Evaluation of High Vacuum Flavor Extraction Device as a Novel Technique for the Extraction of Volatile Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Preparation of Fried Tilapia Mince
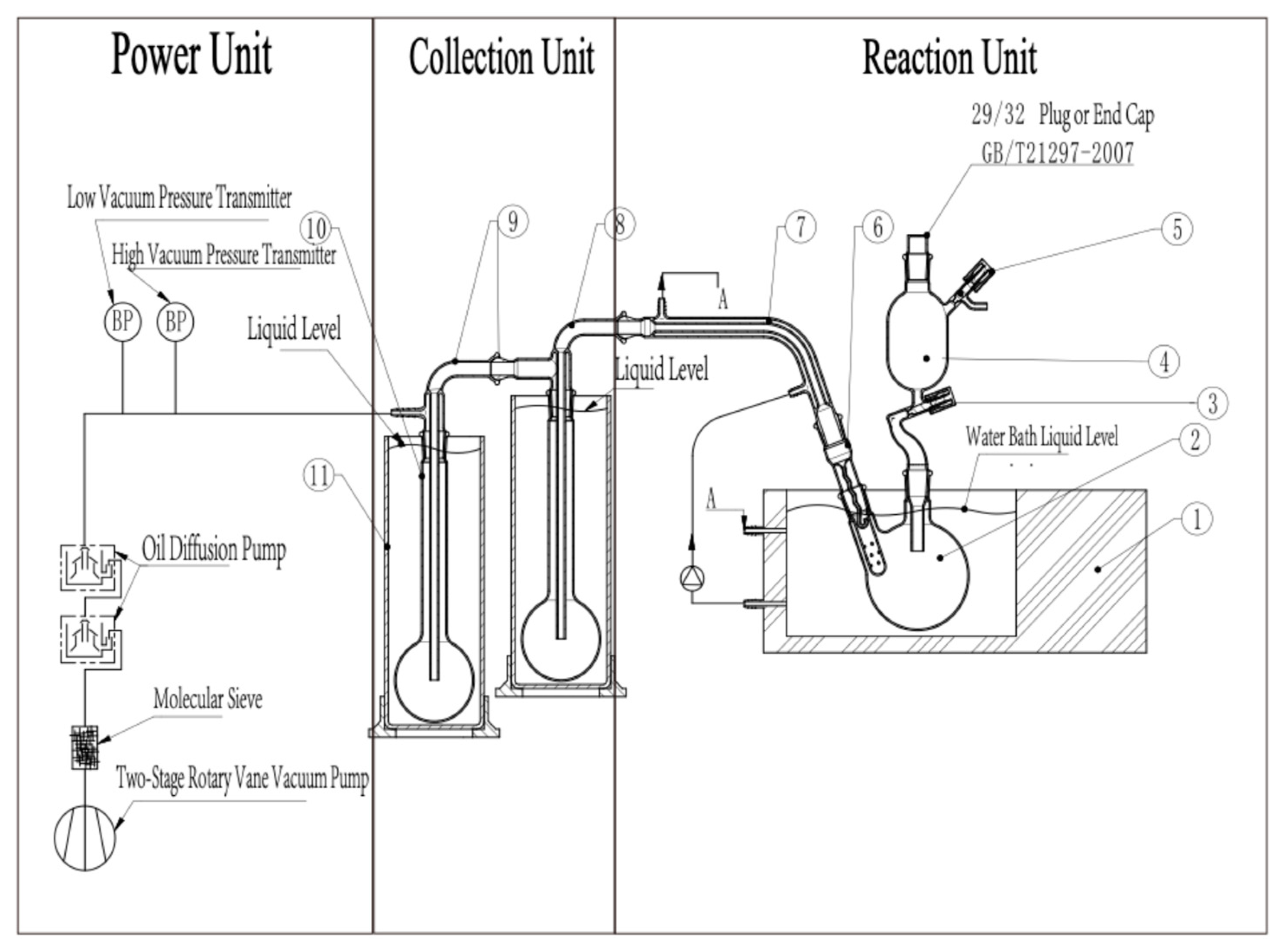
2.3. Manufacturing and Features of HVE
2.4. Precision and Reproducibility Experiment for HVE
2.5. Experiment on the Extraction and Separation of Volatile Compounds in Tilapia
2.5.1. Preliminary Extraction of Key Volatile Flavor Compounds from Fried Tilapia Mince Using Accelerated Solvent Extraction Device (ASE)
2.5.2. Extraction of Key Volatile Flavor Compounds from Fried Tilapia Mince Using HVE
2.5.3. Extraction of Key Volatile Flavor Compounds from Fried Tilapia Mince Using SAFE
2.5.4. Extraction of Key Volatile Flavor Compounds from Fried Tilapia Mince Using SDE
2.6. Gas Chromatography (GC) Analysis of Volatile Compounds
2.7. Triangle Test Method to Assess the Effectiveness of Three Extraction Methods on the Aromatic Substances from Fried Tilapia Mince
2.8. Data Analysis
3. Results and Discussion
3.1. Precision and Reproducibility Results for the HVE Extraction Device
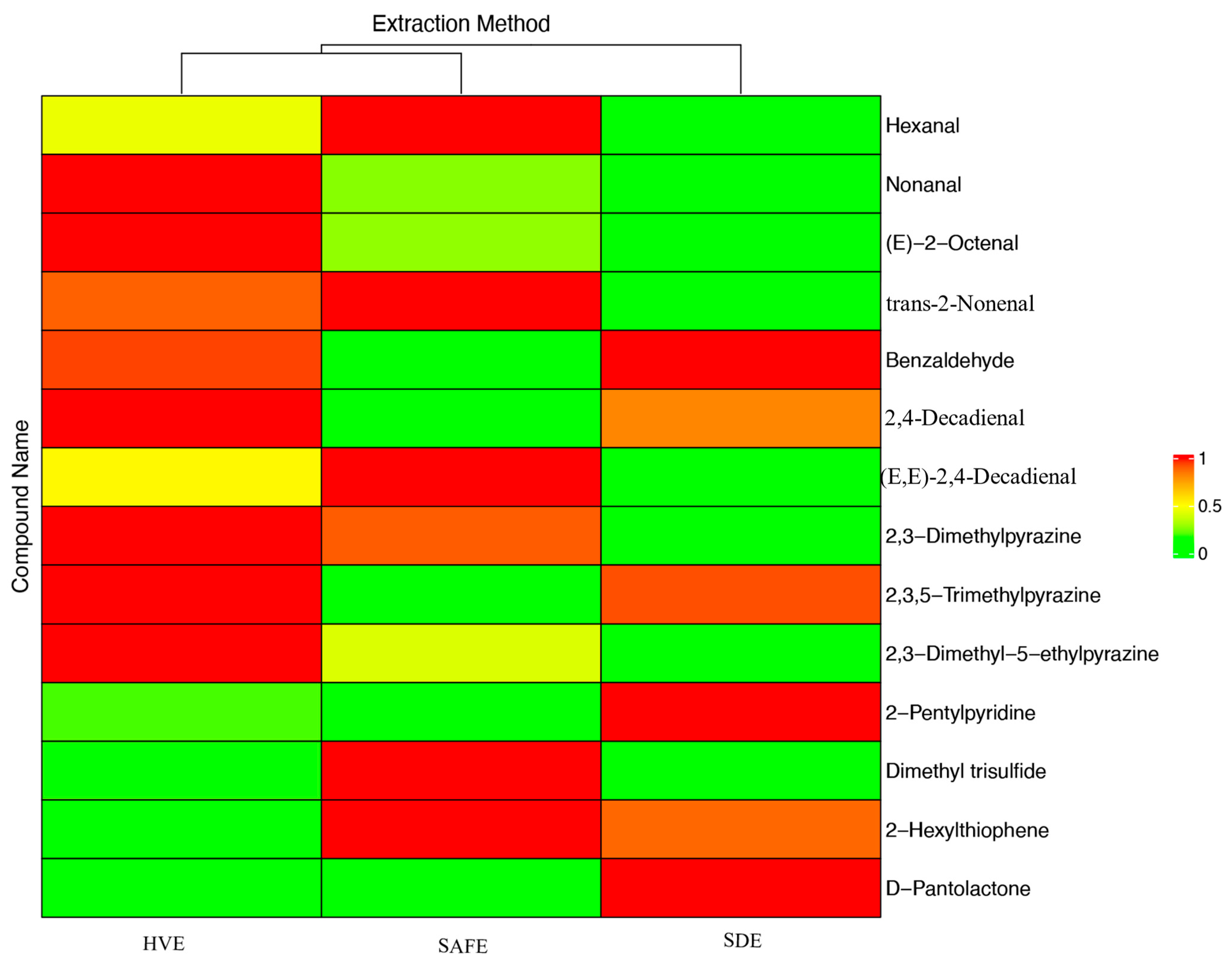
3.2. Study on the Extraction Efficiency of Different Types of Volatile Compounds in Tilapia by Various Extraction Methods
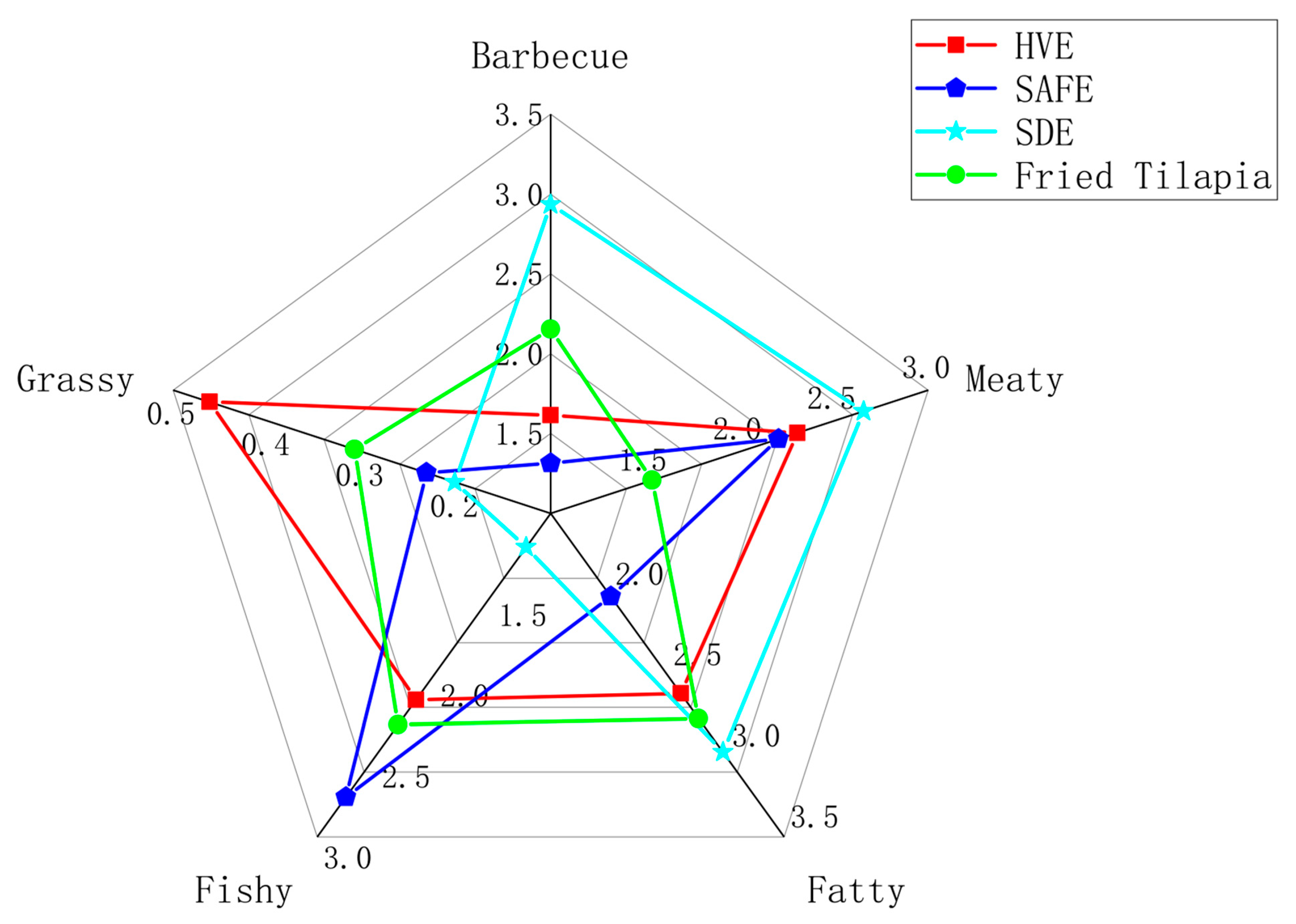
3.3. Comparison of Odor Profiles of Volatile Compounds in Fried Tilapia Mince by Three Different Extraction Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Zhao, X.; Zhao, M.; Liu, X.; Pang, Y.; Zhang, M. Characterization of the Key Aroma Constituents in Fried Tilapia through the Sensorics Concept. Foods 2022, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Qian, Y.L.; Alcazar Magana, A.; Xiong, S.; Qian, M.C. Comparative Characterization of Aroma Compounds in Silver Carp (Hypophthalmichthys molitrix), Pacific Whiting (Merluccius productus), and Alaska Pollock (Theragra chalcogramma) Surimi by Aroma Extract Dilution Analysis, Odor Activity Value, and Aroma Recombination Studies. J. Agric. Food Chem. 2020, 68, 10403–10413. