009, 20, 326–333. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Ma, Z.; Zhang, Q.; Li, H. The occurrence and contamination level of ochratoxin a in plant and animal-derived food commodities. Molecules 2021, 26, 6928. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.C.; Pena, A.; Lino, C.M. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010, 27, 187–198. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants. Food and Agriculture Organization of the United Nations, World Health Organization. Available online: http://apps.who.int/iris/bitstream/handle/10665/43870/9789241209472_eng.pdf;sequence=1 (accessed on 23 November 2022).
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Ji, X.; Li, R.; Yang, H.; Qi, P.; Xiao, Y.; Qian, M. Occurrence of patulin in various fruit products and dietary exposure assessment for consumers in China. Food Control 2017, 78, 100–107. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Xiao, Y.; Wang, W.; Lyu, W.; Wang, X.; Li, Y.; Deng, T.; Yang, H. Mycotoxins in cereal-based infant foods marketed in China: Occurrence and risk assessment. Food Control 2022, 138, 108998. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Sharma, B.; Borah, R.; Haque, S.; Mahmud, M.M.C.; Shah, A.K.; Rawal, D.; Bora, H.; Bui, S. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon 2020, 187, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef]
- Hayat, A.; Paniel, N.; Rhouati, A.; Marty, J.-L.; Barthelmebs, L. Recent advances in ochratoxin A-producing fungi detection based on PCR methods and ochratoxin A analysis in food matrices. Food Control 2012, 26, 401–415. [Google Scholar] [CrossRef]
- Bogs, C.; Battilani, P.; Geisen, R. Development of a molecular detection and differentiation system for ochratoxin A producing Penicillium species and its application to analyse the occurrence of Penicillium in cured meat. Int. J. Food Microbiol. 2006, 107, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Artigot, M.P.; Loiseau, N.; Laffitte, J.; Mas-Reguieg, L.; Tadrist, S.; Oswald, I.P.; Puel, O. Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus. Microbiology 2009, 155, 1738–1747. [Google Scholar] [CrossRef]
- Varga, J.; Due, M.; Frisvad, J.C.; Samson, R.A. Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Stud. Mycol. 2007, 59, 89–106. [Google Scholar] [CrossRef]
- Varga, J.; Rigó, K.; Molnár, J.; Tóth, B.; Szencz, S.; Téren, J.; Kozakiewicz, Z. Mycotoxin production and evolutionary relationships among species of Aspergillus section Clavati. Anton. Leeuw. Int. J. G. 2003, 83, 191–200. [Google Scholar] [CrossRef]
- Vismer, H.F.; Sydenham, E.W.; Schlechter, M.; Brown, N.L.; Hocking, A.D.; Rheeder, J.P.; Marasas, W.F.O. Patulin-producing Penicillium species isolated from naturally infected apples in South Africa. S. Afr. J. Sci. 1996, 92, 530–534. [Google Scholar]
- Svendsen, A.; Frisvad, J.C. A chemotaxonomic study of the Terverticillate penicillia based on high performance liquid chromatography of secondary metaboliti. Mycol Res 1994, 98, 1317–1328. [Google Scholar] [CrossRef]
- Dombrink-Kurtzman, M.A. The sequence of the isoepoxydon dehydrogenase gene of the patulin biosynthetic pathway in Penicillium species. Anton. Leeuw. Int. J. G. 2007, 91, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Filtenborg, O. Terverticillate Penicillia: Chemotaxonomy and mycotoxin production. Mycologia 2018, 81, 837–861. [Google Scholar] [CrossRef]