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Niu, G.; Zhou, X.; Gao, K.; Liu, X. Interactions of Heat-Induced Myosin with Hsian-Tsao Polysaccharide to Affect the Fishy Odor Adsorption Capacity. Food Hydrocoll. 2023, 136, 108282. [Google Scholar] [CrossRef]
- de Sousa Galvão, M.; Narain, N.; do Socorro Porto dos Santos, M.; Nunes, M.L. Volatile Compounds and Descriptive Odor Attributes in Umbu (Spondias tuberosa) Fruits during Maturation. Food Res. Int. 2011, 44, 1919–1926. [Google Scholar] [CrossRef]
- Li, N.; Sun, B.-G.; Zheng, F.-P.; Chen, H.-T.; Liu, S.-Y.; Gu, C.; Song, Z.-Y. Identification of Volatile Components in Yak Butter Using SAFE, SDE and HS-SPME-GC/MS. Nat. Prod. Res. 2012, 26, 778–784. [Google Scholar] [CrossRef]
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F. Comparison of Two Extraction Techniques (SDE vs. SPME) for the Determination of Garlic and Elephant Garlic Volatile Compounds. Food Anal. Methods 2022, 15, 1867–1879. [Google Scholar] [CrossRef]
- Majcher, M.; Jeleń, H.H. Comparison of Suitability of SPME, SAFE and SDE Methods for Isolation of Flavor Compounds from Extruded Potato Snacks. J. Food Compos. Anal. 2009, 22, 606–612. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Jiang, X.; Huang, M.; Liu, H.; Meng, N.; Wu, J.; Zhao, D. Comparative Study on Key Odorants of Jiujiang Fenggang Huangjiu and Their Succession Regularities during Aging Using Sensory-Directed Flavor Analysis. Food Chem. 2024, 430, 137052. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Fu-ping, Z.; Hai-tao, C.; Si-yuan, L.; Chen, G.; Zhen-yang, S.; Bao-guo, S. Identification of Volatile Components in Chinese Sinkiang Fermented Camel Milk Using SAFE, SDE, and HS-SPME-GC/MS. Food Chem. 2011, 129, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Fang, Z.; Chen, J.; Zhang, Q.; Sang, N.; Zheng, T.; Zhang, C.; Yang, G.; Liu, Y. Improving Perception Accuracy of Flexible Capacitive Stretchable Sensors Using Piecewise Power-Law Model. IEEE Sens. J. 2024, 24, 21436–21446. [Google Scholar] [CrossRef]
- ISO. Accuracy (Trueness and Precision) of Measurement Methods and Results; 5725–1; ISO: Geneva, Switzerland, 2023. [Google Scholar]
- Vilas-Franquesa, A.; Juan, B.; Saldo, J. Targeted Analysis of Sea Buckthorn Oil Extracted by Accelerated Solvent Extraction Technique Using Green and Conventional Solvents. LWT 2022, 164, 113643. [Google Scholar] [CrossRef]
- Wang, R.; Wu, L.-X.; Guo, B.-X.; Zhao, P.-H.; Yin, W.-T.; Liu, H.-M.; Mei, H.-X.; Duan, Y.-H. Characterization of Aroma-Active Compounds in Sesame Hulls at Different Roasting Temperatures by SAFE and GC-O-MS. Food Chem. X 2024, 21, 101203. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhao, M.; Zhen, D.; Tan, J.; Wang, T.; Xie, J. Key Aroma Compounds in Chinese Fried Food of Youtiao. Flavour Fragr. J. 2020, 35, 88–98. [Google Scholar] [CrossRef]
- Xie, J.; Sun, B.; Zheng, F.; Wang, S. Volatile Flavor Constituents in Roasted Pork of Mini-Pig. Food Chem. 2008, 109, 506–514. [Google Scholar] [CrossRef]
- Grigorakis, K.; Taylor, K.D.A.; Alexis, M.N. Organoleptic and Volatile Aroma Compounds Comparison of Wild and Cultured Gilthead Sea Bream (Sparus aurata): Sensory Differences and Possible Chemical Basis. Aquaculture 2003, 225, 109–119. [Google Scholar] [CrossRef]
- Fan, W.; Qian, M.C. Characterization of Aroma Compounds of Chinese “Wuliangye” and “Jiannanchun” Liquors by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Cheng, Y.; Liu, Y. Effect of Frying Oils’ Fatty Acid Profile on Quality, Free Radical and Volatiles over Deep-Frying Process: A Comparative Study Using Chemometrics. LWT-Food Sci. Technol. 2019, 101, 331–341. [Google Scholar] [CrossRef]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.-P.; Zhang, Y.; Dai, W.