- Andersen, B.; Smedsgaard, J.; Frisvad, J.C. Penicillium expansum: Consistent production of patulin, chaetoglobosins, and other secondary metabolites in culture and their natural occurrence in fruit products. J. Agric. Food Chem. 2004, 52, 2421–2428. [Google Scholar] [CrossRef]
- Moake, M.M.; Padilla-Zakour, O.I.; Worobo, R.W. Comprehensive review of patulin control methods in foods. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef]
- Raiola, A.; Meca, G.; Garcia-Llatas, G.; Ritieni, A. Study of thermal resistance and in vitro bioaccessibility of patulin from artificially contaminated apple products. Food Chem. Toxicol. 2012, 50, 3068–3072. [Google Scholar] [CrossRef]
- Zheng, X.; Xia, F.; Li, J.; Zheng, L.; Rao, S.; Gao, L.; Yang, Z. Reduction of ochratoxin A from contaminated food by Lactobacillus rhamnosus Bm01. Food Control 2023, 143, 109315. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, L.; Xia, F.; Li, J.; Zhou, W.; Yuan, L.; Rao, S.; Yang, Z. Biological control of blue mold rot in apple by Kluyveromyces marxianus XZ1 and the possible mechanisms of action. Postharvest Biol. Technol. 2023, 196, 112179. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, W.; Rao, S.; Gao, L.; Li, H.; Yang, Z. Degradation of patulin in fruit juice by a lactic acid bacteria strain Lactobacillus casei YZU01. Food Control 2020, 112, 107147. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.; Deng, Q.; Sun, L.; Wang, R.; Ye, L.; Tao, S.; Liao, J.; Gooneratne, R. Fungal diversity and mycotoxin contamination in dried fish products in Zhanjiang market, China. Food Control 2021, 121, 107614. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Lam-Tung, N.; Schmidt, H.A.; von Haeseler, A.; Bui Quang, M. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2015, 50, 401–404. [Google Scholar] [CrossRef]
- Zhong, Q.D.; Li, G.H.; Wang, D.B.; Shao, Y.; Li, J.G.; Xiong, Z.H.; Wu, Y.N. Exposure assessment to ochratoxin a in Chinese wine. J. Agric. Food. Chem 2014, 62, 8908–8913. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Niu, Y.; Ma, T.; Xu, P.; Huang, W.; Zhan, J. Determination, content analysis and removal efficiency of fining agents on ochratoxin A in Chinese wines. Food Control 2017, 73, 382–392. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Han, S.Y.; Li, M.; Ma, T.Z.; Sheng, W.J.; Zhu, X. Simultaneous analysis of 20 mycotoxins in grapes and wines from hexi corridor region (China): Based on a QuEChERS-UHPLC-MS/MS Method. Molecules 2018, 23, 1926. [Google Scholar] [CrossRef]
- Bessaire, T.; Perrin, I.; Tarres, A.; Bebius, A.; Reding, F.; Theurillat, V. Mycotoxins in green coffee: Occurrence and risk assessment. Food Control 2019, 96, 59–67. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Yan, Z.; Li, Z.; Cheng, Y.; Chang, W. Occurrence and co-occurrence of mycotoxins in nuts and dried fruits from China. Food Control 2018, 88, 181–189. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zhang, H.; Apaliya, M.T.; Zhang, X.; Zhao, L.; Jiang, Z.; Yang, Q.; Gu, X. Identification and toxicological analysis of products of patulin degradation by Pichia caribbica. Biol. Control 2018, 123, 127–136. [Google Scholar] [CrossRef]




| Sample Classification | Sample Name | Number of Sample Type | Mean ± SD (µg/kg) | OTA Content (µg/kg) | Number of Positive | Number of Exceedance | Risk |
|---|---|---|---|---|---|---|---|
| Cereals related foods | Rice | 7 | / | / | / | / | / |