-D.; Guo, L.; Tan, J.-F.; Peng, Q.-H.; et al. Identification and Quantification of Key Odorants in the World’s Four Most Famous Black Teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Iraqi, R.; Vermeulen, C.; Benzekri, A.; Bouseta, A.; Collin, S. Screening for Key Odorants in Moroccan Green Olives by Gas Chromatography−Olfactometry/Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2005, 53, 1179–1184. [Google Scholar] [CrossRef]
- Salazar, R.; Domenek, S.; Ducruet, V. Interactions of Flavoured Oil In-Water Emulsions with Polylactide. Food Chem. 2014, 148, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Callemien, D.; Dasnoy, S.; Collin, S. Identification of a Stale-Beer-like Odorant in Extracts of Naturally Aged Beer. J. Agric. Food Chem. 2006, 54, 1409–1413. [Google Scholar] [CrossRef]
- Hausch, B.J.; Arpaia, M.L.; Kawagoe, Z.; Walse, S.; Obenland, D. Chemical Characterization of Two California-Grown Avocado Varieties (Persea americana Mill.) over the Harvest Season with an Emphasis on Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2020, 68, 15301–15310. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Xie, J.; Xiao, Q.; Cheng, J.; Chen, F.; Wang, S.; Sun, B. Formation Mechanism of Aroma Compounds in a Glutathione-Glucose Reaction with Fat or Oxidized Fat. Food Chem. 2019, 270, 436–444. [Google Scholar] [CrossRef]
- Corral, S.; Salvador, A.; Flores, M. Elucidation of Key Aroma Compounds in Traditional Dry Fermented Sausages Using Different Extraction Techniques. J. Sci. Food Agric. 2015, 95, 1350–1361. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Yang, W.; Liu, Y.; Zhang, Y.; Huang, M.; Sun, B. Characterization of the Potent Odorants Contributing to the Characteristic Aroma of Beijing Douzhi by Gas Chromatography–Olfactometry, Quantitative Analysis, and Odor Activity Value. J. Agric. Food Chem. 2018, 66, 689–694. [Google Scholar] [CrossRef]
- Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of Key Aroma Compounds in Fresh and Roasted Terebinth Fruits Using Aroma Extract Dilution Analysis and GC–MS-Olfactometry. Microchem. J. 2019, 145, 96–104. [Google Scholar] [CrossRef]
- Gu, Y.; Feng, T.; Song, S.; Yao, L.; Sun, M.; Wang, H.; Liu, Q.; Yu, C. GC-O-MS Analysis of Aroma-Active Compounds of Chinese Almonds Obtained by Different Pretreatment Methods. J. Food Biochem. 2023, 2023, 9950778. [Google Scholar] [CrossRef]
- Miller, M.E.; Stuart, J.D. Comparison of Gas-Sampled and SPME-Sampled Static Headspace for the Determination of Volatile Flavor Components. Anal. Chem. 1999, 71, 23–27. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Song, H.-L. Comparison of Four Extraction Methods, SPME, DHS, SAFE, Versus SDE, for the Analysis of Flavor Compounds in Natto. Food Anal. Methods 2018, 11, 343–354. [Google Scholar] [CrossRef]
- Huang, X.-H.; Zhang, Y.-Y.; Zhu, M.; Zhou, D.-Y.; Du, M.; Zhu, B.-W.; Dong, X.-P.; Fisk, I.; Qin, L. The Effects of Different Extraction Methods on the Aroma Fingerprint, Recombination and Visualization of Clam Soup. Food Funct. 2021, 12, 1626–1638. [Google Scholar] [CrossRef]
- Sarhir, S.T.; Amanpour, A.; Selli, S. Characterization of Ayran Aroma Active Compounds by Solvent-Assisted Flavor Evaporation (SAFE) with Gas Chromatography–Mass Spectrometry–Olfactometry (GC–MS–O) and Aroma Extract Dilution Analysis (AEDA). Anal. Lett. 2019, 52, 2077–2091. [Google Scholar] [CrossRef]
- Yao, L.; Mo, Y.; Chen, D.; Feng, T.; Song, S.; Wang, H.; Sun, M. Characterization of Key Aroma Compounds in Xinjiang Dried Figs (Ficus carica L.) by GC–MS, GC–Olfactometry, Odor Activity Values, and Sensory Analyses. LWT 2021, 150, 111982. [Google Scholar] [CrossRef]
| SN | CC | Compound Name | Standard Curve Equation | RC | Content (mg/L) | RSD (%) | RR (%) |
|---|---|---|---|---|---|---|---|
| 1 | Nitrogen Compounds | 2,3,5-Trimethylpyrazine | y = 28.23 + 12.57x | 0.9992 | 24.15 ± 2.74 | 11.36 | 96.59 |