| Corn grit | 6 | 3.22 b ± 0.63 | 2.90–3.53 | 2 | / | / | |
| Corn flour | 7 | / | / | / | / | / | |
| Corn flakes | 3 | 1.32 c ± 0.07 | 1.32 | 1 | / | / | |
| Sticky rice | 3 | / | / | / | / | / | |
| Millet | 7 | 2.04 c ± 0.05 | 2.04 | 1 | / | / | |
| Black rice | 5 | 6.40 a ± 0.33 | 6.40 | 1 | 1 | serious | |
| Brown rice | 5 | 1.70 c ± 0.22 | 1.70 | 1 | / | / | |
| Wheat flour | 5 | / | / | / | / | / | |
| Oat | 9 | 2.26 c ± 1.72 | 1.22–4.25 | 3 | / | / | |
| Total | 57 | 2.80 ± 1.91 | 1.22–6.40 | 9 | 1 | / | |
| Nuts | Pistachio nuts | 3 | 1.55 c ± 0.06 | 1.55 | 1 | / | / |
| Sinian wood | 2 | / | / | / | / | / | |
| Walnut | 2 | 8.17 a ± 6.24 | 3.75–12.58 | 2 | 1 | serious | |
| Broad bean | 2 | / | / | / | / | / | |
| Macadamia nut | 5 | 8.19 a ± 3.70 | 4.66–12.04 | 3 | 2 | serious | |
| Cashew nut | 4 | / | / | / | / | / | |
| Pecan fruit | 3 | 3.35 b ± 0.13 | 3.35 | 1 | / | / | |
| Melon seed | 2 | 4.20 b ± 0.11 | 4.20 | 1 | / | / | |
| Total | 23 | 4.41 ± 3.40 | 1.55–12.58 | 8 | 3 | / | |
| Coffees | Hazelnut coffee | 4 | 35.61 a ± 0.21 | 35.61 | 1 | 1 | serious |
| Latte | 4 | 40.01 a ± 0.27 | 40.01 | 1 | 1 | serious | |
| White coffee | 3 | 26.15 b ± 31.14 | 4.13–48.17 | 2 | 1 | serious | |
| White peach coffee | 5 | 20.72 c ± 17.35 | 8.45–32.99 | 2 | 2 | serious | |
| Total | 16 | 20.20 ± 18.12 | 4.13–48.17 | 6 | 5 | / | |
| Wine | Wine | 3 | 4.02 a ± 0.79 | 3.12–4.59 | 3 | 3 | serious |
| Total | 3 | 4.02 ± 0.79 | 3.12–4.59 | 3 | 3 | / | |
| Sample classification | Sample Name | Number of Sample Type | Mean ± SD (µg/kg) | PAT content (µg/kg) | Number of positive | Number of exceedance | risk |
| Fruits | Red Fuji Apple | 5 | / | / | / | / | / |
| Fresh-cut apple | 5 | 192.78 ± 7.30 | 192.78 | 1 | 1 | serious | |
| Pear | 3 | / | / | / | / | / | |
| Total | 13 | 192.78 ± 7.30 | 192.78 | 1 | 1 | / | |
| Apple products | Apple juice | 13 | 84.16 ± 52.69 | 26.85–140.44 | 5 | 3 | serious |
| Cider | 3 | 87.14 ± 3.03 | 87.14 | 1 | 1 | serious | |
| Apple jelly | 3 | / | / | / | / | / | |
| Apple cider vinegar | 5 | / | / | / | / | / | |
| Total | 24 | 85.28 ± 39.90 | 26.85–140.44 | 6 | 4 | / | |
| Hawthorn products | Hawthorn preserves | 13 | 63.03 ± 30.11 | 29.83–88.56 | 3 | 2 | serious |
| Total | 13 | 63.03 ± 30.11 | 29.83–88.56 | 3 | 2 | / | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Q.; Zhang, Z.; Huang, P.; Wang, B.; Zheng, X. Assessment of Fungal and Contamination of Ochratoxin A and Patulin in Foods Susceptible to Contamination in the Yangzhou Market, China. Foods 2024, 13, 3205. https://doi.org/10.3390/foods13193205
Gong Q, Zhang Z, Huang P, Wang B, Zheng X. Assessment of Fungal and Contamination of Ochratoxin A and Patulin in Foods Susceptible to Contamination in the Yangzhou Market, China. Foods. 2024; 13(19):3205. https://doi.org/10.3390/foods13193205
Chicago/Turabian StyleGong, Qinghua, Zihan Zhang, Peiwen Huang, Bo Wang, and Xiangfeng Zheng. 2024. "Assessment of Fungal and Contamination of Ochratoxin A and Patulin in Foods Susceptible to Contamination in the Yangzhou Market, China" Foods 13, no. 19: 3205. https://doi.org/10.3390/foods13193205
APA StyleGong, Q., Zhang, Z., Huang, P., Wang, B., & Zheng, X. (2024). Assessment of Fungal and Contamination of Ochratoxin A and Patulin in Foods Susceptible to Contamination in the Yangzhou Market, China. Foods, 13(19), 3205. https://doi.org/10.3390/foods13193205