| 2 | 2,3-Dimethyl-5-ethylpyrazine | y = 36.64 + 13.17x | 0.9991 | 21.39 ± 2.78 | 12.99 | 85.54 | |
| 3 | 2,3-Dimethylpyrazine | y = 30.37 + 12.76x | 0.9993 | 21.56 ± 2.73 | 12.67 | 86.24 | |
| 4 | Sulfur Compounds | 2-Hexylthiophene | y = 32.90 + 16.20x | 0.9994 | 25.80 ± 2.86 | 11.08 | 85.99 |
| 5 | Dimethyl trisulfide | y = 11.47 + 3.32x | 0.9992 | 22.30 ± 2.78 | 12.45 | 89.19 | |
| 6 | Aldehydes | (E)-2-Octenal | y = 30.82 + 12.37x | 0.9991 | 22.22 ± 1.98 | 8.90 | 88.86 |
| 7 | (E,E)-2,4-Decadienal | y = 22.97 + 9.35x | 0.9997 | 22.38 ± 2.20 | 9.85 | 89.53 | |
| 8 | Benzaldehyde | y = 10.05 + 6.48x | 0.9995 | 21.88 ± 2.51 | 11.46 | 87.53 | |
| 9 | trans-2-Nonenal | y = 20.36 + 11.39x | 0.9994 | 24.71 ± 1.98 | 8.01 | 98.83 | |
| 10 | Hexanal | y = 27.85 + 9.58x | 0.9990 | 21.88 ± 2.95 | 13.49 | 87.54 | |
| 11 | Ketones | Hydroxyacetone | y = 5.99x + 0.37 | 0.9997 | 37.33 ± 4.84 | 12.97 | 93.32 |
| 12 | Esters | D-Pantolactone | y = −3.59 + 2.57x | 0.9993 | 24.37 ± 3.41 | 14.01 | 97.48 |
| SN | CC | Compound Name | Standard Curve Equation | RC | HVE (mg/kg) | SAFE (mg/kg) | SDE (mg/kg) |
|---|---|---|---|---|---|---|---|
| 1 | Aldehydes | Hexanal | y = 68.57x − 1.57 | 0.9998 | 60.48 ± 7.41 a | 99.30 ± 11.82 b | 27.72 ± 1.43 c |
| 2 | Nonanal | y = 6.68x + 0.28 | 0.9990 | 258.16 ± 36.47 a | 225.63 ± 5.29 b | 213.99 ± 7.87 b | |
| 3 | (E)-2-Octenal | y = 8.82x + 1.02 | 0.9994 | 47.065 ± 6.687 a | 27.19 ± 1.91 b | 19.51 ± 3.19 b | |
| 4 | trans-2-Nonenal | y = 37.46x + 1.81 | 0.9996 | 55.09 ± 5.29 a | 58.74 ± 8.36 a | 22.81 ± 1.76 b | |
| 5 | Benzaldehyde | y = 43.16x + 0.38 | 0.9998 | 131.61 ± 14.67 a | 29.77 ± 2.02 b | 137.70 ± 12.20 a | |
| 6 | 2,4-Decadienal | y = 26.18x − 0.23 | 0.9995 | 60.80 ± 7.49 a | 9.09 ± 0.79 b | 51.511 ± 3.272 c | |
| 7 | (E,E)-2,4-Decadienal | y = 18.40x + 2.92 | 0.9993 | 341.05 ± 35.59 a | 477.51 ± 68.90 b | 195.08 ± 0.65 c | |
| 8 | Nitrogen Compounds | 2,3-Dimethylpyrazine | y = 30.27x + 1.70 | 0.9997 | 35.76 ± 3.82 a | 33.57 ± 1.82 a | 12.25 ± 1.05 b |
| 9 | 2,3,5-Trimethylpyrazine | y = 33.32x + 2.01 | 0.9998 | 34.81 ± 4.55 a | 27.65 ± 3.73 a | 34.30 ± 5.12 a | |
| 10 | 2,3-Dimethyl-5-ethylpyrazine | y = 32.41x + 2.56 | 0.9998 | 26.61 ± 1.83 a | 25.07 ± 1.65 a | 23.98 ± 3.06 a | |
| 11 | 2-Pentylpyridine | y = 39.95x + 1.66 | 0.9997 | 53.82 ± 8.21 a | 2.35 ± 0.38 b | 265.29 ± 32.61 c | |
| 12 | Sulfur Compounds | Dimethyl Trisulfide | y = 8.82x + 1.02 | 0.9994 | 60.65 ± 10.00 a | 77.35 ± 9.33 a | 57.18 ± 3.16 a |
| 13 | 2-Hexylthiophene | y = 13.09x − 0.23 | 0.9995 | 5.55 ± 0.37 a | 23.29 ± 3.15 b | 21.22 ± 0.10 b | |
| 14 | Esters | D-Pantolactone | y = 21.20x − 1.09 | 0.9999 | 126.09 ± 9.64 a | 128.30 ± 3.07 a | 197.48 ± 24.71 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhou, J.; Qin, J.; Qin, Z.; Jiang, J.; Yu, F.; Chen, M.; Liu, X.; Zhang, M. Evaluation of High Vacuum Flavor Extraction Device as a Novel Technique for the Extraction of Volatile Compounds. Foods 2024, 13, 3206. https://doi.org/10.3390/foods13193206
Liu M, Zhou J, Qin J, Qin Z, Jiang J, Yu F, Chen M, Liu X, Zhang M. Evaluation of High Vacuum Flavor Extraction Device as a Novel Technique for the Extraction of Volatile Compounds. Foods. 2024; 13(19):3206. https://doi.org/10.3390/foods13193206
Chicago/Turabian StyleLiu, Mingyuan, Jie Zhou, Jingkai Qin, Zhongyi Qin, Jiequn Jiang, Futian Yu, Mei Chen, Xiaoling Liu, and Meishuo Zhang. 2024. "Evaluation of High Vacuum Flavor Extraction Device as a Novel Technique for the Extraction of Volatile Compounds" Foods 13, no. 19: 3206. https://doi.org/10.3390/foods13193206
APA StyleLiu, M., Zhou, J., Qin, J., Qin, Z., Jiang, J., Yu, F., Chen, M., Liu, X., & Zhang, M. (2024). Evaluation of High Vacuum Flavor Extraction Device as a Novel Technique for the Extraction of Volatile Compounds. Foods, 13(19), 3206. https://doi.org/10.3390/foods13193206





